Impact of cinnamic acid on physiological and anatomical changes in maize plants ( Zea mays L.) grown under salinity stress
Автор: Singh Pramod Kumar, Chaturvedi Varun Kumar
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 2 т.10, 2014 года.
Бесплатный доступ
The environmental contamination with high salt is the elementary intimidation to the agriculture. Maize plants were deeply affected due to salinity worldwide and a severe problem to scientists. A probable survival strategy of the plants under unpleasant environmental circumstances is to use of endogenous metabolites that could ameliorate the harsh effect of salinity. Current study was under taken to observe the effect of cinnamic acid (CA), a central molecule of phenylpropanoid pathway (Secondary metabolism) on the growth and development of maize plants under NaCl stress conditions. CA is rapidly produced by plants in response to stressful condition. Response to maize seed to the presoaking treatment 0.05mM CA was deliberated under different concentration of NaCl stress such as 50, 100, 150, 200, mM NaCl for 14 days. The injurious effects of salinity on growth and development were manifested by decreased fresh weight, dry weight, and relative water content (RWC) and chlorophyll pigment contents. Degree of lipid peroxidation turned down through the significant decrease in MDA content in maize seedlings. CA induced the anatomical properties under salinity in present exploration. The cortical cells were induced in root in response to CA than stress. Here, the present study was undertaken with the aim of determining salt induced anatomical and morphological alteration in the presence of exogenous CA. The major reduction in dimension of cortical cells was observed which indicate that salt stress reduced the tolerance of cortical cell more than treatment in maize root. We conclude that CA is a potential phenylpyranoid for protecting crop plant under saline environment.
Adaptation, cinnamic acid, cortical parenchyma, lipid peroxidation, phenypyrenoids, salt stress, zea mays l
Короткий адрес: https://sciup.org/14323874
IDR: 14323874
Текст научной статьи Impact of cinnamic acid on physiological and anatomical changes in maize plants ( Zea mays L.) grown under salinity stress
The problem of soil salinity has been aggravated such as saline irrigation and use of chemical by extensive use of modern agricultural practices fertilizers. To achieve improved salt tolerance in crop plants it is important to prevent cellular damage and to re-establish the homeostatic conditions in the new, stressful environment. These environmental stresses often impair the availability of water for its biological functions like its role as a solvent, transpirant, electron donor; transport medium, etc. (Zhu, 2002, Zolla et al., 2010). Effect due to salt on plant with time and degree of ions accumulation which eventually rises to toxic level and impose an addition stress on physiological process (Muns, 2002). As some morphological parameters were negatively affected by salinity, some anatomical variables were diminished as well. Cortical parenchyma and vascular cylinder were reduced (Ceccoli et al., 2011). High salt content, especially chloride and sodium sulphates, affects plant growth by modifying their morphological, anatomical (Szepesi et al., 2009) and physiological traits (Singh et al., 2013). Reduction in growth under saline conditions is a consequence of several physiological responses, including modification of ion balance, water status, mineral nutrition, stomatal behavior, photosynthetic efficiency, and carbon allocation and utilization (Munns and Tester, 2008).Soil salinization is continuously reducing the arable land for conventional agriculture and resulting in severe agricultural losses (Parida and Das, 2005). Development of methods for inducing stress tolerance in plants against adverse environmental conditions is vital and necessary and is a major focus of research over many decades (Zuchi and Astolfi, 2012). The present study is an aim to assess fundamental effect of CA on biochemical changes such as photosynthetic pigment, lipid peroxidation and anatomical changes in two week old maize seedlings under saline and non saline condition. Here we also ascertained the counteraction of CA on growth characteristics such as dry weight, fresh weight root length, shoot length and RWC under NaCl stress.
MATERIALS AND METHODS
Plant material and growth conditions
Seeds of Z ea mays L. (Var. Jaunpuri) were procured from Genetics and Plant Breeding Department, Institute of Agricultural Sciences, BHU, India. Seeds were surface sterilized with 0.01% HgCl 2 followed thorough washing with glass-distilled water. Homogenous surface sterilized seeds were presoaked in different treatment for six hrs as follows, Control in distilled water; 0.05 mM CA in distilled water; 50, 100, 150, and 200 mM of NaCl and 0.05 mM CA with each level of salinity. Presoaked seeds were placed in acid washed petridishes lined with moistened whatman no. 1 filter paper in an incubator at 27±2°C for germination. And transferred to pots with perforated plastic tops. Plants were grown under artificial light (in the range of 27±2°C air temperature, 450-500 μmol m-2 s-1 light intensity and 75% relative humidity). Each pot contains four plants and supplied with Hoagland nutrient solution at alternate days (Hoagland and Arnon 1950) (half strength) along with different salinity levels (0, 50, 100, 150, 200 mM NaCl).
Plant measurement and analysis
Growth dynamics of corn ( Zea mays L.) plants were analyzed in two weeks old maize seed lings. Plants were uprooted carefully and washed in distilled water for the measurement of root length, shoot length, fresh weight and dry weight according to standard methods. Plants had been placed in a thermo ventilated oven at 70 °C until a constant dry weight was obtained and their weight expressed as g plant-1 basis.
Relative water content (%)
The effect of salt stress on leaf relative water contents (RWC) was observed and calculated by method of (Yamasaki and Dillenburg 1999). Third leaves of the two weeks old corn plants were removed to observe fresh mass (FM) and were floated in distilled water inside a closed Petri dish for turgid mass(TM). Thereafter, periodical weight during the imbibitions period, leaves were after gently wiping the water from the leaf surface. At the end of imbibitions period, leaf sample were placed in a pre heated oven at 80ºCfor 48h in order to obtain dry mass (DM).Value of FM, TM.DM were used to calculate LRWC using the equation:
LRWC (%) = [(FM - DM)/ (TM - DM)] 100.
Where FM is fresh mass, DM is dry mass, and TM is turgid mass.
Measurement of leaf chlorophyll contents
Photosynthetic pigments (chlorophyll a, b and carotenoids) were measured in fresh leaf samples before harvesting. Leaf samples (0.5 g) were homogenized with acetone (90% v/v), filtered and made up to a final volume of 50 ml. Pigment concentrations were calculated from the absorbance of extract at 663, 645 and 470 nm using the formula of (Lichtenthaler, 1987).
Chlorophyll a (mg/g FW) = (11.75 x A663 – 2.35 x A645) x 50/500
Chlorophyll b (mg/g FW) = (18.61 x A645 – 3.96 x A663) x 50/500
Carotenoid (mg/g FW) = [(1, 000 x A470) – (2.27 x Chl a)] – (81.4 x Chl b)/227) x 50/500].
Determination of MDA content
Lipid peroxidation in leaf was evaluated by malondialdehyde (MDA) content following the method of (Heath and Packer, 1968). For the determination of MDA, 0.5 g leaf tissue was homogenized in 10 ml of 0.1% trichloroacetic acid (TCA) in an ice bath. Then homogenate was transferred to a tube and centrifuged at 15,000 g for 5 min at 4oC. To 10 ml aliquot of the supernatant 4.0 ml of 0.5% thiobarbituric acid in 20% TCA was added into a new tube. The mixture was incubated at 95°C for 30 min and then quickly cooled at room temperature and centrifuged at 10,000g for 10 min, absorbance of the supernatant was recorded at 532 nm by spectrophotometric analysis. The value for non-specific absorption at 600 nm was subtracted. The MDA equivalent was calculated as follows,
MDA (nmol / ml FW) = {(A 532 – A 600) /155, 000} x 106
Study of changes in root anatomy
Fresh free hand cross sections were prepared from adventitious root of 14 days old maize root (Lux et al. 2005). Cross sections were arranged from middle of root in different level of salinity (0, 50, 100, 150, 200 mM NaCl) in presence or absence of 0.05 mM CA. Sections of root were arranged in orders to control, stress, treatment and stress with treatments, were deliberated. The sections were fixed for 48 h by immersion [5% formaldehyde, 5% acetic acid, 90% ethanol (70%)]. Dehydration of the tissue sections were accomplished in a graded ethanol series (50, 70, 95, and 100%, 30 min each) followed by immersion in tert-butanol (8 h) and embedding in Paraplast Plus. After hardening, 12 μm thick cross-sections were cut with a rotation microtome. Cross-sections were collected on glass slides. The tissue sections were deparaffinized in xylene (3× 10 min) and rehydrated (ethanol 100, 95, 70, and 50%, 5 min each). The washed sections (H2O, 1 min) were successively stained with safranin (0.5%) (0.5%; (Ruzin, 1999) and references within), cleared with 100% xylene (3× 10 min), and air dried. Digital images were taken. Measured parameters included epidermal layer, cortex parenchyma, endodermis, stele, and vascular cylinder. The crosssections were analyzed under light microscopy for differences in the cortical cell, vascular cylinder and the lignisation of endodermis and peri-/exodermis after staining with safranine (Brundrett et al., 1988).
Statistical analysis
Results were presented as means values ± standard error designed with five replications. Data were analyzed by one way analysis of variance (ANOVA). Means were compared by the least significant difference test of the 0.05% level of significance.
RESULTS
Growth response to salt stress
Fresh weight/ Dry matter
Influence of CA on growth measurements of maize plants under salinity stress were analyzed by accumulation of root shoot weight. Fresh weight of maize plants gradually decreased with increasing concentrations of salt and was significant at 100 mM of NaCl (Table-1). Further, fresh weight of two week old maize plants sharply reduced at higher doses of salinity (51.53 % at 150 mM and 57.64 % at 200 mM of NaCl) in comparison to aqueous control. Application of CA showed increase in fresh weight of plants in both saline and non-saline conditions. 7 % increase in fresh weight (g-1 plant) was recorded in non-saline condition, however under saline conditions CA enhanced the growth by 21.15 % in comparison to 150 mM of NaCl- stressed plants and alleviates the destructive effects of salt.
Dry matter of plants decreased significantly with exposure to NaCl salinity and the reduction was severe at 200 mM of NaCl treatment without CA. Exogenously applied 0.05 mM CA increased dry yield in both saline and non-saline conditions (Table-1). However, this effect of CA was more pronounced and significant in saline conditions. Salinity affected reduction in growth parameters such as root and shoot length was greater in the absence of CA, whereas in the presence of exogenous CA, effects of salinity were countered significantly.
Root length/ Shoot length
Root length of two week old maize plants decreased upto 28 % at 200 mM of NaCl in comparison to aqueous control whereas exogenous CA application alleviated the damaging effect of deleterious salt concentration by 16 % of aqueous control (Table-1). Similar trend was observed in the case of shoot length; however reduction in shoot length was high in comparison to that of root. Shoot length decreased upto 60.4 % at 200 mM of NaCl in comparison to aqueous control plants. CA control plants showed 9 % increase in shoot length as compared to CA deficient control. At higher doses of salinity exogenous CA application alleviated the effect of salt and the decrease was 47 % at 200 mM of NaCl.
Relative Water Content
The relative water content in the leaves decreased gradually with the increasing concentration of NaCl and the decrease was 17 % and 25 % at 150 mM and 200 mM of NaCl respectively, whereas the subsequent treatment of CA significantly increased its level over the control (an increase of 4 %) and also overcome the toxic effects generated by NaCl (7 % and 15 % at 150 mM and 200 mM of NaCl respectively) Table-2. Salinity in plant tissue is the decrease of relative water contents in stress plants may be associated with the decrease of plant dynamism. Leaf water potential also decreased significantly under salinity stress .CA treatment induced an increase in RWC of the stress seedlings.
Photosynthetic pigments
The contents of photosynthetic pigments (chlorophyll a, b and carotenoids); especially chlorophyll a decreased sharply with increasing stress levels. High doses of NaCl appeared with deleterious effects (81.20 % at 150 mM and 92.86 % at 200 mM) on the content of chlorophyll a in the absence of exogenous CA as compared to the aqueous control. Although in presence of 0.05 mM CA, effects of NaCl stress were counteracted and pigments level were increased significantly 72% (Table-2). This restoratory effect of CA pretreatment on the reduction in the content of chlorophyll a in maize seedlings under saline condition was 9 % at 150 mM and 14 % at 200 mM of NaCl.
Chlorophyll b also decreased sharply with increasing stress levels. High doses of NaCl appeared with deleterious effects (79 % at 150 mM and 94 % at 200 mM) on the content of chlorophyll b in the absence of exogenous CA as compared to the aqueous control. Although in presence of 0.05 mM CA, effects of NaCl stress were counteracted and pigments level were increased significantly 76 % (Table-2). This restoratory effect of CA pretreatment on the reduction in the content of chlorophyll b in maize seedlings under saline condition was 32 % at 150 mM and 4 % at 200 mM of NaCl. Similar trend was observed with carotenoids content.
Malondialdehyde content (lipid peroxidation)
Degree of lipid peroxidation was measured as accumulation of MDA (malondialdehyde) content in leaf tissues of 2-week-old maize seedlings. Accumulation of MDA in NaCl-stressed (200 mM) seedlings of maize was very high than that of control in the absence of CA, whereas in the presence of 0.05 mM exogenous CA content of MDA (a product of lipid peroxidation) was reduced significantly (Fig. 1). Present observation shows that the rate of lipid peroxidation under saline conditions was counteracted by CA treatment in maize seedlings. Salinity impaired the membrane permeability increasing electrode leakage however application of CA partly maintained membrane permeability. This result agree with those of (Bor et al., 2003) who found salt stress increases the lipid peroxidation in the leaves of two beet species Grains soaking presowing with CA led to a significant decrease in level of lipid peroxidation. It has been noted that the maintaining integrity of cellular membrane under stress condition is considered an integral part of salinity tolerance.CA reduced the degree of ion leakage under salinity stress (salt–stress)/or maintain the ionic balance under adverse environmental conditions.
Anatomical changes
Cross-section area of the vascular cylinder showed significant differences in which vascular cylinder was effected in salinity and Cinnamic acid induced these properties under stress. Major changes were found in the transverse section of maize root .In our study we found that the cortex was reduced in stressed root as compared to control. On observe to parenchyma high salt level was significantly different to control and due to presence of CA salt effect was reduced. Reduction in vascular cylinder and parenchyma were proportional to each other at high salt and CA played a significant role for inducing their reduction. Changes of shape, size and thickness of cortical cells also observed in stress condition.
The cortex thickness increased significantly (P < 0.01) in higher salt concentration but not significant different treatment and control. Treated cortical cell was not differentiated between epidermis and cortex. The proportion of the stele portion decreased significantly (P < 0.001) in stress plant. However, Cinnamic acid induced the vascular cylinder by reducing salt stress. Differences in the peri- and exothermal layers and the lignisation of the endodermis and the peri-/exodermis were found between treatments and control (Fig. 2). It is more in stress plants. Result showed that the crosssectional area of the vascular cylinder have significant differences between treatments decreasing from control to high NaCl concentration.
On observing to the parenchyma, only the highest salt level was significantly different to control but not Low NaCl concentration and cinnamic acid was played a contrasting role in stress to reduce salinity. The vascular cylinder/cortical parenchyma ratio was affected by salinity as levels of NaCl were different from no NaCl added (control plants). However, even with no differences were detected between treatment and control.
Finally we concluded that High salt content, especially chloride and sodium affects plant growth by modifying their anatomical trait .this is due to osmotic effects and ionic imbalances affecting plant metabolism. Anatomically, it affects cell division and expansion processes and reduces the size of meristems, cortex and vascular cylinder.
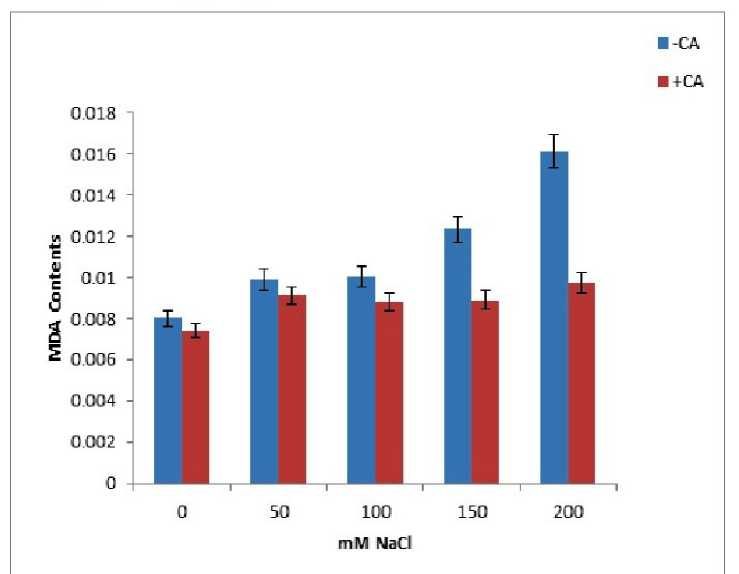
Figure 1. Effect of increasing NaCl concentrations on anatomical changes in two weeks old maize plants in absence and presence of CA (0.05 mM).
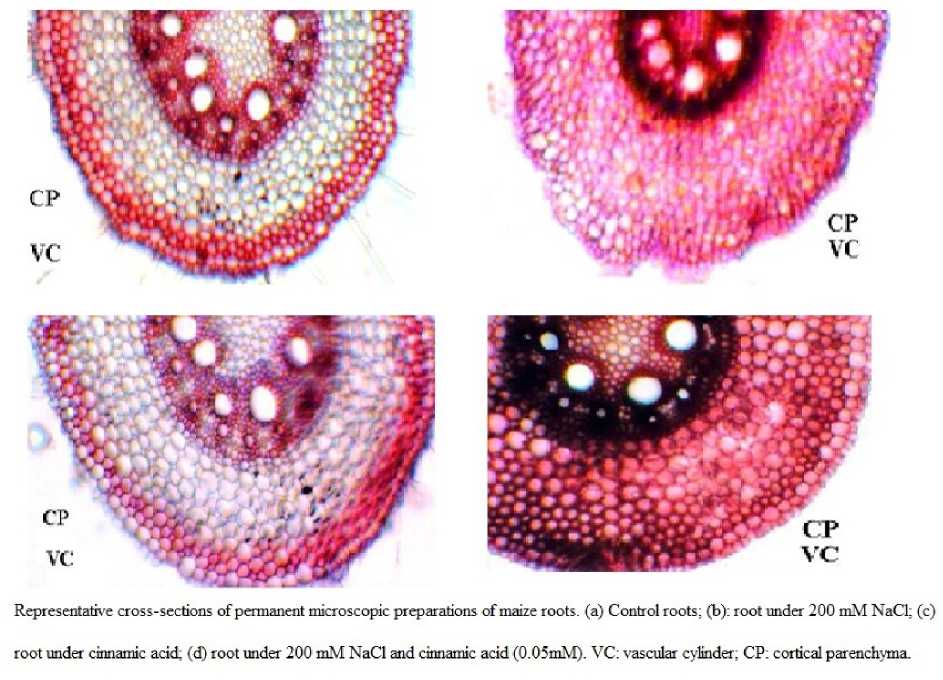
Representath'
root under cinnamic acid; (d) root under 200 mM NaCl and cinnamic acid (0.05mM). VC: vascular cylinder: CP: cortical parenchyma.
sections of permanent microscopic preparations of maize roots, (a) Control roots; (b): root under 200 mM NaCl: (c)
Figure 2. Effect of increasing NaCl concentrations on changes in MDA contents in two weeks old maize plants in absence and presence of CA (0.05 mM).
Table 1. Effects of increasing NaCl concentrations on changes in growth characteristics in two weeks old maize plants in absence or presence of CA (0.05mM).
|
NaCl |
Fresh weight |
Dry weight |
Root length |
Shoot length |
||||
|
(g plant-1) |
(g plant-1) |
(cm) |
(cm) |
|||||
|
(mM) |
CA - |
CA + |
CA - |
CA + |
CA- |
CA+ |
CA- |
CA+ |
|
0 |
5.70 |
7.6 |
0.78 |
1.00 |
6.5 |
7.1 |
9.1 |
10 |
|
±0.013 |
± 0.033 |
±0.004 |
±0.003 |
±1.45 |
±1.59 |
±2.03 |
±2.24 |
|
|
50 |
5.69 |
5.8 |
0.75 |
0.76 |
6.2 |
6.6 |
8.2 |
9.2 |
|
±0.008 |
± 0.039 |
±0.002 |
±0.004 |
±1.39 |
±1.48 |
±1.83 |
±2.06 |
|
|
100 |
4.3 |
4.8 |
0.46 |
0.62 |
6.1 |
6.4 |
7.4 |
7.9 |
|
±0.103 |
± 0.051 |
±0.003 |
±0.003 |
±1.36 |
±1.43 |
±1.65 |
±1.77 |
|
|
150 |
2.51 |
2.6 |
0.23 |
0.25 |
5.8 |
6.1 |
5.1 |
6.2 |
|
±0.009 |
± 0.070 |
±0.005 |
±0.003 |
±1.30 |
±1.36 |
±1.14 |
±1.39 |
|
|
200 |
2.30 |
2.4 |
0.22 |
0.27 |
4.7 |
5.5 |
3.6 |
4.8 |
|
±0.010 |
± 0.070 |
±0.003 |
±0.004 |
±1.05 |
±1.23 |
±0.81 |
±1.07 |
|
|
LSD |
0.032 |
0.052 |
0.041 |
0.062 |
0.022 |
0.153 |
0.022 |
0.024 |
Data presented are mean ± SE (n = 5). LSD values were determined at p< 0.05
Table 2. Effect of increasing NaCl concentrations on changes in chlorophyll a, chlorophyll b, carotenoids and relative water content in two weeks old maize plants in absence and presence of CA (0.05 mM).
|
NaCl (mM) |
Chlorophyll a (mg g-1 FW) |
Chlorophyll b (mg g-1 FW) |
Carotenoids (Mg g-1 FW) |
Relative water content (%) |
||||
|
CA- |
CA+ |
CA- |
CA+ |
CA- |
CA+ |
CA- |
CA+ |
|
|
Control |
2.66 |
4.57 |
1.49 |
2.62 |
1.84 |
2.98 |
80.1 |
83.2 |
|
±0.59 |
±0.102 |
±0.33 |
±0.59 |
±0.41 |
±0.67 |
±17.91 |
±18.60 |
|
|
50 |
2.25 |
4.07 |
1.08 |
2.49 |
1.36 |
2.57 |
79.6 |
81.1 |
|
±0.50 |
±0.91 |
±0.24 |
±0.56 |
±0.30 |
±0.57 |
±17.79 |
±18.14 |
|
|
100 |
1.28 |
3.44 |
0.54 |
2.27 |
0.90 |
2.23 |
76.1 |
80.3 |
|
±0.29 |
±0.77 |
±0.12 |
±0.51 |
±0.20 |
±0.49 |
±17.02 |
±17.96 |
|
|
150 |
0.50 |
2.91 |
0.31 |
1.97 |
0.70 |
2.01 |
66.5 |
73.9 |
|
±0.11 |
±0.65 |
±0.07 |
±0.44 |
±0.16 |
±0.45 |
±14.87 |
±16.53 |
|
|
200 |
0.19 |
2.30 |
0.09 |
1.43 |
0.50 |
1.81 |
60.2 |
67.7 |
|
±0.0.04 |
±0.51 |
±0.02 |
±0.32 |
±0.11 |
±0.40 |
±13.48 |
±15.14 |
|
|
LSD |
0.023 |
0.022 |
0.020 |
0.020 |
0.022 |
0.026 |
0.024 |
0.020 |
Data presented are mean ± SE (n = 5). LSD values were determined at p< 0.05
DISCUSSION
The results clearly indicate that the NaCl has adverse effect on growth of maize seedling and the harshness increased at high salt concentration and Growth of the seedling was retarded under salinity stress condition in maize plant. Seedling growth was significantly retarded in salinity compared to treatment and control at increasing concentration of NaCl at 14 days. The growth retardation was harshly affected at 150 mM NaCl and 200 mM NaCl concentration however the reduction of seedling growth was less treatment with respective control at increasing concentration of NaCl. Relative water contents were significantly lowered with increasing concentrations of NaCl compared to control but it was induced with cinnamic acid (Table 2). The present results are in line with findings with NaCl regarding to reduction of water content percentage due to increasing NaCl salinity. It is due to deficiency of water supply to the cells by increasing Na+ ions in cytoplasm compete with K+ ions led to lowering osmotic potential in cell cytoplasm of all stress condition (Malagoli et al ., 2008; Rewald et al .,
2012). Salinity induced structural changes in xylem in root. In salt stress plants vascular cell thickness was much larger than control and it was reduced with CA. Salt stress plants showed higher thickness in vascular tissue and cortex thickness was reduced in increasing concentration of NaCl in comparison to CA treated plants. Contents of photosynthetic pigments, chlorophyll a, b and carotenoids decreased significantly under salinity stress whereas in the presence of 0.05 mM CA, effects of NaCl stress on the contents of photosynthetic pigments were reduced up to 50%. CA is supposed to increase the functional state of the photosynthetic machinery in plants either by the mobilization of internal tissue nitrate or chlorophyll biosynthesis. CA has also been reported to have stimulatory effects on photosynthetic capacity in maize plants through the induction of Rubisco activity.CA treatment also increased the level of chlorophyll in the present investigation (Table 2), which is well supported by the earlier observations in maize seedlings under stress-free conditions as well as under saline conditions.
Functions of plasma membrane are adversely affected by salt stress that can be measured as level of membrane functions. MDA (a product of membrane lipid peroxidation) content could reflect the degree of membrane lipid peroxidation (Abed-Shalata et al ., 2001). In the present study, MDA concentration increased significantly with increasing concentrations of NaCl stress. The membrane damage is caused by the direct degradation of polyunsaturated fatty acid. However, this damage was improved by the application of exogenous CA. This alleviation in the level of membrane damage, by CA, was obvious significantly at higher doses of salt treatment. The ameliorative effect of CA might be due to its involvement in the protection of membrane deterioration through the reduction in the hydrolysis of membrane lipids. These results are in agreement with the findings of Gunes et al. who have reported the CA induced acclimation in maize plants under salinity stress.
ACKNOWLEDGMENTS
The Authors are thankful to University Grant Commission, New Delhi, India for financial support under Major Research Project (MRP No. F 38255/2009, SR).
Список литературы Impact of cinnamic acid on physiological and anatomical changes in maize plants ( Zea mays L.) grown under salinity stress
- Abed-Shalata, A. and Neumann, P.M. (2001). Exogenous ascorbic acid (Vitamin C) increase resistances to salt stress and reduce lipid peroxidation. J. Exp. Bot. 52: 2207-2211
- Bor, M., Ozdemir, F. and Turkan, I. (2003). The effect of salt stress on lipid peroxidation and Antioxidants in leaves of sugar beet Beta vulgaris L. and wild beet Beta maritime L. Plant Sci. 164: 77-84
- Brundrett, M.C., Enstone, D.E. and Peterson, C.A. (1988). A berberine-aniline blue fluorescent staining procedure for suberin, lignin, and callose in plant tissue. Protoplasma. 146: 133-142
- Ceccoli, G., Ramos, J.C., Ortega, L.I., Acosta, J.M. and Perreta, M.G. (2011) Salinity induced anatomical and morphological changes in Chloris gayana Kunth roots. BIOCELL. 35(1): 9-17
- Heath, R.L. and Packer, L. (1968). Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Archives in Biochemistry and Biophysics. 125: 189-198
- Hoagland, D.R. and Arnon, D.L. (1950). The water culture method for growing plants without soil. Calif Agric. Exp. Sta. Circular. 347
- Lichtenthaler, H.K. (1987). Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 148: 350-382
- Lux, A., Morita, S., Abe, J. and Ito, K. (2005). An Improved Method for Clearing and Staining Free-hand Sections and Whole-mount Samples. Ann. Bot. 96: 989-996
- Malagoli, P., Britto, D.T., Schulze, L.M. and Kronzucker, H.J. (2008). Futile Na+ cycling at the root plasma membrane in rice (Oryza sativa L.): kinetics, energetics, and relationship to salinity tolerance. J. Exp. Bot. 59: 4109-4117
- Munns, R. (2002). Comparative physiology of salt and water stress. Plant Cell Environ. 25: 239-250
- Munns, R. and Tester, M. (2008). Mechanisms of salinity tolerance. Annual Rev. Plant. Biol. 59: 651-681
- Parida, A.K. and Das, A.B. (2005). Salt tolerance and salinity effects on plants: a review. Ecotoxicology and Environmental Safety. 60(3): 324-349
- Reward, B., Raveh, E., Gendler, T., Ephrath, J.E. and Rachmilevitch, S. (2012). Phenotypic plasticity and water flux rates of Citrus root orders under salinity. J. Exp. Bot. 63: 2717-2727
- Ruzin, S.E. (1999). Plant microtechnique and microscopy. New York, Oxford University Press
- Singh, P.K., Singh, R. and Singh, S. (2013). Cinnamic acid induced changes in reactive oxygen species scavenging enzymes and protein profile in maize (Zea mays L.) plants grown under salt stress. Physiol Mol Biol Plants, 19(1): 53-59
- Szepesi, A., Csiszar, J., Gemes, K., Horvath, E., Horvath, H., Simon, M.L. and Tari, I. (2009). Salicylic acid improves acclimation to salt stress by stimulating abscisic aldehyde oxidase activity and abscisic acid accumulation, and increases Na+ content in leaves without toxicity symptoms in Solanum lycopersicum L. Plant Physiol. 166: 914-925
- Yamasaki, S. and Dillenburg, L. R. (1999). Measurements of leaf relative water content in araucaria angustifolia. Revista Brasilleira de Fisiologia Vegetal. 11: 69-75
- Zhu, J.K. (2002). Salt and drought stress signal transduction in plants. Annu. Rev. Plant. Biol. 53: 247-273
- Zolla, G., Heimer, Y.M. and Barak, S. (2010). Mild salinity stimulates a stress induced morphogenic response in Arabidopsis thaliana roots. J. Exp. Bot. 61: 211-224
- Zuchi, S. and Astolfi, S. (2012). Changes in growth irradiance is reflected on H+ATPase activity of plasma membrane enriched vesicles from maize (Zea mays L.) roots. Plant Physiol. 169: 50-54


