In-vitro response of Vigna aconitifolia to drought stress induced by PEG - 6000
Автор: Soni Priyanka, Rizwan M., Bhatt K.V., Mohapatra T., Singh Govind
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 3 т.7, 2011 года.
Бесплатный доступ
This investigation was aimed at investigating effect of low water potential generated by PEG on growth, sugar content and stress related enzymes (catalase, GPOX) at seedling level. Additionally gene expression was also studied using SDS-PAGE. A total of twelve moth bean genotypes comprising of elite moth bean lines were evaluated for desiccation tolerance at germination and seedling stage. Germination was happened differentially at 8% PEG while 15% PEG induced wilting in 7d old seedlings. Longer roots and shoots, higher dry weight of root and shoots both in control and treated seedlings was observed with desiccation tolerance. Drought tolerant and sensitive varieties differed in their protein profiling with newer genes showing their expression in tolerant once. Two genes were found up regulated in all tolerant varieties during PEG induced stress. Higher basal activity of enzymes (GPOX and catalase) and total soluble sugar during water deficit in drought tolerant varieties was reported.
Water deficit, vigna aconitifolia, drought tolerance
Короткий адрес: https://sciup.org/14323525
IDR: 14323525
Текст научной статьи In-vitro response of Vigna aconitifolia to drought stress induced by PEG - 6000
Abbreviations: DW- Dry weight, FW- Fresh weight, GPOX- Guaicol peroxidase, MS- Muras-hige-skoog, PEG - polyethylene glycol, RDW-Root dry weight, RFW- Root fresh weight, RL-Root length, SDW- Shoot dry weight, SFW- Shoot fresh weight, SL- Shoot length, SDS-PAGE-Sodium dodecyl sulphate–polyacrylamide gel electrophoresis
Water deficit is one of the major abiotic stresses, which adversely affects the crop growth and yield (Jaleel et al, 2009). A plant able to withstand these stresses while maintaining good productive health may only be a solution to continued resource limiting agriculture. In vitro culture techniques minimize environmental variations due to defined nutrient media, controlled conditions and homogeneity of stress application. In addition, the simplicity of such manipulations enables studying large plant population and stress treatments in a limited space and short period of time. Polyethylene glycols (PEG) of high molecular weights have been long used to simulate drought stress in plants as non-penetrating osmotic agents lowering the water potential in a way similar to soil drying (Larher et al, 1993).
Abiotic stress leads to a series of morpho-physiological, biochemical and molecular changes that adversely affect plant growth and productivity. Under more prolonged water stress, dehydration of plant tissue can result in an increase in oxidative stress which causes increase in the activity of antioxidants, production of stress proteins and accumulation of compatible solutes (Zhu, 2002).
The moth bean a crop of hot desert region shows a great deal of tolerance to drought. Such crops investigated systematically could also be a source of efficient alleles for various stress tolerant pathways or mechanisms. This experiment thus examined the effect on germination and seedling growth to drought in twelve varieties of Vigna aconitifolia and examined the associated changes in enzyme, protein and sugar levels under water stress.
MATERIALS AND METHODS
Plant material
Seeds of twelve moth bean genotypes comprising of elite moth bean lines viz., RMO-40, RMO-225, RMO-257, RMO-423, RMO-435, RMM-12-zero, RMM-12-single, RMM-12-poly, RMM-12-double, CZM-04-01, CZM-18 and CZM-105 were used in the experiment.
Optimization of concentration of PEG
Seedling : Four disinfected seeds were transferred on filter paper bridges in each of the three test tubes containing јth liquid MS media. Stress was imposed on seven days old seedlings using PEG-6000 with different concentrations (5, 10, 15, and 20%). For this јth MS liquid medium in test tube was replaced with fresh MS media containing various concentrations of PEG, after seven days of seed placement.
Germination : Seeds were surface sterilized using 0.1% HgCl 2 for 5 min followed by washing in distilled water and four seeds were germinated on filter paper bridges in each test tube containing different concentration of (2 – 10 %) in PEG.
All plant materials were cultured in growth chambers at 26 ° C, 16/8 h light/dark photoperiod and 2500–3000 lux light intensity. All the evaluations were repeated three times.
Measurment of growth
The shoot and root lengths (cm) were measured on seedlings after 7 days of PEG treatment with the help of meter scale. Fresh weight of the root and shoot samples was taken with the help of weighing balance (Mettler Toledo, USA) immediately after taking them out of test tubes and wiping out the moisture with paper towel. The roots and shoots were oven-dried at 800C for 48 h in order to take their dry weight.
Biochemical Parameters: Enzyme extraction was done using 50 mM sodium phosphate buffer (pH 7.0) having 0.1 mM EDTA, 0.1% PVP. Antioxidants (Catalase and oxidase) activities were analyzed in PEG stressed and non stressed seedlings. Total soluble sugars (TSS) concentration was estimated in samples of young leaf tissue (0.5 g), extracted in 5 ml ethanol (70% w/v) and washed twice with ethanol and calculated using the anthrone method with glucose as the standard and were expressed as mg/gm FW.
Protein Extraction: Protein was extracted from leaves of control and PEG treated seedlings after 7 days of PEG treatment. Leaves were frozen in liquid nitrogen and Homogenized in Protein extraction buffer (0.5mM Tris-HCl-2.5 ml, urea-9.6 gm, SDS-1.0 gm, glycerol-4.0 ml, β mercaptoethanol-500 µl) at 4°C. Samples were then centrifuged at 15000 rpm for 10 min. Supernatant was collected in fresh tube and was kept in boiling water for 5 min to denature the protein. These protein samples were stored at -20°C. This supernatant was used as a sample for polyacrylamide gel electrophoresis.
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE): Electrophoresis of equal quantity of protein after measuring with Bradford method carried out on 12 per cent polyacryamide gel. The sample so prepared contained, 34 µ l of extracted protein and 2 µ l of 10x Bromophenol blue dyes. Standard protein marker (Banglore genei) was loaded in a separate well. Gels were stained by Coomassie brilliant blue dye (R).
RESULTS
Visual observation
Germination in all twelve verities all the twelve verities (RMO-423, RMO-435, RMO-40, RMO-225, RMO-257, RMM-12-Single, RMM-12, RMM-12–Poly, RMM-12-Double, CZM-105, CZM-18, and CZM-04-01) of moth bean was completely inhibited at 10% of PEG level. However a differential response was visible at 8% of PEG (Fig. 1). Germination was inhibited at 6% PEG in all but two varieties viz. RMO-435 and RMM-12-Single. The germination was highest for the variety RMM-12-Single and CZM-105 (86%) at 8% of PEG and was least for the variety CZM-04-01(11%).
The seedlings of the twelve verities of moth bean sustained water stress induced by up to 10% of PEG polyethylene glycol-6000 (PEG-6000). Treatment of 20% PEG to seven days old seedlings inhibited growth, induced chlorosis, wilting, burning of roots and leaves in all twelve verities however, at different periods of treatment. Differential visible effect of 15% PEG (Fig 2) was considered suitable for further growth and metabolic studies. Leaf shrinking, yellowing of root tips, leaf curling, diffused growth of roots were the main symptoms observed after seven days of 15% PEG treatment. According to day wise observation no significant change was observed after 1 day of treatment, on 3 rd day some of seedling started showing leaf curling, yellowing of leaves etc. At 5 th day browning of roots was observed in RMM-12- double while RMO-423 showed blackening of roots (Fig 3). At 7 th day of 15% PEG treatment most of plants showed differential visible effect so optimized for screening. More wilting was shown at 9 th day by all the verities. Symptom development rate was high and appeared on lower concentrations of PEG in susceptible genotypes in comparison to tolerant.
The twelve varieties were classified as tolerant, moderate tolerant and sensitive based on germination inhibition at 8% PEG and number of seedling showing wilting symptoms at seedling stage at 15% PEG. Variety RMO-435, RMM-12-Single, RMM-12-Poly and CZM-105 were considered tolerant showing 14 to 19% germination inhibition and 14 to 19% wilted seedlings. Varieties RMM-12-Zero, RMM-12-Double, RM0-423, CZM-18 were considered moderate tolerant with 25 to 33% germination inhibition and 19 to 42% wilted seedlings. Varieties showing more than 55% germination inhibition and more than 72% wilted seedlings (RMO-225, RM0-40, RMO-257, and CZM-04-01) were considered sensitive.
Growth and metabolism
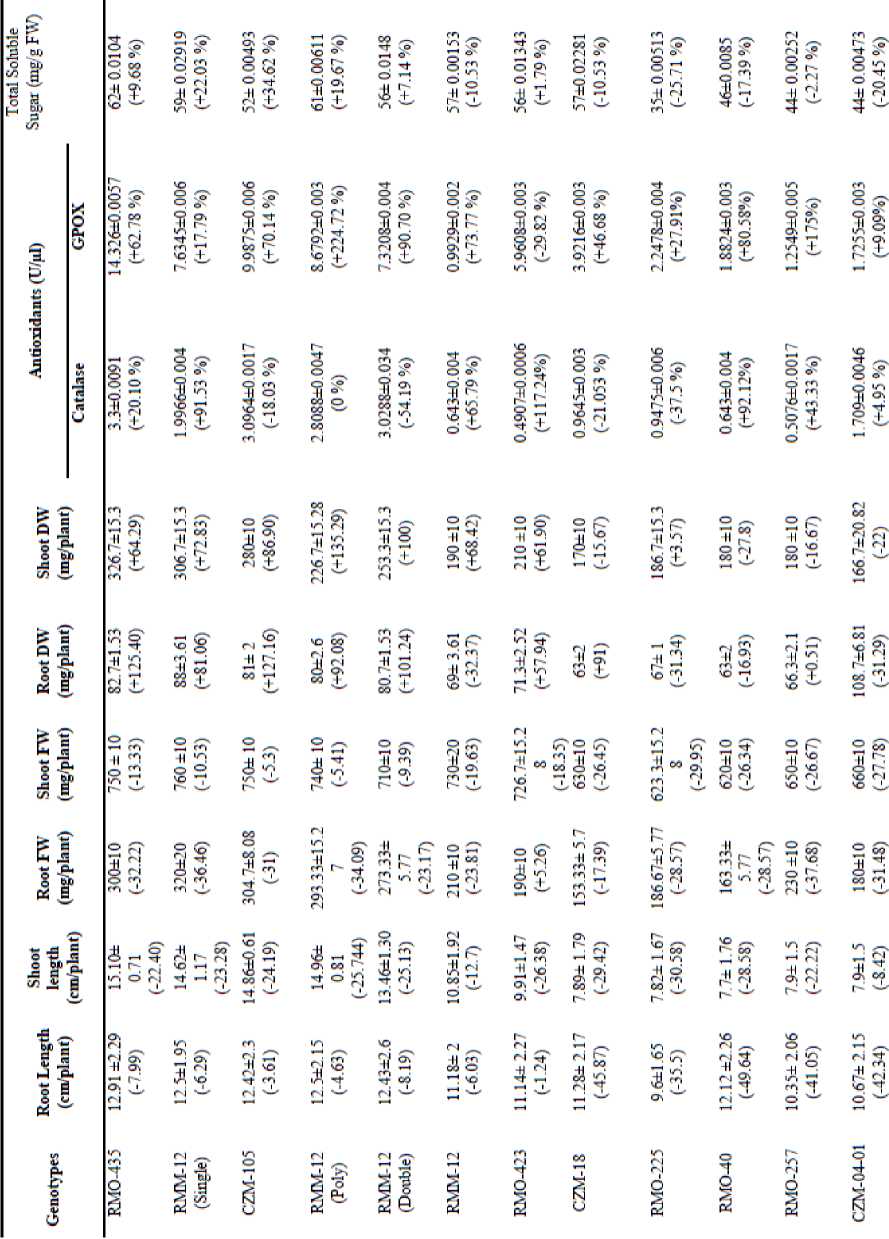
We documented the growth and metabolism in terms of seedling growth, fresh and dry weights of shoots and roots, alterations in the levels of cytosolutes, and activity of antioxidants- catalase and GPOX (Table 1). The responses to water deficit conditions however, were genotype specific. The shoot and root length was reduced in all the genotypes by applying 15% PEG. Root and shoot fresh weights also showed a reducing trend except for the variety RMO-423 which recorded a marginal increase (5.26%) in root fresh weight unlike above traits root and shoot dry weight increased considerably for most of the genotype studied. More than 100% increase was recorded for some of the genotype for both root and shoot dry weight especially in genotypes classified as tolerant on the basis of germination percentage or wilting symptoms. Total soluble sugars and antioxidant system significantly enhanced in tolerant varieties (RMO-435, RMM-12 (Single), RMM-12 (poly) and CZM-105) while in sensitive varieties (RMO-40, RM0-257, RMO-225 and CZM-04-01) their level found very low.
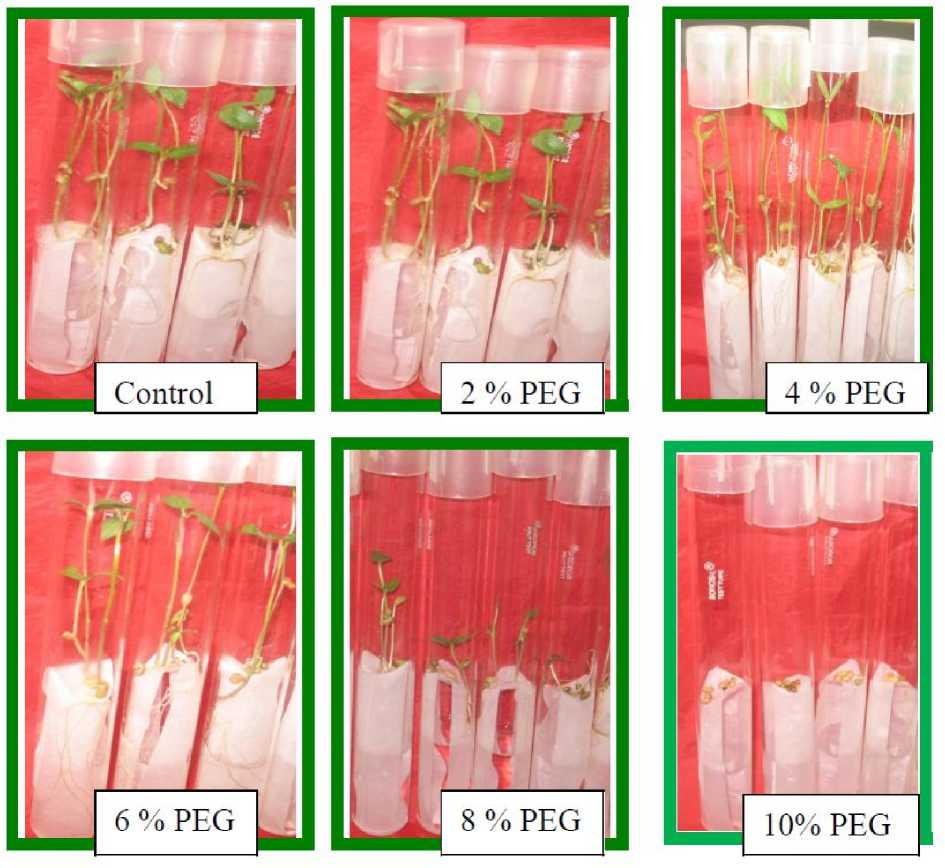
Figure 1. Visible effect of different concentration of PEG (2%-10%) on the morphology of moth bean at germination level (RMO-435)
Table 1. Effect of PEG6000 treatments on root and shoot length, dry and fresh weights of shoots and roots, concentrations of enzyme, TSS after 7 days of seedlings of Vigna aconitifolia . (Values with in parenthesis are percent reduction (-) or increase (+) over control).
*Values are mean of the ten seedlings (± values are SD). Data are means ± SD of 30 plants
Most of the morpho-physiological traits were correlated with each other and with biochemical parameters studied. However root dry weight accumulation was not correlated with any of the other traits studied under unstressed condition, but was positively correlated with all the traits under stress condition. The root length showed correlation at 5% level of significance with root fresh weight, shoot dry weight, root dry weight under stressed condition. Germination under high osmoticum was not correlated with catalase levels under normal growing condition as well as under stress condition (8% PEG). Similarly root dry weight under control condition was not correlated with germination percentage at 8% PEG levels, values being negative (-0.117). Nevertheless germination percentage was positively correlated with root dry weight under stressed condition (0.789). Similarly wilting resistance was not correlated with root dry weight under control condition whereas it had high correlation with root dry weight under stress condition. Root length and shoot length was found highly correlated under drought (0.92) while during control conditions it was 0.81. Maximum correlation was found between RL and SFW i.e. 0.97 during stress. No correlation was found between RDW and other factors under control conditions while under stress it was correlated with each parameter. There was negative correlation between RDW and germination under non stressed conditions. Catalase was having good correlation with others in comparison to control conditions.

Figure 2. Screening of RMO-435 at seedling level on media having different PEG concentrations (5%-20%). A, B, C, D and E are 5 %, 10 %, 15 %, and 20 % respectively PEG treated seedlings.
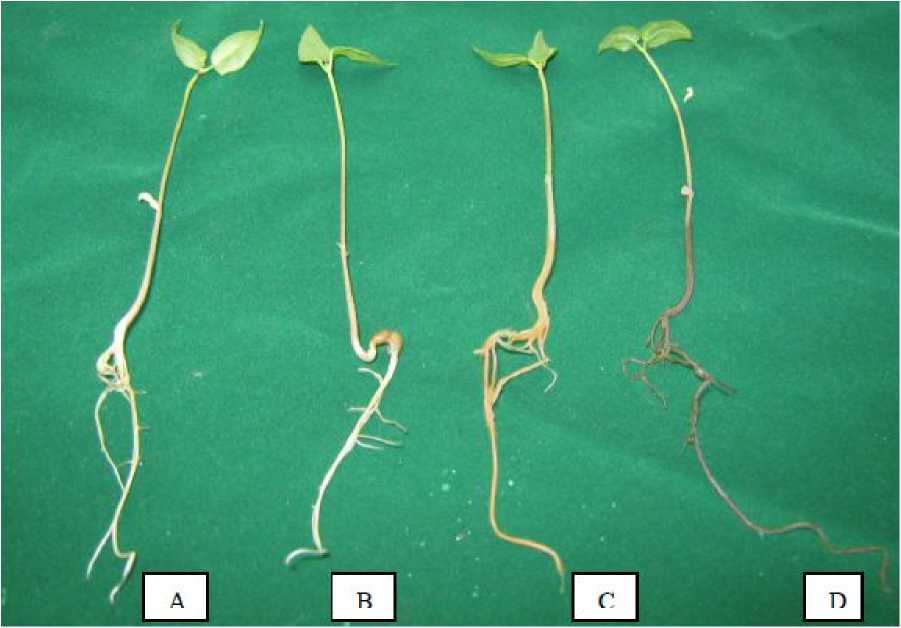
Figure 3. Differential morphological symptoms on the moth bean seedlings after treatment of 15 % PEG.A-RMO-435, B-CZM-105, C-RMM-12-double, and D-RMO-423.
Electrophoretic patterns of proteins under PEG stress
The twelve varieties of moth bean subjected to 15% PEG for Seven days exhibited alterations in their protein profiles (Fig 4). The electrophoretically separated protein in PEG-treated seedlings as compared with control revealed (i) quantitative decline in certain proteins, (ii) some proteins remained unchanged, and (iii) de novo induction of specific proteins. A protein of 97.4 KD was quantitatively high in treated seedlings of all varieties. Proteins of molecular weights 97.4 KD and 48.6 KD were constitutively expressed in all the genotypes in both stressed and non stressed conditions. PEG induced proteins of 54.5 KD and 22.6 KD were expressed only in tolerant genotypes.
A 20.9 KD protein was found upregulated in all genotypes in non stressed condition.
DISCUSSION
Water deficit is one of the most common environmental stresses that affects growth and development of plants (Aslam et al, 2006). The selection of these tolerant genotypes having high yield in drought stress conditions is very important (Richards et al, 2002).
Some promising genes have been identified and cloned and these can be applied biotechnologically in both agriculture and forest and grass for the eco-environmental construction under abiotic stress conditions.
Moth bean is a major crop of arid zone with high tolerance against drought. Water stress often occurs during crop establishment and seedling growth either directly from low available soil moisture or from osmotic effects associated with drought. Studies on this crop are therefore expected to improve our understanding of the effect of water and drought stresses during germination and seedling growth.
Generally evaluation and identification systems of drought-tolerant plants include field testing, controlled drought stress and natural desiccation stress method. Field based screening is simple and reliable but restricted by the season, labordemanding and need controlled drought stress. The chemical identification method with polyethylene glycol (PEG) to make osmotic stress can overcome the shortcoming of the above field screening. Various research groups have been using high osmotic solutions such as PEG to screen and evaluate the genotypes. PEG is described as a nonionic water soluble polymer, which does not penetrate to intact plant tissues rapidly and is widely used to induce water stress in higher plants (Nepomuceno et al, 1998). Several authors reported the use of PEG for in vitro drought screening in crop plants (Gopal and Iwana, 2007).
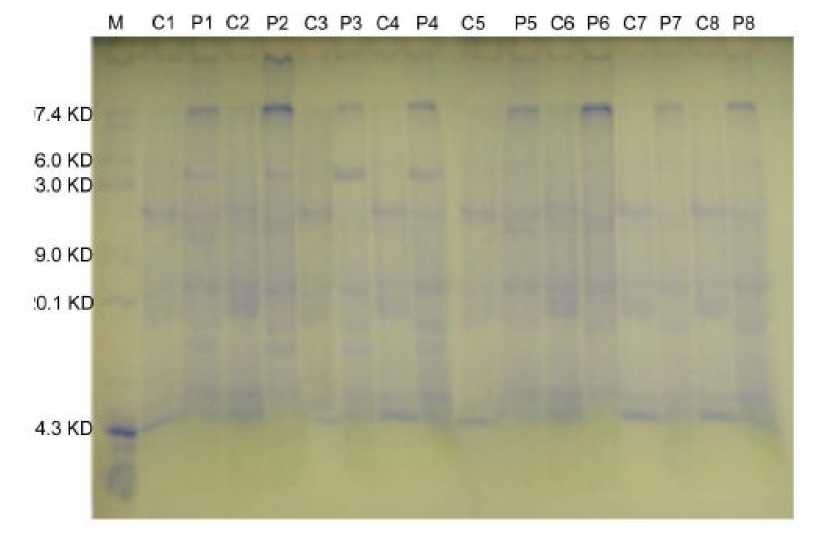
Figure 4. Protein profile of 8 culvitars of Vigna aconitifolia seedlings extracted after 7 days of PEG (15%) induced water stress treatment. Control: Seedlings grown in absence of PEG, M-Marker, T-Treated, 1-RMM-12(single), 2-RMM-12(poly), 3-CZM-105, 4-RMO-435, 5-RMO-40, 6-RMO-225, 7-RMO-257, 8-CZM-04-01.
This study sheds light on the relationship between drought stress and its effect on morpho-physiological (root length, shoot length, fresh and dry weights of shoots and roots), biochemical
(cytosolutes, and activity of antioxidants- catalase and GPOX) parameters of Vigna aconitifolia.
PEG inhibited the germination and caused wilting in seedlings. Germination was however inhibited at much lower concentration of PEG (8%)
compared to the concentration that induced wilting (15%). Hence moth bean is more sensitive to water stress at germination level than seedling stage. This could be part of survival strategy of the species under stress conditions where availability of sufficient water for further growth only can induce germination. Low water uptake and restricted metabolic activities were assigned to decrease in the germination. However, genotypes showing higher level of germination (RMo-435, CZM-105, RMM-12 (single), RMM-12 (poly) under stressed condition, were also more tolerant at seedling stage.
On the basis of percent germination and development of wilting symptoms in seedlings all the genotypes were categorized and tolerant (RMO-435, RMM-12-Single, RMM-12-Poly, CZM-105), moderately tolerant (RMM-12-Zero, RMM-12-Double, RM0-423, CZM-18) and susceptible (RMO-225, RM0-40, RMO-257, and CZM-04-01). Further discussion explores and explains the differences in these three categories of genotypes in terms of various tolerance imparting mechanisms studied.
All of the genotypes show reduction in growth in response to PEG-induced stress. Though, root and shoot length were reduced in all the genotypes the length under unstressed condition was more and reduction under stressed condition was comparatively less in tolerant genotypes identified on the basis of germination and seedling stage wilting percentage except CZM-18 which showed greater reduction in both root and shoot length although it was selected as moderately tolerant genotype. Water deficit reduced the plant growth under drought stress in pearl millet (Kusaka et al, 2005).
Drought stress decreased the root length in various plant species wheat and maize (Nayar and Gupta, 2006). The basal level of root length was also high in tolerant and moderately tolerant genotypes as compared to sensitive genotypes except in RMO-40 implying that root length is important for a plant to exploit the available water.
Difference in sensitivity and response of plants to PEG at cultivar level largely depends upon genetic constitution of the plants (Garsia-Reina et al, 1988). Root length is known important trait in selection of drought resistant variety. According to the Imanparast and Hassanpanah, (2009) genotypes that had good coleoptiles length had excess germination percentage too and seeds had good root growth could have better settlement and had high yield under insufficient environmental condition. Our results for seedling level stress tolerance were correlated with the findings of Fischer and Maurer, (1978).
In this study drought stress caused the germination percentage decrease in all of the genotypes. PEG causes the seed reserves materials hydrolysis decrease and finally the germination percentage decrease (Munns and Weir, 1981). The germination speed of tolerant genotypes to the drought stress was more than the sensitive genotypes germination speed like the germination percentage.
Growth parameters like fresh and dry weights are known to have a profound effect on water limited conditions. In the present study a reduction in root and shoot fresh weight was recorded in stressed conditions in all the genotypes although a marginal increase in root fresh matter (5.26%) was reported in var. RMO-423. However, basal (unstressed control) level of fresh mass was more in tolerant varieties as compared to sensitive varieties.
Most of the tolerant genotypes accumulated more dry matter under control that too increased under stress (15% PEG) however moderately tolerant or susceptible genotypes recorded comparatively less increase or even decrease in dry weight of roots and shoots. Moreover, the ratio of dry matter over fresh weight also increased in most genotypes increase being much higher in tolerant genotypes both for root and shoot. PEG-induced water deficit produced substantial dehydration that led to elevated dry matter content in shoots and roots of moth bean genotypes. However, this can not explain the absolute increase in dry matter. Probably, associated increase in antioxidant activity while helped maintain high metabolic rates accumulation of more sugars resulted in increased dry weight. Some studies have reported that biomass accumulation increased (Fischer and Maurer, 1978) that supports our study too while others have found that it decreased (Hanson et al, 1994) or remained unchanged (Morgan, 1992) during stress conditions. In Brassica napus fresh weight of stressed plants was reduced but dry weight increased considerably compared to unstressed plants (Siddiqui et al, 2008). Gill et al (2001) also reported significant increase in dry weight (relative to control) in different tissues of treated sorghum seedlings in response to NaCl and PEG treatments. In addition, Al Hakimi et al (1995) presented evidence that PEG-induced drought stress increased water soluble carbohydrate concentrations in all soybean cultivars. In addition, accumulation of solute either actively or passively is an important adaptation mechanism for plant in response to osmotic stress like water deficit. In response to this condition, many organisms synthesize solute that helps to retain water within cells or protect cellular components from the injury caused by dehydration. According to Al Hakimi et al (1995) DW increase is associated with cell division and new material synthesis. Hence, this gain in biomass might be attributed to the increased synthetic activity which is visible from the DW values. The established increase after stress imposition probably reflects an increase in carbohydrate metabolism (Sunderland, 1960). Our results suggest that genotype specific response for dry matter accumulation under water stress could be attributed to associated levels of soluble sugar content and increased metabolism under the influence of increased anti-oxidants. Higher accumulation of dry matter in desiccations tolerant genotypes both in root and shoot is suggestive of its role in maintenance of higher osmoticum required for continued absorption of water.
In this experiment, only the total sugar content was determined without the identification of specific sugar components. Imposition of PEG treatments to varieties of moth seedlings significantly increased total soluble sugar content. In our study the concentration of soluble sugar increased under drought stress in most of tolerant varieties of moth bean genotypes except in two moderately tolerant genotypes (RMM-12–zero and CZM-18) however all the tolerant genotypes had higher basal level of total sugars under control conditions. On the other hand in all sensitive genotypes basal sugar content was less that too decreased under stress condition. It is therefore total amount of soluble sugar is more important then its increase under the influence of water stress. The accumulation of soluble sugars is strongly correlated to the acquisition of drought tolerance in plants (Hoekstra et al, 2001).
Protection against dehydration by the former sugars was correlated with the increase in shoots and roots (Nayer Mohammadkhani and Reza Heidari, 2008).
Present study suggests that the high level or accumulation of soluble sugars under stress in tolerant genotypes may be correlated to the longer root and shoot lengths, more dry matter accumulation and in turn acquisition of drought tolerance in moth bean.
Drought stress causes the production of reactive oxygen radicals or species (ROS). Mechanisms of ROS detoxification exist in all plants and can be categorized as enzymatic (SOD, APX, POX, GR, etc) and non-enzymatic (AA, flavanones, anthocyanins, etc) (Shao et al, 2008).
A considerable increase in accumulation of catalase +117.24% was recorded in RMO-423 as compared to other cultivars in the present study. High activity increase of GPOX was found (+224.72%) in RMM-12 poly as compared to other cultivars maintains efficient antioxidant system during drought stress. In general the tolerant genotypes either had high basal activity of the two enzymes studied or was increased to higher level with water deficiency. However, it was noteworthy that in some genotypes level of enzyme activity reduced with PEG induced stress. However, having higher basal activity (unstressed) still imparted them the tolerance. The mutants down regulating these enzymes probably survived by being associated with efficient other protecting mechanisms or their own high basal level. The peroxidase and catalase activity in Vigna unguiculata L., was associated with desiccation tolerance level of variety (Nair et al, 2008).
We found moth bean to be tolerant to the administered drought stress, protecting itself from oxidative damage by higher basal or increased antioxidant activity in leaves. These studies suggest that high contents of total soluble sugars as well as high activity of antioxidants may contribute towards better drought adaptation capabilities of RMO-435, RMM-12-Single, RMM-12–Poly and CZM-105 as compared to the other cultivars of moth bean considered for study. The alterations in protein profiles were recorded in various cultivars of moth bean subjected to PEG stress. In the present investigation up-regulation of polypeptides of 54.5 KD and 22.6 KD in the PEG-tolerant cultivars was recorded. Presence of low level of this protein even in control seedlings suggests its constitutive expression. This demonstrates alteration in metabolic activities under the influence of water stress leading to variation in drought tolerance ability of genotypes and associated modification in development and growth pattern. Proteins which are up regulated by stress condition (stress protein) have been observed in response to high and low temperature, salinity, drought and several other stress factors (Viswanathan and Khanna-Chopra, 1996).
All the growth and biochemical parameters viz root length, shoot length, root fresh weight, shoot fresh weight, root dry weight, shoot dry weight, catalase, GPOX, total soluble sugar were significantly correlated with each other and with germination and wilting resistant percentage recorded for stress condition except a non significant positive correlation between germination percentage and catalase. Similar results were obtained for unstressed condition also. However under unstressed condition catalase was not associated with total soluble sugar in addition to germination percentage taken for stressed condition. The correlation study thus suggests catalase activity is not required for faster or better germination. However it is seeds intrinsic capacity to produce healthier seedlings with longer shoot and root with better fresh weight along with shoot dry weight and more total soluble sugar and to stand water stress at seedling stage. However root dry weight is probably the function of solute concentration in the medium that increased with increase in solute concentration to maintain osmotic potential for proper absorption.
Root dry weight was not associated with any of traits studied under control, showed a significant association with most growth and biochemical parameters suggesting that accumulation of dry matter with increase in solute concentration is associated with drought tolerance and maintenance of growth under stress. A high correlation of germination percentage in control as well as in stress condition with wilting resistance and other trait suggest that germination test can be satisfactorily used for large in vitro scale screening of germplasm for drought tolerance using PEG.
ACKNOWLEDGEMENT
This study is a part of the NAIP component-4 (National Agriculture Innovation Project), India and Plant Biotechnology Centre, COA, SKRAU, Bikaner.
REFRENCES
Al Hakimi A., Monneveux P. and Galiba G. (1995). Soluble sugars, proline and relative water content (RWC) as traits for improving drought tolerance and divergent selection for RWC from T. polonicum into T. durum . J. Gen. Breed., 49 : 237-244.
Aslam M., Khan I.A., Saleem M. and Ali Z. (2006). Assessment of water stress tolerance in different maize accessions a germination and early growth stage. Pak. J. Bot., 38 (5): 15711579.
Fischer R.A. and Maurer R. (1978). Drought resistance in spring wheat cultivars. I. Grain yields responses. Aust. J. Agri. Res ., 29 :897912.
Garsia-Reina G., Moreno V. and Luque A. (1988). Selection for NaCl tolerance in cell culture of three canary island tomato land races. Recovery of tolerant plantlets from NaCl tolerant cell strains . J. Pl. Phy. , 133 :1-6.
Gill P.K., Sharma A.D., Singh P. and Bhullar S.S. (2001) . Effect of various abiotic stresses on the growth, soluble sugar and water relation of sorghum seedlings growth in light and darkness. Bulg. J. Pl. phyl., 27 (1–2): 72-84.
Gopal J. and Iwana K. (2007). In vitro screening of potato against potato against water stress mediated through sorbital and polyethylene glycol. Pl. Cell Report , 26 :693-700.
Hoekstra, F.A., Golovina, E.V. and Buitink, J. (2001). Mechanism of plant desiccation tolerance. Trends Pl. Sci ., 9 :431- 439.
Imanparast L. and Hassanpanah D. (2009). Response of Onobrychis genotypes to PEG 10000 induced osmotic stress. Biotechnology, 8 (3): 365-369.
Jaleel C.A., Manivannan P., Wahid A., Farooq M., Al-Juburi H.J., Somasundaram R. and Panneerselvam R. (2009). Drought stress in plants: A review on morphological characteristics and pigment composition. Int. J. Agri. Biol ., 11 :100-105.
Kusaka M., Lalusin A.G. and Fujimuram T. (2005). The maintenance of growth and turgor in pearl millet ( Pennisetum glaucum L. Leeke) cultivars with different root structures and osmoregulation under drought stress. Pl. Sci., 168 : 1– 14.
Morgan J.M. (1992). Osmotic components and properties associated with genotypic differences in osmoregulation in wheat. Aust. J. Pl. Phy., 19 : 67-76.
Munns R., Weir R. (1981) Contribution of sugars to osmotic adjustment in elongating and expanding zones of wheat leaves during moderate water deficits at two light levels. Australian Journal of Plant Physiology 8 , 93105
Nair A.S., Abraham T.K. and Jaya D.S. (2008). Studies on the changes in lipid peroxidation and antioxidants in drought stress induced cowpea ( Vigna unguiculata L.) varieties. J. Envir. Biol., 29 (5) 689-691.
Nayar H. and Gupta D. (2006). Differential sensitivity of C3 and C4 plants to water deficit stress: association with oxidative stress and antioxidants. Envir. Exp. Bot ., 58 :106-113.
Nayer Mohammadkhani and Reza Heidari (2008). Drought-induced Accumulation of Soluble Sugars and Proline in Two Maize Varieties. World Applied Sciences Journal 3 (3): 448-453
Nepomuceno A.L., Oostrerhuis D.M. and Stewart J.M. (1998). Physiological reponses of cotton leaves and roots to water deficit induced by Polythylene Glycol. Envir. Exp Bot ., 40 :29-41.
Richards R.A., Rebetzke G.J. Condon A.G. and van Herwaarden, A.F. (2002). Breeding opportunities for increasing the efficiency of water use and crop yield in temperate cereals. Crop. Sci., 42: 111-121.
Shao H.B., Chu L.Y., Lu Z.H., Kang C.M. (2008). Primary oxidant scavenging and redox signaling in higher plants. Int J Biol Sci ; 4 :8–14
Siddiqui Z., Khan M.A., Kim B.G., Huang J.S., Kwon T.R. (2008). Physiological Responses of Brassica napus Genotypes to Combined Drought and Salt Stress. Plant Stress, 2 (1): 7883.
Sunderland N. (1960). Cell division and expansion in the growth of the leaf. J. Exp. Bot., 11 : 6880.
Viswanathan C. and Khanna-Chopra R. (1996). Heat shock proteins - Role in thermotolerance of crop plants. Curr. Sci ., 71 :275-284.
Zhu J.K. (2002). Salt and drought stress signal transduction in plants. Annu Rev Plant Bio.,l 53 :247-73
Список литературы In-vitro response of Vigna aconitifolia to drought stress induced by PEG - 6000
- Al Hakimi A., Monneveux P. and Galiba G. (1995). Soluble sugars, proline and relative water content (RWC) as traits for improving drought tolerance and divergent selection for RWC from T. polonicum T. durum. J.Gen. Breed., 49: 237-244.
- Aslam M., Khan I.A., Saleem M. and Ali Z. (2006). Assessment of water stress tolerance in different maize accessions a germination and early growth stage. Pak. J. Bot., 38(5): 1571-1579.
- Fischer R.A. and Maurer R. (1978). Drought resistance in spring wheat cultivars. I. Grain yields responses. Aust. J. Agri. Res., 29:897-912.
- Garsia-Reina G., Moreno V. and Luque A. (1988). Selection for NaCl tolerance in cell culture of three canary island tomato land races. Recovery of tolerant plantlets from NaCl tolerant cell strains. J. Pl. Phy., 133:1-6.
- Gill P.K., Sharma A.D., Singh P. and Bhullar S.S. (2001).Effect of various abiotic stresses on the growth, soluble sugar and water relation of sorghum seedlings growth in light and darkness. Bulg. J. Pl. phyl., 27(1-2): 72-84.
- Gopal J. and Iwana K. (2007). In vitro screening of potato against potato against water stress mediated through sorbital and polyethylene glycol. Pl. Cell Report, 26:693-700.
- Hanson A.D., Rathinasabapathi B., Rivoal J., Burnet M., Dillon M.O. and Gage D.A. (1994). Osmoprotective compounds in the Plubaginaceae: A natural experiment inmetabolic engineering of stress tolerance. Proc. Nat. Acad.Sci. USA, 91:306-310.
- Hoekstra, F.A., Golovina, E.V. and Buitink, J. (2001). Mechanism of plant desiccation tolerance. Trends Pl. Sci., 9:431-439.
- Imanparast L. and Hassanpanah D. (2009). Response of Onobrychis genotypes to PEG 10000 induced osmotic stress. Biotechnology, 8(3): 365-369.
- Jaleel C.A., Manivannan P., Wahid A., Farooq M., Al-Juburi H.J., Somasundaram R. and Panneerselvam R. (2009). Drought stress in plants: A review on morphological characteristics and pigment composition. Int. J. Agri. Biol., 11:100-105.
- Kusaka M., Lalusin A.G. and Fujimuram T. (2005). The maintenance of growth and turgor in pearl millet (Pennisetum glaucum L. Leeke) cultivars with different root structures and osmo-regulation under drought stress. Pl. Sci., 168: 1-14.
- Larher F., Leport L., Petrivalsky M. and Chappart M. (1993). Effectors for the osmoinduced praline response in higher plants. Pl.Phy. Bioch., 31(6), 911-922.
- Morgan J.M. (1992). Osmotic components and properties associated with genotypic differences in osmoregulation in wheat. Aust. J. Pl. Phy., 19: 67-76.
- Munns R., Weir R. (1981) Contribution of sugars to osmotic adjustment in elongating and expanding zones of wheat leaves during moderate water deficits at two light levels. Australian Journal of Plant Physiology 8, 93-105
- Nair A.S., Abraham T.K. and Jaya D.S. (2008). Studies on the changes in lipid peroxidation and antioxidants in drought stress induced cowpea (Vigna unguiculataL.) varieties. J. Envir. Biol.,29(5) 689-691.
- Nayar H. and Gupta D. (2006). Differential sensitivity of C3 and C4 plants to water deficit stress: association with oxidative stress and antioxidants. Envir. Exp. Bot., 58:106-113.
- Nayer Mohammadkhani and Reza Heidari (2008). Drought-induced Accumulation of Soluble Sugars and Proline in Two Maize Varieties. World Applied Sciences Journal 3 (3): 448-453
- Nepomuceno A.L., Oostrerhuis D.M. and Stewart J.M. (1998). Physiological reponses of cotton leaves and roots to water deficit induced by Polythylene Glycol. Envir. Exp Bot., 40:29-41.
- Richards R.A., Rebetzke G.J. Condon A.G. and van Herwaarden, A.F. (2002). Breeding opportunities for increasing the efficiency of water use and crop yield in temperate cereals. Crop. Sci., 42: 111-121.
- Shao H.B., Chu L.Y., Lu Z.H., Kang C.M. (2008). Primary oxidant scavenging and redox signaling in higher plants. Int J Biol Sci; 4:8-14
- Siddiqui Z., Khan M.A., Kim B.G., Huang J.S., Kwon T.R. (2008). Physiological Responses of Brassica napus Genotypes to Combined Drought and Salt Stress. Plant Stress, 2 (1): 78-83.
- Sunderland N. (1960). Cell division and expansion in the growth of the leaf. J. Exp. Bot.,11: -80.
- Viswanathan C. and Khanna-Chopra R. (1996). Heat shock proteins -Role in thermotolerance of crop plants. Curr. Sci., 71:275-284.
- Zhu J.K. (2002). Salt and drought stress signal transduction in plants. Annu Rev Plant Biol., 53:247-73


