Impact of Triazole on Antioxidants activity and Lipid peroxidation at Cicer arietinum (Chick pea)
Автор: Aabshar Khan, Umesh Kumar
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 4 т.16, 2020 года.
Бесплатный доступ
The present study was carry out to evaluate the impact of pesticide Triazole on Chick pea (Cicer arietinum).Our experiments observed that the antioxidants activity increased with the treatments of Triazole concentration at 100ppm, 200ppm, and 400ppm as compare to control of Chick pea plant (Cicer arietinum). Enzymatic antioxidants i.e Superoxide Dismutase (SOD), Catalase (CAT), and Guaicol peroxidase (GPOD) were enhance of the plant as compare to control and in the leaves of pesticides-stressed plants, Lipid peroxidation (LPO) activities significantly increased with increasing pesticides concentrations (100ppm, 200ppm, and 400ppm) as compared to the control plants. The aim of study was to determine parameters which can be used to identify chick pea plant tolerance to pesticides stress.
Chick pea, Cicer arietinum, Pesticides, Triazole, Lipid peroxidation (LPO), Antioxidants activity, Superoxidase Dismutase (SOD), Catalase (CAT), Guaicol peroxidase (GPOD).
Короткий адрес: https://sciup.org/143173869
IDR: 143173869
Текст научной статьи Impact of Triazole on Antioxidants activity and Lipid peroxidation at Cicer arietinum (Chick pea)
Chick pea (“Cicer arietinum”) is the most important stable food in several developing countries and chemical fertilizers are the most important input required for chick pea cultivation. Chick pea contain higher amount of protein (23%), dietary fiber, carbohydrates (64% total carbohydrates), and minerals i.e calcium, potassium, zinc, iron, and phosphorus (Thudi et al. , 2011). In 2016, chick pea was cultivated on 10.7 million hectares (Mha) around the world. India, Pakistan, and Iran grew more than 8 Mha, and the rest of cultivated area included countries in Asia, the middle East, and Canada (FAOSTAT 2006; Varshney et al. , 2011).
Negative environmental conditions like pesticides, salt-stress, temperature, heavy metal, and air pollution can increase the generation of ROS such as, 1O 2 , O 2 -, H 2 O 2 , and OH in plant. Plant have cells, and organelles i.e the mitochondria, chloroplast, and peroxisomes activate antioxidants defense system (Tuteja, 2007). The antioxidants defense mechanism are divided into two classes such as enzymatic, and non-enzymatic antioxidants. Enzymatic antioxidants include SOD, CAT, and GPOD, and non-enzymatic antioxidants include Proline (Pro), Total phenol content, and non protein thiol (Gill et al. , 2010). The antioxidants defence mechanism plays an important role in detoxification, and removal of toxic, there by hindering their negative effect.
MATERIALS AND METHODS
Field experiments on chick pea (Cicer arietinum) was conducted at the Mohammad Ali Jauhar University, Rampur (U.P), India during winter season. Chick pea seeds was surface sterilized for 20 minutes in 1% HgCl2, and seeds were finally washed in sterile distilled water few time. Further, seeds were germinated by placing them on petri plate in the dark on the floating plastic net . After germination, seedling were placed in pots contain 3 kg soil with 1/4 strength modified Hoagland nutrient solution. After growing, they were treated with 100ppm, 200ppm, and 400ppm concentration of pesticides 10 DAS after the treatments indicated above, the shoots of seedlings were harvested and immediately frozen and stored in an -80°C freezer. Lipid peroxidation (LPO) was measured as described by (Hedges et al., 1999). The estimation of Enzymatic antioxidants identified i.e catalase (CAT), was measured in the samples based on a previously established protocol by (Hosetti 1994), superoxide dismutase (SOD) activity was estimate by (Bauchamp 1971), and Guaiacol peroxidase (GPOD) was measured according to (Hammerschmidt et al., 1982).
RESULTS AND DISCUSSION
Impact of Pesticides (Triazole) on Superoxidase Dismutase activity (SOD)) in chick pea plant
In our experiment, superoxidase dismutase activity (SOD) in chick pea plant was increased with different concentration of pesticides at (100ppm, 200ppm, and 400ppm) as compare with control (Fig. 1). Similarly, a literature survey indicates that triazoles have enhance the activity of antioxidant potential (Sivakumar, 2011), which helps plants environmental stress conditions. These results are in agreement with those of (Nair et al. , 2012) who reported that triazols cause an enhance in the activities of antioxidant enzymes, such as superoxide dismutase (SOD), peroxidase and polyphenol oxidase. Furthermore, (Fletcher et al. , 2000) indicated that triazole-induced stress tolerance in plants may be caused, at least in part, by increased antioxidant activity, which reduces oxidative injury to membrane and enzyme activity.
Impact of Pesticide Triazole on catalase activity (CAT) in chick pea plant
The present study showed that in chick pea ( Cicer arietinum) plant the catalase activity (CAT) increased with the age in control with various concentration of pesticides at (100ppm, 200ppm, and 400ppm). At 400ppm showed higher enhancement of CAT as compared with non-treated plants (Fig. 2).Our results based on catalase activity increased in bananas treated by TDM. Furthermore, TDM treatment in plant and increased PPO activity, Phenol levels were reduce in plants and enhance levels of auxin therefore improves plant growth (Kishorekumar et al., 2008). Changes in catalase activity depends on the intensity and duration of stress, and can induce new isozymes (Jaleel et al., 2007).
Impact of Pesticides (Triazole) on Guaiacol peroxidase (GPOD) in chick pea plant
Present study observed that Guaiacol peroxidase (GPOD) activity increased with increament of pesticides concentration at (100ppm, 200ppm, and 400ppm) as compare with control in chick pea (“Cicer arietinum”) plant (Fig. 3). Similarly, the result performed at an increase in Guaiacol peroxidase (GPOD) activity under the influence of the aromatic herbicide 1,10-phenantroline in the leaves of some species of plants (Herman et al., 1998).
SOD
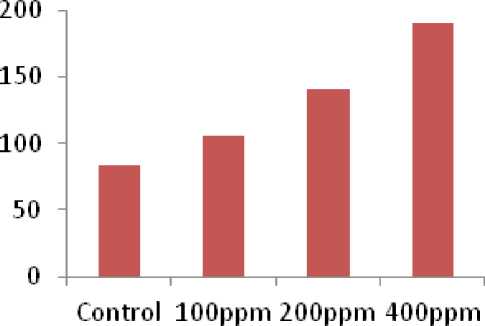
Figure 1. Impact of Triazole with different concentration on superoxide dismutase (SOD) activity in chick plant (Cicer arietinum).
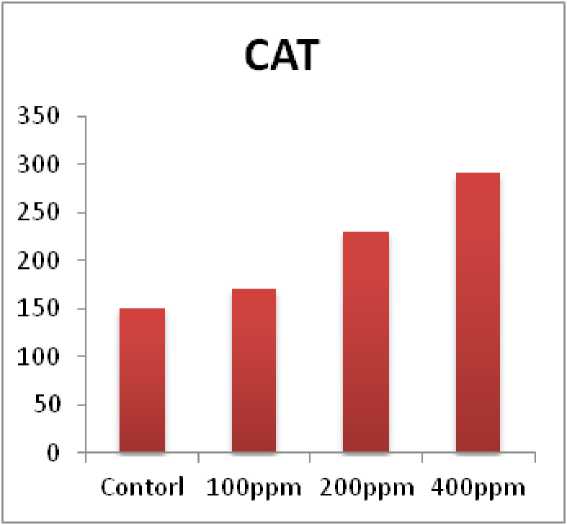
Figure 2. Impact of Triazole with various concentration on catalase activity (CAT) in chick pea plant ( Cicer arietinum ).
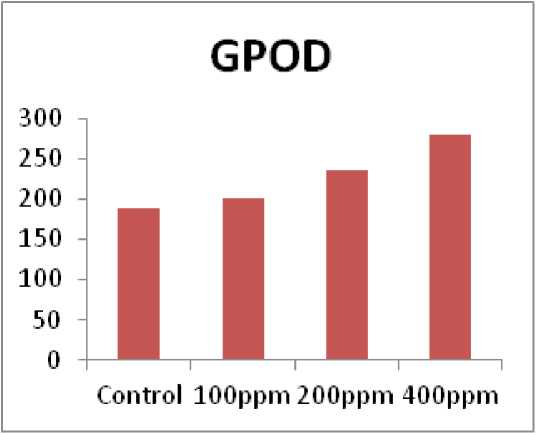
Figure 3. Impact of Triazole with increasing concentration on Guaiacol peroxidase (GPOD) in chick pea plant ( Cicer arietinum ).
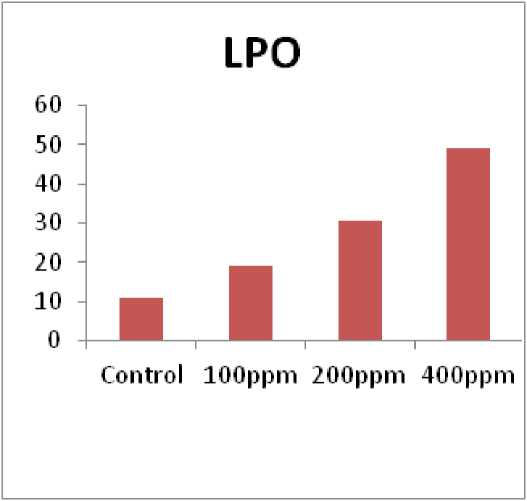
Figure 4. Impact of Triazole with enhancing the concentration on Lipid peroxidation (LPO) in chick pea plant ( Cicer arietinum ).
Impact of Pesticide Triazole on Lipid peroxidation (LPO) in chick pea plant
In our experiment observed that in chick pea plant the lipid peroxidation activity increased with different concentration of pesticides at (100ppm, 200ppm, and 400ppm) as compare to untreated plant. Lipid peroxidation activity highly increased at 400ppm than compare to (100ppm, and 200ppm) when compared to control in chick plant (Cicer arietinum) (Fig. 4). Similarly results based on Lipid peroxidation in E. adenophorum and E. odoratum was determined by measuring the malondialdehyde (MDA) content, a product of lipid peroxidation, in 1 g leaf fresh weight following (Madhava, 2000). Exposure of Glycine max. L. to insecticide deltamethrin or other pesticides led to increase in lipid peroxidation in leaves and root (Bashir et al., 2007; Song et al., 2007).
CONCLUSION
Present study observed that Guaiacol peroxidase
(GPOD) activity increased with increament of pesticides concentration at (100ppm, 200ppm, and 400ppm) as compare with control in chick pea ( Cicer arietinum ) plant. Similarly, the result is similar with an increase in Guaiacol peroxidase (GPOD) activity under the influence of the aromatic herbicide 1,10-phenantroline in the leaves of some species of plants (Herman et al. , 1998).
ACKNOWLEDGMENT
Список литературы Impact of Triazole on Antioxidants activity and Lipid peroxidation at Cicer arietinum (Chick pea)
- Bashir, F., Siddiqi, TO., Mahmooduzzafar, Iqbal, M. (2007). The anti oxidative response system in Glycine max (L.) Merr. exposed to deltamethrin, a synthetic pyrethroid insecticide. Environ Pollut 147: 94–100.
- Beauchamp, C., and Fridovich, I. (1971). Superoxide Dismutase: Improved Assay and an Assay Application to Acrylamide Gels, Anal Biochem. 44: 276.
- FAOSTAT, (2006). Press Release/faostat.fao.org/ Fletcher, R.A., Gilley, A., Davis, T.D., and Sankhla, N. (2000). Triazole as plant growth regulators and stress protectants. Hort Rev, 24: 55–138.
- Gill, S., Tuteja, N. (2010). Reactive oxygen species and antioxidants machinery in abiotic stress tolerance in crop plants. Plant Physiol. And Biochem, 48: 909-930.
- Hammerschmidtr, N., and kucj. (1982). Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiological Plant Pathology. 20: 73–82.
- Hedges, D. M., De Long, J. M., Forney, C. F., and Prange, R. K. (1999). Improving the thiobarbituric acid reactive substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interferin compounds. Planta. 207: 604-661.
- Herman, B., Biczak, R., and Gurgul, E. (1998). Effect of 1,10phenanthroline on peroxidase and catalase activity and chlorophyll, sugar, and ascorbic acid contents. Biol. Plant. 41: 607.
- Hosetti, B.B., and Frost, S. (1994). Catalase activity in waste water, Water Res. 28: 497-500.
- Jaleel, C.A., Gopi, R., Manivannan, P., Sankar, B., Kishorekumar, A., and Panneerselvam, R., (2007). Antioxidant potentials and ajmalicine accumulation in Catharanthus roseus after treatment with giberellic acid, Colloids Surf. B: Biointerfaces 60: 195–200.
- Kishorekumar, A., Jaleel, C.A., Manivannan, P., Sankar, B., Sridharan, R.,and Murali, P.V. (2008). Comparative effects of different triazole compounds on antioxidant metabolism of S. rotundifolius. Colloids Surfact B: Biointerfaces 62: 307-311.
- Madhava, Rao., KV, Sresty TVS. (2000). Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan L. “Millspaugh”) in response to Zn and Ni stresses. Plant Sci. 157: 113–128.
- Nair, V.D., Gopi, R., Mohankumar, M., Kavina, J., and Panneerselvam, R. (2012). Effect of triadimefon: a triazole fungicide on oxidative stress defense system and eugenol content in Ocimum tenuiflorum L. Acta Physiol Plant. 34: 599-605.
- Sivakumar, T., and Panneerselvam, R. (2011). Triadimefon mediated changes in antioxidant and indole alkaloid content in two species of Datura. Amer J Plant Physiol. 6: 252-260.
- Song, NH., Yin, X., Chen, GF., and Yang, H. (2007). Biological responses of wheat (Triticum aestivum) plants to the herbicide chlorotoluron in soils. Chemosphere 69:1779–1787.
- Thudi, M., Bohra, A., Nayak, S. N., Varghese, N., Shah, T, M., Penmetsa, R. V., and Thirunavukkarasu, N., et al. (2011). Novel SSR Markers from BACEnd Sequences, Dart Arrays and a Comprehensive Genetic Map with 1,291 Marker Loci for Chick pea (Cicer arietinum L.). PLoS ONE, 6(11): e27275.
- Tuteja, N. (2007). Mechanisms of high salinity tolerance in plants. Methods in Enzymology, 428(07): 419-438.
- Varshney, R. K., Pazhamala, L., Kashiwagi, J., Gaur, P. M., Krishnamurthy, L., and Hoisington, D. (2011). Physiology and Genomic Approaches for Root Trait Breeding to Improve Drought Tolerance in chick pea (Cicer arietinum L.) In A. Costa De Oliveira & R. K. Varshney (Eds), Root Genomics (pp. 233-250). Springer Berlin Heidelberg.


