Improving of barley seedling growth by seed priming under water deficit stress
Автор: Jalilian Jalal, Khalilzadeh Razieh, Khanpaye Edris
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 2 т.10, 2014 года.
Бесплатный доступ
Seed priming was used to reinforcement of barley seedling growth under water deficit stress in a greenhouse condition. Barley seeds were primed with humic acid, Pseudomonas Spp., Marmarin, distilled water (hydropriming) and none (as control) under four levels of water deficit stress (irrigation at 20 (I 1), 40 (I 2), 60 (I 3) and 80% (I 4) field capacity). Results indicated that all measured parameters were decreased with increasing the stress levels, except root length and root-shoot ratio. The highest value of root length (18.42 cm) and root-shoot ratio (2.84) was obtained in the I 1 irrigation regime. However, I 2, I 3 and I 4 irrigation regimes did not significantly affect on barley seedling traits, but I 1 irrigation regime exhibited better growth. Seed priming with Pseudomonas affected root length, root and shoot dry weight, plant height and SPAD, significantly. Seed priming with Marmarin showed best results on SPAD. The maximum (0.37 g/plant) shoot dry weight was obtained from I 2 irrigation regime with hydropriming treatment. Whereas, the lowest shoot dry weight (0.14 g/plant) was observed in I 1 irrigation regimes in control condition. Hydropriming shows better response in root length, root and shoot and plant height and was equal with Pseudomonas, so it could be considered as a suitable substitute in organic agricultural systems.
Humic acid, irrigation regimes, plant height, pseudomonas, root
Короткий адрес: https://sciup.org/14323852
IDR: 14323852
Текст научной статьи Improving of barley seedling growth by seed priming under water deficit stress
Plant growth, development and production are affected by natural stresses in the form of biotic and abiotic stresses such as drought, salinity and freezing, inversely (Abdalla and Elkhoshiban, 2007). Water deficit and salt stresses are global issues to ensure survival of agricultural crops and sustainable food production (Jaleel et al., 2007). With increasing drought stress, water availability decreases, changing the percentage and velocity of germination and growth of seedlings adversely (Kaya et al., 2006). seedling growth and establishment (Gamze et al., 2005), root/shoot ratio and root length at early stages of plant growth (Dhanda et al., 2004) and survival of wheat seedlings after desiccation have been suggested as useful traits for improving yield under dry conditions.
Barley ( Hordeum vulgare L.) is one of the most widely grown crops in arid and semiarid regions of the world. The seed of barley shows a delayed or reduced germination when the water potential of surrounding medium decreases (Tabatabaei, 2013). The improved seed performance could be attributed partially to osmotic adjustment, metabolic repair processes or a buildup of germination metabolites during treatments (Haghpanah et al., 2009).
Priming is one of the effective strategies to overcome drought stress (Yagmur and Kaydan, 2008). Seed priming techniques such as hydropriming, hardening, osmo-conditioning, osmo- hardening, and hormonal priming have been used to accelerate emergence of roots and shoots, more vigorous plants, and better drought tolerance in many field crops like wheat (Iqbal and Ashraf, 2007), chickpea (Kaur et al., 2002), sunflower (Kaya et al., 2006) and cotton (Casenave and Toselli, 2007). Nutrient priming has been proposed as a novel technique that combines the positive effects of seed priming with an improved nutrient supply (Al Mudaris and Jutzi, 1999). In nutrient priming, seeds are pretreated (primed) in solutions containing the limiting nutrients instead of being soaked simply in water. Bio-priming affects growth rate by increasing the solubility of minerals in soil and absorption of nutrition by barley root (De Freitas and Germida, 1990).
Earlier researchers (Afzal et al., 2005; Basra et al., 2006; Roy and Srivastava, 2000) suggested that the adverse and depressive effects of water stress on germination can be alleviated by various seed priming treatments. Therefore, the aim of this study was to evaluate the effect of seed priming with water, and new sources of materials such as Marmarin, Humic acid and Pseudomonas in order to achieve an effective solution for optimizing the plant seedling growth and establishment under limited water condition in the arid and semi-arid areas.
MATERIALS AND METHODS
The experiment was conducted as factorial based on completely randomized design with three replications, under greenhouse conditions, in Urmia University (39°59΄N and 50°75΄E), Iran during 2011. Seed priming (without priming, hydropriming, priming with Marmarin, Humic acid and Pseudomonas Spp. ) and water deficit stress (irrigation at 20(I 1 ), 40(I 2 ), 60(I 3 ) and 80% (I 4 ) of field capacity) were set as experimental treatments. Daily and night temperatures of greenhouse were maintained at 20 and 15 °C, respectively.
Humic acid (Humax®, JH Biotech, Inc., Ventura, CA) contains of 12 % humic acid, 3 % folic acid, and 3 % K 2 O. Humic acid were applied according to factory recommendations (5 g per lit). Marmarin, contains Organic matter 20-22 %, Growth regulator (Cytokinin) 400 ppm and total amino acid 6.5 % (w/v). Seeds were placed in the mentioned liquid priming media at 25ºC for 8 hour in the dark. Seeds were covered with plastic bags to refuse moisture loss. After soaking, seeds were washed with distilled water, and dry in the incubator at 25ºC in the dark.
In each priming and water treatment, each replication was represented by five plants. Five barley seeds ( Hordeum vulgare cv. Valfajer) were sown in each PVC pots (10 cm diameter × 20cm length) were filled with a 500 g mixture of sand , soil and organic dry matter (2:1:1).
All pots were watered at the field capacity until the emergence of the first leaf. At this growing stage, water was withheld to induce water deficit as irrigation regimes treatments. Soil moisture content was kept at needs amount of field capacity during the period of the experiment by weighing the pots daily and adding by distilled water to obtain the needs wet weight. The measurement of soil humidity was done by weighing pot (Sahnoune et al., 2004). The soil water measurements were done on three randomly pot for each experimental treatment.
After germination, plants were thinned to one plant per pot, and after 40 days from sowing plants were harvested. Plant height, leaf width and length were measured with a ruler with a precision of 1 mm. In order to eliminate the residue, roots were washed by water. The length of roots (in cm), was measured. Roots volume was evaluated according to the method of Musick et al. (1965) by immersion in a graduate test tube and measure of the displaced water volume. Roots and shoots were separated gently and were dried at 75 oC for 48 hours and weighed to determine the average root and shoot dry weights (Al-Niemi and Dohuki, 2010). Root-shoot length ratio was estimated by dividing root length to shoot length. Chlorophyll concentration was assessed using a chlorophyll meter (SPAD CCM-200, USA), measurements being taken at three points of each leaf (upper, middle and lower part). The average of these three readings was considered as SPAD reading of the leaf.
The data were analyzed by analysis of variance using the general linear model procedure in the SAS (SAS Institute). Means were separated using Fisher’s protected Duncan's Multiple Range (DMR) test at the 95% level of probability.
RESULTS AND DISCUSSION
In this study, all the examined parameters were decreased with increasing the water deficit stress levels, except root length and root-shoot ratio (Table 1). The largest root length (18.42 cm) was obtained in I1 irrigation regime whereas the lowest (16.92 cm) was in I3 irrigation regime (Table 2). It is reported drought conditions influence the root length and root elongation process by affecting cell division and turgidity of the cells (Golbashy et al., 2012). Reducing water supply in soil achieved a situation for plant to pursue root growth though soil depth. This shows that in order to resist drought stress, the plant employed this strategy throughout individual survival straggle by drought conditions. These findings are in agreement with the observations of Ajayi and Olufayo (2004) in sorghum.
Our results showed that seed priming show significant response in terms of root length, root and shoot dry weight, plant height and SPAD (Table 1). Mean comparison showed that the highest root length was achieved under hydropriming (Table 3). Priming in distilled water enhanced root length in asparagus seeds (De Carvalho Bittencourt et al., 2005) support our results of improved performance of hydroprimed seeds over the unprimed ones. Faster emergence rate after priming may be due to increased rate of cell division in the root tips of seedlings from primed seeds as reported in wheat (Basra et al., 2006) and sunflower (Kaya et al., 2006).
There are positive relationships between root volume and field survival and/or performance (Jacobs et al., 2005).The highest and lowest root volume were observed in irrigation at 60% field capacity (I3) and irrigation at 20% field capacity( I1 respectively (Table 2). There is evidence that planting seedlings with large root volumes may reduce moisture stress and subsequent transplant shock (Haase and Rose, 1993). Our results confirm the findings of Degu et al. (2008), who reported drought stress reduced root growth activity of barley, as is observed by other plants species.
Root dry matter was significantly affected by irrigation treatments and seed priming (Table 1). Highest root dry weight belonging to I 2 condition (40% field capacity) that was 46.66 percent higher than I 1 condition (20% field capacity) (Table 2). Reduction in root dry weight in I 1 condition could be due to reduction in root volume as indicated in Table 2 which is results of low water absorbance by germinated seeds (Zaefizadeh et al., 2011). By increased root length in I 1 , root weight decreased. Therefore the weight of roots states that the relationship between root weight and root length is a negative relationship with water stress (Bahrami, 2012).
Four seed treatments gave better performance than control (untreated) with clear effectiveness of hydropriming in improving the root dry weight. So, the highest (0.25 g/plant) and lowest (0.19 g/plant) root dry weight was obtained from the hydropriming and control, respectively (Table 3). The beneficial effects of hydropriming have been attributed to the mobilization in embryonic tissues of enzyme activities required for rapid seed germination and of compounds such as free amino acids, proteins, and soluble sugars from storage organs (Ashraf and Foolad, 2005). The improved seedling fresh and dry weight of barley may be attributed to optimum availability of nutrients to seedling soon after their emergence, which enhanced the early growth and thus resulted in improved fresh and dry weight of barley in the nutrient primed seed treatments. Better performance of seedlings raised from seeds primed with Marmarin, Humic acid and Pseudomonas might be due to increased the root weight, stimulate production of photosynthetic dyes, thus growing plant vitality (Sultana et al., 2005).
The shoot dry weight was affected by the interaction of seed priming and irrigation regimes (Table 1). The highest shoot dry weight (0.37 g/plant) was obtained from I 2 irrigation regimes with hydropriming treatment. Whereas, the lowest shoot dry weight (0.14 g/plant) was observed in I 1 irrigation regimes in control condition (Table 4). The inhibitory and deleterious effects of water stress seemed to be decreased by seed priming with plant regulation. Additionally, the role of plant growth regulators in overcoming the harmful effects of water definite on growth may be due to the change in the endogenous growth regulators which affects plant water balance and or decreasing root resistance to water flow (Mac Robbie, 1981).
Plant height of the barley was influenced by irrigation regimes (Table 1). It is obvious from Table 2 that increasing drought stress had significantly reduced plant height of barley. I 4 irrigation regimes and I 2 irrigation regimes produced the maximum (9.66 cm) and minimum (6.52 cm) plant height. Irrigation at I 1 condition (irrigation at 20% field capacity) leads to decrease 32.51% plant height of the barley as compared to irrigation at I 4 condition (Irrigation at 80% field capacity). It is reported that reductions in plant height could be due to decline in cell division and enlargement caused by water stress (Bahrami, 2012).
Irrigation regimes had a significant effect on leaf length (Table 1). The highest (11.83 mm) and lowest (9.06 mm) leaf length was observed in I4 and I1 irrigation regimes, respectively (Table 2). The effect of stress during the vegetative stage is the development of smaller wider and flat leaf surface which can reduce the leaf area index at maturity and result in less light interception by the crops (Jones and McLeod, 1990).
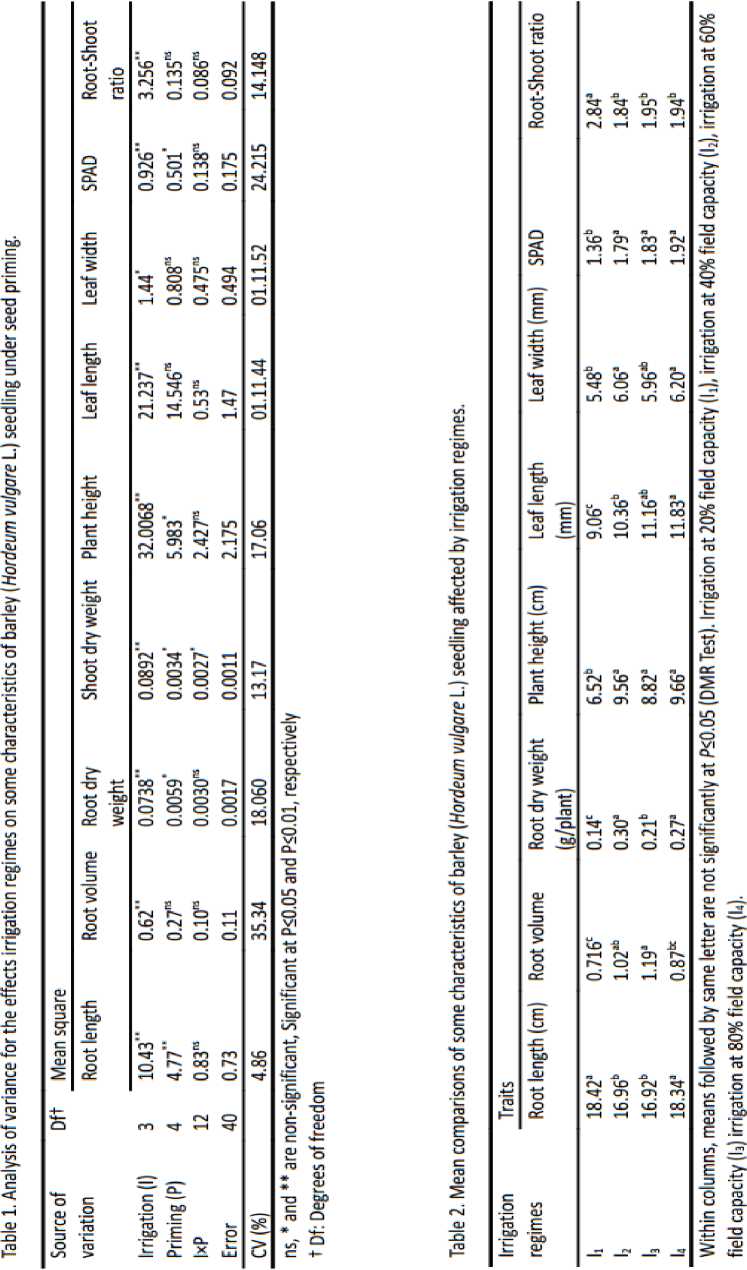
Table 3. Mean comparisons of some characteristics of barley ( Hordeum vulgare L.) seedling affected by different seed priming treatments.
|
Priming treatment |
Traits Root length Root dry weight Shoot dry weight Plant height (cm) SPAD (cm) (g/plant) (g/plant) |
|
Control Hydropriming Humic acid Marmarin Pseudomonas |
16.87c 0.19b 0.22b 7.69b 1.39b 18.54a 0.25a 0.26a 9.64a 1.88a 17.66b 0.24a 0.26a 8.60ab 1.74ab 17.30bc 0.23a 0.25a 8.43ab 1.90a 17.93ab 0.23a 0.26a 8.85ab 1.73ab |
The means followed by same letter are not significant at P ≤0.05 (DMR Test).
Table 4. Means comparison of interaction between irrigation regimes and seed priming application on shoot dry weight (g/plant) of barley ( Hordeum vulgare L.) seedling.
|
Treatments |
Shoot dry weight (g/plant) Irrigation regimes I 1 I 2 I 3 I 4 |
|
Control Hydropriming Humic acid Marmarin Pseudomonas |
0.140k 0.316abc 0.243efg 0.203fghij 0.170hijk 0.370a 0.220efghi 0.306abcd 0.150jk 0.353a 0.326ab 0.233efg 0.163ijk 0.363a 0.270bcde 0.230efgh 0.190ghijk 0.350a 0.256cdef 0.25defg |
Within columns, means followed by same letter are not significantly at P ≤0.05 (DMR Test). Irrigation at 20% field capacity (I 1 ), irrigation at 40% field capacity (I 2 ), irrigation at 60% field capacity (I 3 ) irrigation at 80% field capacity (I 4 ).
Considerable decrease in leaf width was observed, depending on the increase in water stress levels (Table 1). The highest leaf width was observed in I 4 condition (6.20 mm), whereas the lowest was noted at I 1 condition (5.48 mm) (Table 2).
Significant differences were observed among irrigation regimes and seed priming treatments with respect to SPAD (Table 1). The maximum (1.92 spad) was obtained by 80% field capacity and minimum (1.36 spad) was gained in 20% field capacity. SPAD is a reliable method to assess the changes in the function of maximal efficiency of PSII photochemistry (Fv/Fm) under stress conditions (Broetto et al., 2007). So during the development of water stress a gradual decline of the ratio Fv/Fm occurred. Also, Boussadia et al. (2008) and Ranjbarfordoei et al. (2006) showed that Fv/Fm was reduced significantly in plants submitted to water deficit which was possibly due to the reduction restriction of CO2 for photosynthesis and indicated photo inhibition. De souza et al. (1997) reported that there was no significant difference in leaf chlorophyll content of soybean between irrigations of field capacity and 60% of field capacity, but irrigation at 30% of field capacity caused a significant reduction in leaf chlorophyll.
Results of mean comparison for SPAD content indicated that the highest and lowest root volume were observed in Marmarin and control (no priming) seed treatment, respectively (Table 3). Yang et al. (2004) who stated that, seed priming sources could play an important role in the growth enhancement through their stimulation for metabolic processes as the biosynthesis of chlorophyll.
The effect of irrigation regimes on root-shoot ratio was significant (Table 1). Furthermore, the shoots were more sensitive than roots (Saboora et al., 2006). Table 2 clearly indicates that average root length is greater than shoot length and root-shoot ratio. It is found higher yield (2.84) in irrigation at 20% field capacity (I1) and Irrigation at 40% field capacity (I2) produced minimum yield (1.84) rootshoot ratio. When water supply is limiting, allocation of assimilates tend to be modified in root growth and leads to increase root dry weight and consequently the root-shoot ratio increases (Kaydan and Yagmur 2008).The inhibition effects of drought stress on growth parameters of plants might be due to inhibits the growth through reduced water absorption, changes in water relations of tissues exposed to low water potential, accumulation of ions in tissues and stomata conductance of leaves (Blum, 2005).
CONCLUSION
Generally, root volume, root dry weight, plant height, leaf width, leaf length, and SPAD were found to be sensitive to irrigated at 20 % field capacity (I 1 ) in comparison to others. Shoot dry weight is best at 40 % field capacity (I 2 ) and can be improved by hydropriming. Among different priming sources, hydropriming improved root length, root dry weight, plant height and SPAD of barley seedling.
Список литературы Improving of barley seedling growth by seed priming under water deficit stress
- Abdalla, M.M. and El-Khoshiban N.H. (2007) The influence of water stress on growth, relative water content, photosynthetic pigments, some metabolic and hormonal contents of two Triticium aestivum cultuivars. J. Appl. Sci. Res., 3(12), 2062-2074
- Afzal, I., Basra, S.M.A. and Iqbal, A. (2005) The effects of seed soaking with plant growth regulators on seedling vigor of wheat under salinity stress. J. Stress Physiol. Biochem., 1(1), 6-14
- Ajayi, A.E. and Olufayo, A.A. (2004) Evaluation of two temperature stress indices to estimate grain sorghum yield and evapotranspiration. Agron. J., 96 (5), 1282-1287
- Al Mudaris, M.A. and Jutzi, S.C. (1999) The influence of fertilizer-based seed priming treatments on emergence and seedling growth of Sorghum bicolor and Pennisetum glaucum in pot trials under greenhouse conditions. J. Agron. Crop Sci., 182 (2), 135-142
- Al-Niemi, S.N. and Dohuki, M.S.S. (2010) Seed size and seed quality effects on seedling growth of barley varieties grown in Fe and Zn deficient calcareous soil. Mesopotamia J. Agric., 38(4), 1-5
- Ashraf, M. and Foolad, M.R. (2005) Pre-sowing seed treatment-a shotgun approaches to improve germination, plant growth, and crop yield under saline and non-saline conditions. Adv. Agron., 88, 223-271
- Bahrami, H., Razmjoo, J. and Ostadi Jafari, A. (2012) Effect of drought stress on germination and seedling growth of sesame cultivars (Sesamum indicum L.). Int. J. Agr. Sci., 2(5), 423-428
- Basra, S.M.A., Afzal, I., Anwar, S., Anwar-ul-haq, M., Shafq, M. and Majeed, K. (2006) Alleviation of salinity stress by seed invigoration techniques in wheat (Triticum aestivum L.). Seed Technol., 28(1), 36-46
- Blum, A. (2005) Drought resistance, water-use efficiency, and yield potential-are they compatible, dissonant, or mutually exclusive? Aust. J. Agr. Res., 56, 1159-1168
- Boussadia, O., Ben Mariem, F., Mechri, B., Boussetta, W., Braham, M. and Ben El Hadj, S. (2008) Response to drought of two olive tree cultivars (cv. Koroneki and Meski). Sci. Hort., 116(4), 388-393
- Broetto, F., Duarte, H.M. and Lüttge, U. (2007) Responses of chlorophyll fluorescence parameters of the facultative halophyte and C3-CAM intermediate species Mesembryanthemum crystallinum to salinity and high irradiance stress. J. Plant Physiol., 164(7), 904-912
- Casenave, E.C. and Toselli, M.E. (2007) Hydropriming as a pre-treatment for cotton germination under thermal and water stress conditions. Seed Sci. Technol., 35(1), 88-98
- De Carvalho Bittencourt, M.L., dos Santos Dias, D.C.F., dos Santos Dias, L.A. and Araujo, E.F. (2005) Germination and vigor of primed Asparagus seeds. Sci. Agric. (Piracicaba, Braz.)., 62(4), 319-324
- De Freitas, J.R. and Germida, J.J. (1990) Plant growth promoting rhizobacteria for winter wheat. Can. J. Microbiol., 36(4), 265-272
- De Souza, P.I., Egli, D.B. and Bruening, W.P. (1997) Water stress during seed filling and leaf senescence in soybean. Agron. J., 89 (5), 807-812
- Degu, H.D., Ohta, M. and Fujimura, T. (2008) Drought tolerance of Eragrostis tef and development of roots. Int. J. Plant. Sci., 169(6), 768-775
- Dhanda, S.S., Sethi, G.S. and Behl, R.K. (2004). Indices of drought tolerance in wheat genotypes at early stages of plant growth. J. Agron. Crop. Sci., 190(1), 6-12
- Gamze, O., Mehmet, D.K. and Mehmet, A. (2005) Effects of salt and drought stresses on germination and seedling growth of pea (Pisum sativum L.). Turk. J. Agric. for., 29, 237-242
- Golbashy, M., Ebrahimi, M., Khavari Khorasani, S. and Mostafavi, K. (2012) Effects of drought stress on germination indices of corn hybrids (Zea mays L.). Electronic J. Plant Breeding., 3(1), 664-670
- Haase, D.L. and Rose, R. (1993) Soil moisture stress induces transplant shock in stored and unstored 2+0 Douglas-fir seedlings of varying root volumes. For. Sci., 39(2), 275-294
- Haghpanah, A., Younesi, O. and Moradi, A. (2009) The effect of priming on seedling emergence of differentially matured sorghum (Sorghum bicolor L.) seeds. J. Appl. Sci. Res., 5(7), 729-732
- Iqbal, M. and Ashraf, M. (2007) Seed treatment with Auxins modulates growth and ion partitioning in salt-stressed wheat plants. J. Integr. Plant Biol., 49(7), 1003-1015
- Jacobs, D.F., Salifu, K.F. and Seifert, J.R. (2005) Relative contribution of initial root and shoot morphology in predicting field performance of hardwood seedlings. New For., 30, 235-251
- Jaleel, C.A., Manivannan, P., Sankar, B., Kishorekumar, A., Gopi, R., Somasundaram, R. and Panneerselvam, R. (2007) Water deficit stress mitigation by calcium chloride in Catharanthus roseus: effects on oxidative stress, proline metabolism and indole alkaloid accumulation. Colloids Surf B., 60(1), 110-116
- Jones, R.H. and McLeod, K.W. (1990) Growth and photosynthetic responses to a range of light environments in Chinese tallow tree and Carolina ash seedlings. For. Sci., 36(4), 851-862
- Kaur, S., Gupta, A.K. and Kaur, N. (2002) Effect of osmo-and hydropriming of chickpea seeds on seedling growth and carbohydrate metabolism under water deficit stress. Plant. Growth. Regul., 37, 17-22
- Kaya, M.D., Okçu, G., Atak, M., Çıkıli, Y. and Kolsaric, O. (2006) Seed treatments to overcome salt and drought stress during germination in sunflower (Helianthus annuus L.). Europ. J. Agronomy., 24(4), 291-295
- Kaydan, D. and Yagmur, M. (2008) Germination, seedling growth and relative water content of shoot in different seed sizes of triticale under osmotic stress of water and NaCl. Afr. J. Biotechnol., 7(16), 2862-2868
- MacRobbie, E.A.C. (1981) Effect of ABA in isolated guard cells of Commelina communis L. J. Exp. Bot., 32 (3), 563-572
- Musick, G.J., Fairchild, M.L., Ferguson, V.L. and Zuber, M.S. (1965) A method of measuring root volume in corn (Zea mays L.). Crop Sci., 5(6), 601-602
- Ranjbarfordoei, A., Samson, R. and Van Damme, P. (2006) Chlorophyll fluorescence performance of sweet almond [Prunus dulcis (Miller) D. Webb] in response to salinity stress induced by NaCl. Photosynthetica., 44(4), 513-522
- Roy, N.K. and Srivastava, A.K. (2000) Adverse effect of salt-stress conditions on chlorophyll content in wheat (Triticum aestivum L.) leaves and its amelioration through pre-soaking treatments. Indian J. Agric. Sci., 70(11), 777-778
- Saboora, A., Kiarostami, K., Behroozbayati, F. and Hajihashemi, S. (2006) Salinity (NaCl) tolerance of wheat genotype at germination and early seedling growth. Pak. J. Biol. Sci., 9(11), 2009-2021
- Sahnoune, M., Adda, A., Soualem, S., Harch, M.K. and Merah, O. (2004) Early water-deficit effects on seminal roots morphology in barley. Comptes Rendus Biol., 327 (4), 389-398
- Sultana, V., Ehteshamul-Haque, S., Ara, J. and Athar, M. (2005) Comparative efficacy of brown, green and red seaweeds in the control of root infecting fungi and okra. Int. J. Environ. Sci. Tech., 2 (2), 129-132
- Tabatabaei, S.A. (2013) Effect of osmo-priming on germination and enzyme activity in barley (Hordeum vulgare L.) seeds under drought stress condition. J. Stress Physiol. Biochem., 9(4), 25-31
- Yagmur, M. and Kaydan, D. (2008) Alleviation of osmotic stress of water and salt in germination and seedling growth of triticale with seed priming treatments. Afr. J. Biotechnol., 7(13), 2156-2162
- Yang, C.M., Chang, I.F., Lin, S.J. and Chou, C.H. (2004) Effects of three allelopathic phenolics on chlorophyll accumulation of rice (Oryza sativa) seedlings: II. Stimulation of consumption-orientation. Bot. Bull. Acad. Sin., 45, 119-125
- Zaefizadeh, M., Jamaati-e-Somarin, S., Zabihi-e-Mahmoodabad, R. and Khayatnezhad, M. (2011) Discriminate analysis of the osmotic stress tolerance of different sub-convars of durum wheat during germination. Adv. Environ. Biol., 5(1), 74-80


