In vitro screening for drought tolerance in different sorghum ( Sorghum bicolor (L.) Moench) varieties
Автор: Tsago Yohannes, Andargie Mebeaselassie, Takele Abuhay
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 3 т.9, 2013 года.
Бесплатный доступ
Drought is one of the complex environmental factors affecting growth and yield of sorghum in arid and semi-arid areas of the world. Sixteen elite sorghum ( Sorghum bicolor (L) Moench) genotypes were evaluated for their genetic potential to drought tolerance at callus induction and plant regeneration stage for drought tolerance. The non-ionic water soluble polymer polyethylene glycol (PEG) of molecular weight 6000 was used as osmoticum to simulate water stress. The factorial experiment was laid down in a completely randomized design which comprised of a combination of two factors (genotypes and five PEG stress level; 0, 0.5, 1.0, 1.5, and 2.0% (w/v) treatments). Data were recorded for callus induction efficiency, callus fresh weight, embryogenic callus percentage and plant regeneration percentage. Significant differences were observed among the genotypes, treatments and their interactions for the evaluated plant traits suggesting a great amount of variability for drought tolerance in sorghum. The correlation analysis also revealed strong and significant association between embryogenic callus percent and plant regeneration percent as well as between embryogenic callus percent and plant regeneration percent. By taking into consideration all the measured traits, Mann Whitney rank sum test revealed that 76T1#23 and Teshale followed by Meko, Gambella-1107 and Melkam showed better drought stress tolerance. Therefore they are recommended to be used as parents for genetic analysis, gene mapping and improvement of drought tolerance while Chelenko, Hormat and Raya appear to be drought sensitive.
Callus culture, polyethylene glycol (peg), sorghum bicolor l, water stress
Короткий адрес: https://sciup.org/14323789
IDR: 14323789
Текст научной статьи In vitro screening for drought tolerance in different sorghum ( Sorghum bicolor (L.) Moench) varieties
Sorghum (Sorghum bicolor (L) Moench) is a major crop in many parts of Africa, Asia and Latin America (ICRISAT, 2006). It is the second crop next to maize grown across all agro-ecologies in Africa (Wortmann et al. 2006). In Ethiopia, it is the fifth major cereal crop in terms of area and production next to teff (Eragrostis teff), barley (Hordeum vulgare), wheat (Triticum aestivum) and maize (Zea mays) (CSA 2011). Sorghum is usually grown in arid and semi-arid parts of the tropics and sub-tropics where it is affected by drought during various growth stages (Amjad et al. 2009). This problem is intensified by the possible global climate change scenarios (IPCC 2001). For example, Simon (2009) reported that recurring drought due to deficit of rainfall in semi-arid and dry sub-humid areas of the country is one of the major causes of underproduction of sorghum.
For the last half century variety development for lowland parts of Ethiopia has focused on the selection of early maturing varieties that can escape drought. A number of early sorghum open-pollinated varieties were developed and released for these areas (Asfaw 2007). There are, however, disadvantages to early maturity. Cultivars that mature extremely early tend to be lower in yield because the plants have a shorter growth period to flower and store nutrients in the grain (Sleper and Poehlman 2003).
On the other hand, in vitro culture studies play tremendous role by providing efficient way of understanding plant genetic processes in short period of time in a controlled environment . In vitro culture of plant cells and tissue has attracted considerable interest over recent years because it provides the means to study plant physiological aspects and genetic processes in addition to offering the potential to assist in the breeding of improved cultivars by increasing genetic variability (Wani et al. 2010).
For in vitro drought stress induction, one of the most popular approaches is to use high molecular weight osmotic substances, like PEG. PEG6000 is a non penetrable and nontoxic osmotic substance which is used to lower the water potential of the culture medium and it has been used to simulate drought stress in cultured plant tissues (Muhammad et al. 2010). With this background, the present study was aimed to assess the effect of PEG induced stress on genotypic capacity of callusing, callus growth, embryogenesis and plant regeneration of sorghum genotypes to identify the superior genotypes for drought tolerance.
MATERIALS AND METHODS
Plant material
The seeds of the genotypes were provided by the National sorghum improvement project of Melkassa agricultural research center, Ethiopia. Sixteen sorghum varieties from more than 19 released varieties were selected based on their agro-ecological adaptation. Five PEG concentrations (0-2%) were used. The experiment of the study was laid out in a completely randomized design in factorial arrangement with three replications (Gomez and Gomez 1976).
Callus induction under PEG stress
Murashige Skoog (MS) media (Murashige and Skoog 1962) were prepared as described by Zhao et al. (2010) for callus induction, pH was adjusted and autoclaved. Thirty seeds were cultured per jar.
After four weeks of incubation, the induced calli were excised and sub-cultured, under the same growth conditions, and in the same MS medium to which five level concentrations of polyethylene glycol (PEG) (6000) (0, 0.5, 1.0, 1.5, 2.0 %) has been added (Wani et al. 2010). The incubation period was four weeks in which the media is replaced with the same media in two weeks.
Plant regeneration from induced callus in PEG stress condition
Resulting calli were excised, transferred, to jars containing 25ml MS basal salts medium supplemented with plant hormones and growth regulators as described by Zhao et al. (2010) for shoot initiation in conditions in which PEG (6000)
(0, 0.5, 1.0, 1.5, 2.0 %) has been added. The incubation period was four weeks.
The jars were placed in a growth chamber under fluorescent light and at an ambient temperature of 25 ± 2ºC. The medium was changed in 15 days and after four weeks, calli with clearly differentiated shoots were scored as regenerating callus. Rooting was initiated on MS medium supplemented with 3 mg l-1 indole-3-butyric acid (IBA) under the same conditions. Regenerating calli, showing shoot and root formations, were transferred to MS basal medium with no phytohormones to sustain growth of plantlets for four weeks.
Data recorded and statistical analysis
After the end of callus induction period callus induction efficiency (CIE) was recorded as the number of calli induced divided by total number of seeds cultured × 100. After four weeks of culture, callus was excised and callus fresh weight (CFW) was determined. In the mean time, embryogenic callus percent (ECP) was recorded as the number of embryogenic callus obtained divided by total number of induced callus X 100. Finally, at the end of plant regeneration, data for plant regeneration percent (PRP) was recorded as the number of plantlets obtained divided by total number of embryogenic calli cultured × 100.
Data were analyzed using SAS software 9th Edition. The data analysis was carried out using analysis of variance for each parameter while the differences between treatment means was evaluated at 1% level of significance using Fischer’s LSD.
RESULTS AND DISCUSSION
Callus induction efficiency (CIE)
Callus induction was observed following 4 weeks of culture. The amount and type of callus varied with genotypes. Sorghum genotypes exhibited a significant interaction with PEG stress levels for CIE (Figure 1). In control (0.0% PEG), almost all explants initiated callus. With the increasing level of PEG, the mean percentage of callus induction reduced significantly.
Increasing level of PEG from 0 to 2% had lowest effect in mean CIE for genotypes Meko and Seredo indicating their relative tolerance for drought stress whereas the highest effect of PEG on CIE was observed on genotype Chelenko and Raya respectively which indicated the relative susceptibility of these genotypes to PEG induced stress. In Meko callus, induction percentage on callus induction medium containing 0.5, 1.0, 1.5 and 2 g l-1 PEG was 78.9, 78.7, 78.7 and 77.8% respectively, against 80.0% in the control set whereas in Chelenko callus, induction percentage on callus induction medium containing 0.5, 1.0, 1.5 and 2 g l-1 PEG was 52.2, 42.2, 36.6 and 24.4% respectively, against 62.2% in the control set.
Generally the mean CIE decreased drastically in genotypes under higher PEG treatments than lower PEG treatments (Fig. 2). Decrease in CIE is a typical response of explants of crop genotypes when subjected to PEG stress (Matheka et al. 2008; Biswas et al. 2002; Abdel-Ghany et al. 2004). However, the response of CIE for PEG treatment was genotype dependent. The difference in decreasing trend in CIE of the genotypes might further explain difference in osmotic regulation among genotypes, which enables them to maintain osmotic balance to assist initiation of callus cells under severe stress conditions or might be due to either water shortage which led to profuse mutation in cellular metabolism including protein functioning and alteration in amount of proteins
(Plomion et al. 1999) or altered gene expression controlling this trait (Visser 1994).
Callus Fresh Weight (CFW)
Calli were sub-cultured for a 4-week period on MS with different levels of PEG and PGRs and fresh weights were determined. Total callus fresh weight varied among genotypes, as indicated on Figure 3 and in all genotypes, effect of the PEG treatments resulted in decrease of the mean CFW with increasing level of the PEG from 0 to 2%. Genotype Abeshir produced the highest callus fresh weight (345.1mg) whereas the lowest was recorded in Birmash (315.01mg) indicating the highest damage to callus growth in the latter genotype due to the PEG stress.
The major effect of PEG stress in callus growth is mainly observed in the form of decreasing the callus fresh weight which is a typical response in callus tissue of many crop plants (Sakthivelu et al. 2008; Lutts et al. 1996; Khalequzzaman et al. 2005; Wani et al. 2010). Such a decrease in callus fresh weight in response to PEG stress might be due to water shortage which affects development and growth of cells. Addition of PEG-6000 in solid media lowers water potential of the medium that adversely affect cell division leading to reduced callus growth (Ehsanpour and Razavizadeh 2005; Sakthivelu et al. 2008). Cell division and cell growth are the two primary processes involved in increase of fresh weight. In general, cell division is considered to be less sensitive to drought when compared with cell enlargement or growth (Sakthivelu et al. 2008). However, both cell expansion and cell division can be influenced by relatively mild osmotic stress. Generally, the results of this experiment showed that genotypic differences in callus proliferation are genetically determined in sorghum like other cereals.
Embryogenic Callus Percent (ECP)
More embryogenic callus (EC) generally portents high plantlet regeneration potential, which is an important parameter in in vitro culture systems. In the present study, in the entire genotypes mean ECP increased with increasing level of PEG treatment from 0 to 1.5% and declined at 2% PEG level (Fig.4). However, in varieties Hormat, ESH-2, and Raya there was an overall reduction in ECP of 1.73%, 1.50% and 1.40% respectively, indicating the highest effect of PEG treatment on ECP of these genotypes. The highest increase in ECP was attributed to varieties Girana-1(-3.34), Teshale (3.92) and Meko (-3.88), which is an indication of lowest effect of PEG stress on embryogenic callus formation of these genotypes.
Inconsistent variation in ECP was recorded among genotypes in response to the five PEG stress levels. The inconsistency could be attributed to the difference in genotypes' strategies and responses to the induced water deficit conditions. In line with this, Moon and Park (2008) reported significant increase in embryogenesis more than doubling the average number of somatic embryos with doubling PEG concentration. It has been reported that PEG generally increases total soluble and reducing sugars (Handar et al. 1983), which serve as an osmoticum or as respiratory substrates to endure the stress shock; inter alia , by retarding cell growth, increasing L-proline level, and changing isozyme profiles especially of malate dehydrogenase, acid phosphatase, and peroxidase (Srivastava et al. 1995).
Establishment and propagation of embryogenic callus cultures is an essential prerequisite in cereal tissue cultures for genetic manipulation (Maggioni et al. 1989). In the present investigation, EC percentage decreased with increasing concentrations of PEG in all genotypes particularly at the highest PEG level. It is pertinent to note that the maximum ECP was produced in Teshale, more than in other genotypes on stressed and nonstressed media. Therefore this genotype may be employed on a large scale for developing embroygenic calluses and simultaneously to develop drought-tolerant lines through in vitro screening.
Plant Regeneration Percent (PRP)
Shoot regeneration after sub-culturing on fresh MS media supplemented with different levels of PEG started after four weeks of callus growth. The medium was changed in 15 days and after four weeks, calli with clearly differentiated shoots were scored as regenerating callus, regardless of the number of shoots. Calli regenerating plantlets were revealed a highly significant variation among the tested genotypes (Fig.5).
The highest effect of the PEG stress in PRP was recorded in genotype Chelenko (26.39%) (Fig.6) followed by Raya (23.81%) and Girana-1 (23.61%). In Gambella-1107 (11.94%), Birmash (11.94%) and Teshale (10.33%), PEG treatment imposed the lowest reduction in PRP indicating that these genotypes are the least affected by PEG treatment in terms of plant regeneration.
The results of the present study showed that an increment in PEG stress level caused reduction of PRP in sorghum genotypes which is a typical response of callus cells of many plants (Begum et al. 2011; Wani et al. 2010; Smith et al. 1985). The typical decrease in plant regeneration in callus cells of crop plants in response to water stress is due to water shortage in the cells which leads to a decrease in cell turgor and eventually cell growth. Addition of PEG-6000 in culture media lowers water potential of the medium that affect cell division leading to reduced callus growth and consequently influences regeneration (Ehsanpour and Razavizadeh 2005; Sakthivelu et al. 2008).
Association between In vitro Traits
Callus induction efficiency had positive significant correlation with traits such as callus fresh weight and plant regeneration percent and callus fresh weight had a positive significant correlation with plant regeneration percent in addition embryogenic callus percent showed a positive significant correlation with plant regeneration percent (Table 1). In contrast, callus induction efficiency showed a significant negative correlation with embryogenic callus percent. However, no significant correlation was observed between callus fresh weight and embryogenic callus percent. This suggests that genotypes with high callus induction also caused an increase in the number of plants obtained from regeneration medium. The presence of significant correlation between callus induction efficiency and regeneration capacity of callus might indicate that callus induction and regeneration capacity may be controlled by the same mechanisms.
The highly significant and strong correlation observed between the ability of cultivars to produce embryogenic callus and their capacity for regeneration indicate that embryogenic callus percentage constitute a good index for callus ability to regenerate later on plantlets. The cultivars that presented high embryogenic callus percentages at the first weeks of culture have high chance to regenerate plants after several weeks of culture.
Mann–Whitney–Wilcoxon Rank Sum Test of the Measured Traits
To determine the most desirable drought tolerant genotypes based on all traits measured, mean rank sum method was used according to Ezatollah et al. (2012). In this method, all the indices, mean rank and standard deviation of ranks of all in vitro drought tolerance criteria were calculated and summed (RS) (Table 2). Based on this criterion the most desirable drought tolerant genotypes were identified.
By taking in to consideration all indices, genotypes: Teshale (RS= 8.23) and 76T1#23 (RS= 8.39) followed by genotypes: Meko (RS= 9.29), Gambella-1107(RS= 10.56) and Melkam (RS=11.91) were the most drought tolerant genotypes, respectively. Therefore they are recommended to be used as parents for genetic analysis, gene mapping and improvement of drought tolerance.
While genotypes: Chelenko (RS =16.36), Hormat (RS = 15.74) and Raya (RS= 15.44) were the most sensitive to drought, therefore they are recommended for crossing and genetic analysis of drought tolerance using diallel mating design.
The detection of superior genotypes Teshale, 76T1#23, Meko, Gambella-1107 and Melkam for drought tolerance at cellular level together with their high potential for callus induction leads to the conclusion that a hybridization breeding procedure using these superior plant materials supplemented with in vitro selection for drought tolerance might be beneficial for improving these traits in sorghum. Hence, callus culture can be useful to speed up sorghum improvement.
Table 1. Correlation coefficient (r) of measured traits
|
CIE |
CFW ECP PRP |
|
|
CIE |
1 |
|
|
CFW |
0.349* |
1 |
|
ECP |
-0.125* |
0.001 1 |
|
PRP |
0.332* |
0.731* 0.783* 1 |
|
CIE=Callus Induction Efficiency CFW=Callus Fresh Weight PRP=Plant Regeneration Percent |
||
Table 2. Rank sum of measured traits
|
Variety |
CIE |
CFW |
ECP |
PRP |
Rank Mean |
Rank SD |
RS |
|
Abshir |
08 |
02 |
12 |
06 |
9.1 |
5.88 |
14.98 |
|
Chelenko |
15 |
14 |
03 |
16 |
10.6 |
5.76 |
16.36 |
|
Raya |
16 |
15 |
13 |
15 |
10.8 |
4.64 |
15.44 |
|
Hormat |
11 |
16 |
15 |
12 |
12.1 |
3.63 |
15.74 |
|
Gubiye |
05 |
10 |
07 |
04 |
08.5 |
3.72 |
12.22 |
|
Gambella-1107 |
07 |
07 |
04 |
02 |
06.5 |
4.06 |
10.56 |
|
Birmash |
13 |
11 |
06 |
03 |
08.8 |
3.77 |
12.57 |
|
Meko |
01 |
01 |
01 |
05 |
05.0 |
4.29 |
09.29 |
|
Macia |
12 |
08 |
16 |
13 |
09.7 |
3.86 |
13.56 |
|
Seredo |
02 |
12 |
09 |
07 |
07.7 |
4.90 |
12.60 |
|
Misikir |
14 |
13 |
08 |
09 |
10.4 |
3.72 |
14.12 |
|
Melkam |
06 |
05 |
10 |
10 |
08.2 |
3.71 |
11.91 |
|
76t1#23 |
04 |
04 |
11 |
08 |
04.7 |
3.53 |
08.39 |
|
ESH-2 |
10 |
03 |
14 |
11 |
10.4 |
3.47 |
13.88 |
|
Girana-1 |
09 |
06 |
02 |
14 |
08.0 |
5.54 |
13.54 |
|
Teshale |
03 |
09 |
05 |
01 |
05.3 |
3.09 |
08.23 |
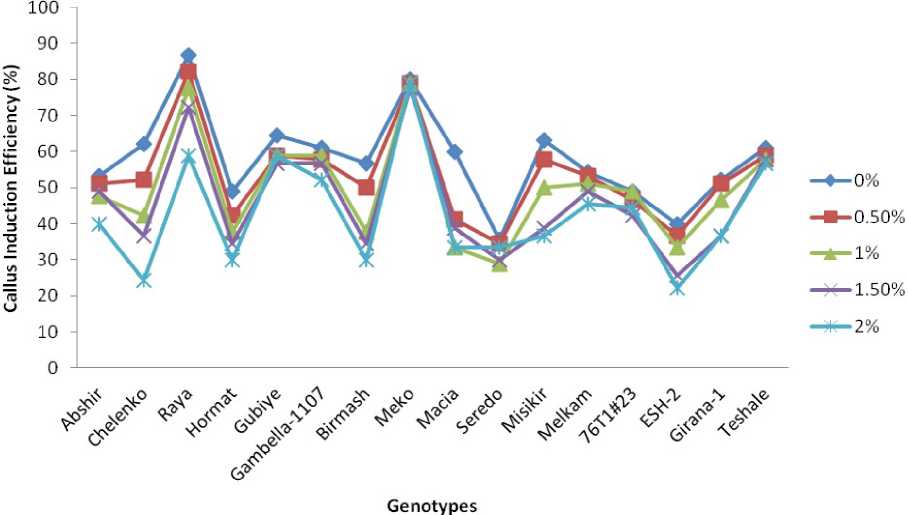
Figure 1. Mean callus induction efficiency (CIE) of genotypes for each PEG treatment level

Figure 2. Callus induction as differed across PEG treatment levels
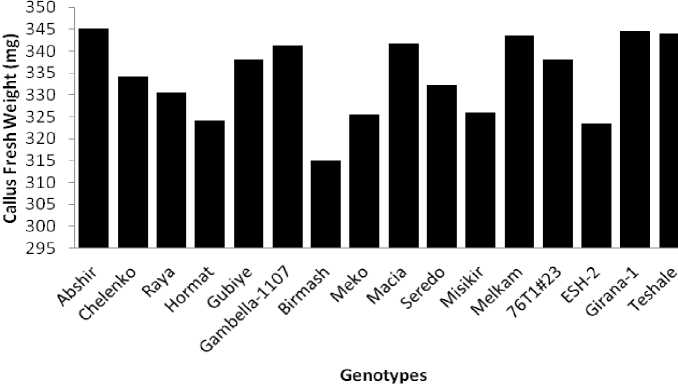
Figure 3. Mean callus fresh weight of the sorghum genotypes
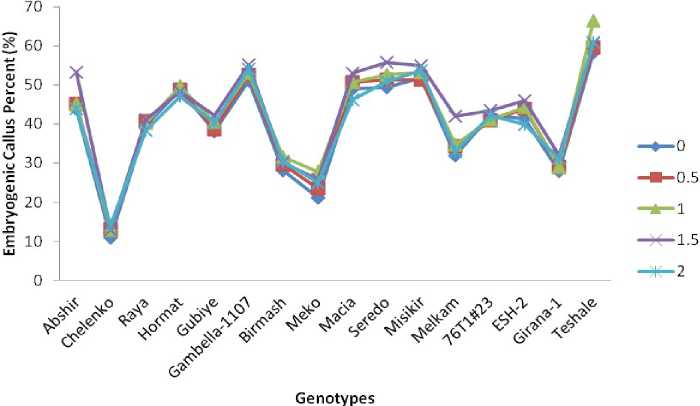
Figure 4. Mean embryogenic callus percent (ECP) of genotypes for each PEG treatment level
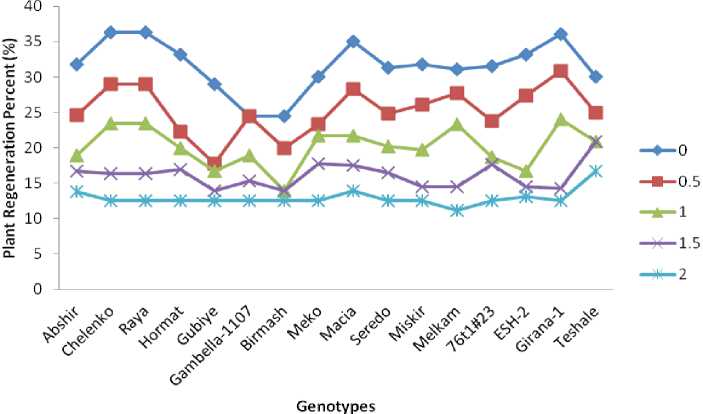
Figure 5. Mean plant regeneration percent (PRP) of genotypes for each PEG treatment level

Figure 6. Plant regeneration as differed across PEG treatment levels in Chelenko genotype
ACKNOWLEDGMENT
This work was financially supported by the Ethiopian Ministry of Education which is gratefully acknowledged. We would like to thank also Melkassa Agricultural Research Center (MARC) for providing the sorghum seeds.
Список литературы In vitro screening for drought tolerance in different sorghum ( Sorghum bicolor (L.) Moench) varieties
- Abdel-Ghany, H.M., Nawar, A.A., Ibrahim, M.E., El-Shamarka, A., Selim, M.M., and Fahmi, A.I. (2004). Using tissue culture to select for drought tolerance in bread wheat. Proceedings of the 4th International Crop Science Congress. Brisbane, Australia, 26 Sep -1 Oct.
- Amjad, M.A., Niaz, S., Abbas, A., Sabir, W.,and Jabran, K. (2009). Genetic diversity and assessment of drought tolerant sorghum landraces based on morph physiological traits at different growth stages. Plant Omics Journal. 2(5):214-227.
- Asfaw, A. (2007). Assessment of yield stability in sorghum. African Crop Science Journal. 15(2): 83 -92.
- Begum, M.K., Islam, M.O., Miah, M.A., Hossain, M.A., and Islam, N. (2011). Production of somaclone in vitro for Drought Stress Tolerant Plantlet Selection in Sugarcane (Saccharum officinarum L.). The Agriculturalist. 9(1 and 2): 18-28.
- Biswas, J., Chowdhury, B., Bhattacharya, A., and Mandal, A.B. (2002). In vitro screening for increased drought tolerance in rice. In Vitro Cell Developmental Biolology. 38:525-530.
- CSA (Central Statistical Authority of Ethiopia): Area, Production and Yield of Crops for Private
- Ehsanpour, A.A., and Razavizadeh, A. (2005). Effect of UV-C on drought tolerance of alfalfa (Medicago sativa) callus. American Journal of Biochemistry and Biotecnology. 1(2): 107-110.
- Ezatollah, F., Jamshidi, B., Cheghamirza, K., and Teixeira da Silva, J.A. (2012). Evaluation of drought tolerance in bread wheat (Triticum aestivum L.) using in vivo and in vitro techniques. Annals of Biological Research. 3(1):465-476.
- Gomez, K.A., and Gomez, A.A. (1976). Statistical Procedures for agricultural Research with Special Emphasis on rice. Philippines, International Rice Research.
- Handar, A.K., Bressen, R.A., Handa, S., and Hasegawa, P.M. (1983). Clonal variation for tolerance to PEG induced water stress in cultured tomato cells. Plant Physiology. 80:938-945.
- ICRISAT (International Crops Research Institute for the Semi-Arid Tropics). (2006). Sorghum Genetic Enhancement: Research Process, Dissemination and Impacts. Andhra Pradesh, India.
- IPCC (Intercontinental Panel on Climate Change). (2001). Climate Change in 2001. Available at http://www. ipcc.ch; accessed on sep.1, 2011.
- Khalequzzaman, M., Haq, N., Hoque, M.E., and Aditya, T.L. (2005). Regeneration efficiency and genotypic effect of 15 Indica type Bangladeshi rice (Oryza sativa L.) landraces. Plant Tissue Culture. 15: 33-42.
- Lutts, S., Kinet, J.M., and Bouharmont, J. (1996). Effects of salt stress on growth, mineral nutrition and proline accumulation in relation to osmotic adjustment in rice (Oryza sativa L.) cultivars differing in salinity resistance. Plant Growth Regulation. 19:207-218.
- Maggioni, L., Lusardi, M.C., and Lupotto, E. (1989). Effects of cultural conditions on callus induction and plant regeneration from mature and immature embryos of rice varieties (Oryza sativa L.). Journal of Genetics and Breeding. 43:99-106.
- Matheka, J.M., Magiri, E., Rasha, A.O., and Machuka, J. (2008). In vitro selection and characterization of drought tolerant somaclones of tropical maize (Zea mays L.). Biotechnology. 7(4): 641-650.
- Moon, H.K., and Park, S.Y. (2008). Somatic embryogenesis and plantlet production using rejuvenated tissues from serial grafting of a mature Kalopanax septemlobus tree. In Vitro Cellular and Developmental Biology. 44:119-127.
- Muhammad, H., Khan, S.A., Shinwari, Z.K., Khan, A.L., Ahmad, N., and Lee, J. (2010). Effect of polyethylene glycol induced drought stress on physio-hormonal attributes of soybean. Pakistan Journal Botany. 42(2): 977-986.
- Murashige, T., and Skoog, F.A. (1962). A revised medium for rapid growth and bioassays with tobacco tissues culture. Physiology of Plants. 15: 473-497. Peasant Holdings for Meher Season 2010/11 (2003 E.C.). Addis Ababa, Ethiopia; 2011.
- Plomion, C., Costa, P., Dubos, C., Frigerio, J.M., Guehl, J.M., and Queyrens, A. (1999). Genetical, physiological and molecular response of Pinus pinaster to a progressive drought stress. Journal of Plant Physiology. 155: 120-129.
- Sakthivelu, G., Devi, M.K.A., Giridhar, P., Rajasekaran, T., Ravishankar, G.A., Nedev, T., and Kosturkova, G. (2008). Drought-induced alterations in growth, osmotic potential and in vitro regeneration of soybean cultivars. General and Applied Plant Physiology Special Issue 34 (1-2): 103-112.
- Simon, K. (2009). Crop monitoring in Ethiopia. European Commission, Joint Research Center 4: 1-19.
- Sleper, D.A., and Poehlman, J.M. (2003). Breeding Field Crops. 5th Edition, Blackwell Publishing, pp 230.
- Smith, R.H.S., Bhaskaran, F., and Miller, R. (1985). Screening for drought tolerance in sorghum using cell culture. In Vitro Cellular and Developmental Biology 21: 10.
- Srivastava, D.K., Gupta, V.K., and Sharma, D.R. (1995). In vitro selection and characterization of water stress tolerant callus cultures of tomato (Lycopersicon esculentum L.). Indian Journal of Plant Physiology. 38:99-104.
- Visser, B. (1994). Technical aspects of drought tolerance. Biotechnology and Development Monitor 18: 5.
- Wani, S.H., Sofi, P.A., Gosal, S.S., and Singh, N.B. (2010). In vitro screening of rice (Oryza sativa L) callus for drought tolerance. Communications in Biometry and Crop Science. 5(2): 108-115.
- Wortmann, C.S., Mamo, M., Mburu, C., Letayo, E., Girma, A., Kayuki, K.C., Chisi, M., Mativavarira, M., Xerinda, S., and Ndacyayisenga, T. (2006). Atlas of Sorghum (Sorghum bicolor (L.) Moench): Production in Eastern and Southern Africa. University of Nebraska, Lincoln.
- Zhao, L., Liu, S., and Song, S. (2010). Optimization of callus induction and plant regeneration from germinating seeds of sweet sorghum (Sorghum bicolor Moench). African Journal of Biotechnology. 9(16), 2367-2374.


