In-vivo thermal stress induces melatonin receptors and heat shock proteins expression in the spleen of mice in a time and temperature dependent manner
Автор: Acharjee S., Singh Sh.sh.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 3 т.19, 2023 года.
Бесплатный доступ
Heat shock proteins (Hsps) responses against stress conditions. Melatonin completes its stress relieving activities via its MT1 and MT2 receptors. The present study delineates the expression pattern of Hsp70/Hsc70 and MT1/MT2 receptor proteins along with the AANAT gene expression in the splenic tissue of mice subjected to hyperthermic stress in a temperature dependent and time dependent manner. In vivo thermal stress resulted increase in expression of Hsp70, Hsc70 and MT2 receptors proteins in both temperature dependent and time dependent manner. Optimum heat exposure at 430C and maximized Hsps expression was observed after 5 hours of heat exposure. Heat stress caused increase in AA-NAT gene expression of the splenic tissue resulted in the synthesis of melatonin which might act as signal molecule for upregulating the activity and rise of the stress responsive genes and proteins like: Hsp70/Hsc70, whereas simultaneous increase of MT2 expression shows its possible involvement in such mechanism.
Thermal stress, heat shock proteins, melatonin receptors, aa-nat gene expression, time and temperature dependent study in mice
Короткий адрес: https://sciup.org/143180560
IDR: 143180560
Текст научной статьи In-vivo thermal stress induces melatonin receptors and heat shock proteins expression in the spleen of mice in a time and temperature dependent manner
The potentiality of hyperthermic stress for damaging the cellular activity is a well-known fact. Activation of apoptosis process in rapid manner is a suggested possible mechanism through which heat stress causes the cellular loss (Kondo et al. , 2000; Khan and Brown, 2002). Recently a report on focusing the improvement of Arrhenius model of cell death concerning the temperature dependent time delay matter was documented (Pearce, 2015). But before the completion of cellular damage via the programmed cell death process, anti-apoptotic protein performs their activity for the protection of the cell. Heat shock proteins (Hsps) are the molecular chaperones in the cellular system which works against the apoptotic process (Gabai et al. , 1997; Kondo et al. , 2000; Li et al. , 2000). Expression of heat shock protein as a result of thermal shock is a natural phenomenon by which cell protects it-self. Stress at higher temperature causes aggregation of heat shock proteins with different polypeptides for their structural refolding trapped in the aggregates (Liberek et al. , 2008). Among various family members, inducible heat shock protein 70 (Hsp70) plays the vital role in the structural refolding process by actively participating with other chaperones (Kalmar and Greensmith, 2009). Another constitutive family member, heat shock cognate 70 (Hsc70) protein is a sensitive biomarker against the various physiological and environmental assaults (Mukhopadhyay et al. , 2003). Both of these heat shock protein protect other cellular proteins from unfolding, or refold the denatured proteins, or drive them for proteasomal degradation (Torigoe et al. , 2009).
Melatonin, a pineal gland product has multidimensional functional capability in different physiological functions of organisms (Reiter, 1991; Hardeland and Fuhrberg, 1996). Melatonin plays a significant role in circadian rhythm (Berra and Rizzo, 2009), and influences the cardio vascular system (Paulis et al., 2012; Lochner et al., 2013) and immune system activity (Maestroni, 1993; Guerrero and Reiter, 2002). It also works as an anti-stress hormone in the physiological system (Maestroni and Conti, 1991a; Maestroni and Conti, 1991b, Brotto et al., 2001). Melatonin performs its functions either non-receptor mediated or receptor mediated action mechanisms. Melatonin is also recognized as an anti-stress hormone, due to its ability of scavenging the free radicals generated from stress conditions (Reiter et al., 2001; Tan et al., 2003; Berra and Rizzo, 2009; Garcia et al., 2014). Melatonin by involving MT1 and MT2 receptors also mediates its functions (previously identified as Mel1a and Mel1b) (Dubocovich, 1995; Reppert, 1997; Browning et al., 2000). The involvement of melatonin in thermoregulation process after heat stress is well reported (Dawson et al., 1996; McLellan et al., 1999; Aoki et al., 2006). Melatonin also works to improve the negative impacts of heat stress (Gharib et al , 2008).
The expression of melatonin receptors and heat shock proteins in various tissues of the physiological system were documented in different studies in various vertebrate groups (Stolte et al. , 2009; Ozacmak et al. , 2009; Dang et al. , 2010; Lollo et al. , 2013). But, lacking of information remains in mammals which can delineate the immune system interactions to cope up against the stress condition generated effects. The primary lymphoid organ thymus remains most active in newborns, but along with the increase of adulthood it becomes sedentary, whereas the secondary lymphoid organs like: spleen, tonsils, lymph nodes, peyer’s patches etc. works throughout life cycle. Therefore, the responsibility of encountering pathogens and antigens mainly relies on the secondary immune organs of the body. We considered spleen for our study because of its role as a main secondary lymphoid immune organ, which covers the maximum percentage of immune activity throughout the entire life cycle in mammals. Although different reports suggested that heat stress causes the rise of melatonin secretion (Abbas et al. , 2007; Sejian et al. , 2008), but till date, heat stress generated change of melatonin receptors expression pattern was not evaluated. Therefore, we evaluated the changes of expression pattern of melatonin receptors after the hyperthermic stress condition along with the increase of time and temperature within the splenic tissue of adult mice.
The present study was focused to observe the changes of melatonin receptors (MT1 and MT2) expression pattern along with heat shock proteins
(Hsp70 and Hsc70). Heat shock protein expression is considered as a sensitive biomarker for determining or evaluating the stress conditions when in vivo heat stress was provided to mice groups. So, heat shock protein expression (Hsp70 and Hsc70) along with the melatonin receptors (MT1 and MT2) were investigated in the mice groups exposed to different temperature gradients. Secondly, after hyperthermic stress along with the increase of time, how heat shock protein expression level changes along with melatonin receptors is also an important query which should be understood for delineating the recovery process of spleen cells from the adverse effects of heat stress.
MATERIALS AND METHODS
Animal Procurement and Maintenance:
Healthy male laboratory Swiss Albino mice were randomly selected from mice colony. Healthy mice colony was housed at ambient laboratory conditions (under 12L: 12D cycles and 250C-270C temperature). Mice were kept in groups of five in polycarbonate cages (43cm x 27cm x 14 cm) to avoid the population stress. Mice were fed regularly with mice feed and water ad libitum.
All the experiments on the animals were conducted in accordance with institutional practice and within the framework of the revised Animal (Specific Procedure) Act of 2007 of Govt. of India on animal welfare. The study was approved by institutional animal ethics committee (IAEC) with ethical clearance no. TU/IAEC/2014/IX/2-3.
Experimental Design:
The experimental design was divided into two subsets. Five mice were kept in each group of each experimental subset. Experimental design of the two subsets are interrelated or interlinked. The first subset consists of 6 mice groups exposed to thermal stress at 430C temperature for 45 minutes in an isolated thermal chamber with proper ventilation. Then the mice groups subjected to heat stress were sacrificed at 1hour intervals after heat exposure. First group sacrificed after 1hour (hr) and then subsequently remaining groups at 2hr, 3hr, 4hr, 5hr and 6hr of heat exposure, respectively. In the second experimental subset, we divided the mice into three groups subjected to different temperatures. In this subset, the first mice group was treated as control group (Con) without any kind of stress and second and third mice group was exposed to thermal or heat stress (Heat) at 410C and 430C temperature for 45 minutes in the same manner as described earlier. The mice groups subjected to heat stress in the second experimental subset were sacrificed after 5 hours of heat exposures, which we observed as the correct time of sacrifice from the result of first experimental subset. It was also noted that higher mortality was there in the mice group which were exposed to temperature above 430C, whereas at 410C and 430C temperature no mortality was noted during the thermal exposure period and post stress period. Therefore, only 410C and 430C temperature were considered for the study of thermal stress in the concerned mice strain. All experimental groups were sacrificed after anaesthesia and the spleen was dissected out immediately on ice. Spleen of each mouse of each group was divided and immediately stored at -400C for western blot analysis and the second part was fixed in aqueous Bouin’s fluid for immunohistochemical studies. A third part of spleen from the second experimental subset was also immediately processed for RNA isolation and subjected to reverse-transcriptase-PCR for analysing the AANAT gene expression level.
Western Blot Analysis:
Western blot analysis was performed to assess the expression of Hsp70, Hsc70 proteins and melatonin receptors MT1, MT2 in the spleen of experimental mice. Spleen tissues were homogenized and lysed in RIPA buffer [1% (v/v) NP-40, 0.1% w/v sodium dodecyl sulphate (SDS) in PBS containing aprotinin, sodium orthovanadate and phenylmethylsulphonylfluoride (PMSF)] and then quantified by Lowry method (1951). Aliquots containing 100 ^g proteins were resolved by 10% (w/v) SDS polyacrylamide gel electrophoresis followed by electro transfer to nitrocellulose membrane (Santa Cruz Biotech, USA). Immune detection was carried out by using anti-Hsp70, anti-Hsc70, anti-Mel 1AR, anti-Mel 1BR [Hsp70; ab79852 and Hsc70; ab 1427, rabbit polyclonal, Abcam, USA; Mel1AR (MT1); sc-13186 and Mel1BR (MT2); sc-13177, goat polyclonal, Santacruz Biotech, USA, diluted 1:200] and ^ -actin antibody (sc-130656, rabbit polyclonal Santacruz Biotech, USA, diluted 1:500) diluted in PBS contained 5% skimmed milk and 0.01% Tween-20 followed by incubation with horseradish peroxidase conjugated secondary antibodies (goat anti-rabbit IgG for Hsp70, Hsc70 and ^ -actin antisera; diluted 1:1000 and rabbit anti-goat IgG for Mel1AR and Mel1BR antisera; diluted 1:1000). The immune interactions were detected by using Super Signal West Pico Chemiluminescent Substrate (#34080, Thermo Scientific, Rockford, USA). Bands were quantified by measurement of optical density using Scion Image Analysis Software (Scion Corporation, MD, and USA). Values were expressed as ratio of the density of the specific signal to ^ -actin signal and expressed as the % control value (Treeck et al., 2006). Each sample corresponds to tissue from a single animal and at least five gels corresponding to each subunit and experimental conditions were analysed.
Immunohistochemistry:
Immunohistochemical studies of experimental tissues (spleen) were done following the procedure adopted by Savaskan et al. , (2002). Paraffin sections (6 µm) fixed on 1% gelatine coated slides were deparaffinised and rehydrated with alcohol grades. The sections were placed in PBS for 30 minutes and endogenous peroxide activity was blocked by 0.3% H 2 O 2 in methanol for 30 minutes at room temperature (250C). Sections were washed thrice with phosphate buffered saline (PBS: 0.1M Na 2 HPO 4 , NaH 2 PO 4 , 0.9% NaCl, pH=7.4) and were placed in blocking solution (horse blocking serum, diluted 1:200 in PBS, PK -6200, Vector Laboratories, Burlingame, CA). Sections were incubated with primary antibodies [Hsp70; ab79852 and Hsc70; ab1427, rabbit polyclonal, Abcam, USA; Mel1AR (MT1); sc13186 and Mel1BR (MT2); sc13177, goat polyclonal, Santacruz Biotech, USA, diluted 1:200] overnight at 40C. Next day, sections were washed thrice with PBS and incubated with biotinylated secondary antibody (Vectastain ABC Universal Kit, PK-6200, Vector Laboratories, Burlingame, CA, dilution 1:1000). Same sections were again washed thrice with PBS and incubated with preformed AB (Avidin-Biotin) reagent for 30 minutes. The antigens were visualized using the
0.03% peroxidase substrate 3,3ˈ-diaminobenzidine (DAB; Sigma-Aldrich Chemicals, St. Louis, USA) in 0.01M Tris-Cl (pH=7.6) and counterstained with Ehrlich’s haematoxylin. The sections were dehydrated and mounted with DPX. Microphotographs of the stained sections were taken under 40X objective of Leica microscope DM4000. To test the specificity of the used antibodies, the primary antibodies were not added in control sections which were treated as negative control and incubated with same dilution of normal serum for overnight at 40C. Next morning the immunohistochemical protocol was followed under the same conditions. The immunohistochemical studies were interpreted by following the semiquantitative analysis method described by Rezzani et al. (2005).
Reverse-Transcriptase-PCR of AANAT (rate limiting enzyme of melatonin biosynthesis):
Total RNA isolation:
Expression of AANAT gene was studied by semi-quantitative PCR using S-1000 thermal cycler (Biorad, USA). Total RNA was isolated from fresh spleen tissue using Pure OLTM (Biorad, USA) following the manufacturer instruction. The isolated RNA was quantitated by spectrophotometry and its integrity was verified on 1% formaldehyde agarose gel electrophoresis containing 2.2M formaldehyde, 8mM sodium acetate, 1mM EDTA and 20 mM MOPS.
Reverse Transcription Reaction:
Reverse transcription reaction was done for cDNA synthesis of RNA sample using iScriptTM cDNA synthesis kit (Biorad, USA) according to the manufacturer instruction. 1 µg total RNA was used for 20 µl reaction mixture and incubated at 25 °C for 5 min. Mixture was then incubated at 42 °C for 30 min and then incubated at 85 °C for 5 min. After chilling on ice the cDNA was subjected for PCR.
Polymerase Chain Reaction:
PCR was done using GoTaq® Green Master Mix (Promega, USA) and specific oligonucleotide primer as per manufacturer instruction. The primers used for AANAT PCR were 5’-CCTTGCAGTCAGGAGTCTCA-3’ (forward) and 5’-AACTCTGAGGTCCCAAGTGG-3’ (reverse) give a 211bp PCR product. GAPDH amplification was done for control expression. The primers used for GAPDH PCR were 5’-AACTTTGGCATTGTGGAAGG-3’ (forward) and 5’-ACACATTGGGGGTAGGAACA-3’ (reverse) give a 223bp PCR product. All the mice primers used were synthesized by Imperial Life Science, India. 5µl of RT product amplified in total mixture volume of 25µl with 0.4µM of each primer and 12.5µl of 2X GoTaq Green Master Mix. The samples were denatured at 94 °C for 2 min and were further amplified as following: (for both AANAT and GAPDH 35 cycles were performed, 94°C 15 sec, 60°C 30 sec and 72°C 40 sec) with a final extension of 10 min at 72°C. The size of amplification product on the gel was assessed by comparison with 100 bp DNA ladder (StepUpTM 100 bp DNA ladder, MBD, 13J, Merck-Millipore, Bangalore, India). All PCR experiments included negative control, in which template cDNA was omitted. PCR products were electrophoresed on ethidium bromide containing 2% agarose gel. Bands were visualized in a UV-transilluminator and photographed (MiniLumi, DNR Bioimaging System, Israel). The AANAT relative amount was expressed in terms of optical density relative to GAPDH.
Statistical Analysis:
Statistical analysis of the data was performed using SPSS 17.0 (SPSS Corp., USA) programme with one way ANOVA followed by Tukey’s multiple range tests for multiple comparisons. The differences were considered significant when p<0.05.
RESULTS
Western blot Analysis:
We performed western blot analysis to assess the expression of Hsp70, Hsc70 and melatonin receptor (MT1 and MT2) proteins in the immune organ, spleen of laboratory Swiss albino mice at the translational level. Hsp70 and Hsc70 proteins were detected as a single band corresponding to 70kDa, whereas melatonin MT1 and MT2 receptors proteins were detected as a single band in between 35–40 kDa, which precisely corresponded to the predicted molecular mass of the receptor (Ahmad and Haldar 2010).
Heat shock protein 70 (Hsp70, inducible) expression in spleen:
Increasing trend in expression pattern of Hsp70 protein was observed in experimental mice groups sacrificed at 1hr, 2hr, 3hr, 4hr, 5hr and 6hr after the heat exposure to 430C temperature (Fig.1). Hsp70 protein expression also increased significantly (P<0.01) at both 410C and 430C exposed mice groups in comparison with the control of the second experimental subset (Fig.2). The mice group exposed to 430C showed significant (P<0.01) higher expression of Hsp70 protein than the 410C group.
Heat shock cognate 70 (Hsc70, constitutive) expression in spleen:
Expression of Hsc70 protein was also showed similar trend of increase in the experimental mice groups sacrificed at 1hr, 2hr, 3hr, 4hr, 5hr and 6hr after the heat exposure at 430C temperature (Fig.3). Significant (P<0.01) increased expression of Hsc70 protein was also observed in 410C and 430C experimental groups compared to control of the second experimental subset (Fig.4). Significant (P<0.01) higher expression of Hsc70 was noted at 430C than the 410C exposed group of mice. It was noted that within 5hr and 6hr, the increasing expression pattern of both proteins (Hsp70 and Hsc70) was stabilized and maximum expression was noted in mice of 5 hr group of the first experimental subset.
Melatonin receptor MT1 expression in spleen:
MT1 receptor protein showed consecutive decrease in expression pattern in the 1hr, 2hr and 3hr mice groups and then again increased to 5hr group (Fig.5). Significant (P<0.01) decrease was also noted in MT1 protein expression at 410C and 430C temperature exposed mice groups in comparison with control group of the second experimental subset (Fig.6). The mice group exposed to 430C showed significant (P<0.01) decreased expression than the 410C exposed mice and control one.
Melatonin receptor MT2 expression in spleen:
In contrast to MT1 receptor protein expression, MT2 receptor protein showed increased expression pattern along with the increase in time after the heat exposure such as in 1hr, 2hr, 3hr, 4hr, 5hr and 6hr groups (Fig.7). MT2 receptor protein also significantly (P<0.01) increased in the mice groups exposed to 410C and 430C temperature in comparison with the control mice group of the second experimental subset (Fig.8). Mice groups exposed to 430C showed significant (P<0.01) higher expression than the 410C group. Interestingly, MT2 receptor protein showed similar pattern of change in the expression as it was noted in heat shock proteins (Hsp70 and Hsc70).
Immunohistochemistry:
We performed immunohistochemistry for observing and localizing Hsp70, Hsc70 protein and melatonin MT1 and MT2 receptors in the splenic tissue of the experimental mice groups. In negative control sections no reaction were detected. The enlarged view of microphotographs showed the specific binding of MT1 and MT2 receptors and extra and intra cellular Hsp70 and Hsc70 protein in the splenic tissue of the experimental groups. The time gap of the spleen tissue collection from the subject exposed to different temperatures was 5 hours and therefore, the immunohisochemical localization of 1 hour and 5 hour was provided here from the first experimental subset to understand the actual difference of proteins in the splenic tissue.
Heat shock proteins (Hsp70 and Hsc70):
Immunohistochemical study showed immunoreactivity of both Hsp70 and Hsc70 proteins in the splenic tissue of each group after the hyperthermic stress condition (430C). Increased immune reactivity of Hsp70 (Fig.9, B1) and Hsc70 (Fig.9, B2) was noted in the splenic tissue of 5 hour group of mice in comparison to 1 hour group of mice (Fig.9, A1, A2) sacrificed after the heat exposure. In a similar way, strong immunoreactivity of Hsp70 (Fig.10) and Hsc70 (Fig.11) was observed in the extra and intra cellular space of splenic tissue of the 430C exposed mice group compared to the 410C exposed and control mice group of second experimental subset.
Melatonin receptors (MT1 and MT2):
After hyperthermic stress at 430C, MT1 showed similar immunoreactivity in the splenic tissue of mice groups sacrificed at 1 hr (Fig.12, A1) and 5 hr (Fig.12, B1). But MT2 showed increased immunoreactivity in the splenic tissue of 5 hr mice group (Fig.12, B2) than the 1 hr group (Fig.12, A2). MT1 immunoreactivity was also stronger in the spleen of control mice than the 410C and 430C exposed mice groups (Fig.13) of the second experimental set. However, less MT1 immunoreactivity was noted in spleen of mice exposed to 430C than mice exposed to 410C. In contrary, MT2 showed stronger immunoreactivity in both 410C and 430C exposed mice groups than the control one of the second experimental set (Fig.14). However, 430C exposed mice group showed stronger MT2 immunoreactivity than the 410C exposed mice group.
Semiquantitative analysis of Immunohistochemistry:
The immunohistochemical studies were interpreted by following the semiquantitative analysis method described by Rezzani et al. (2005) with a petite modification. Careful photographic examination of the immunohistochemical figures showed no reactivity in the negative control sections, which was presented by ‘-’. In a similar way, the experimental groups which showed less immunoreactivity presented by ‘+’, medium immunoreactivity presented by ‘+ +’, high immunoreactivity presented by ‘+ + +’ within the splenic tissue of mice groups. The table no.1 depicts the semiquantitative analysis immunohistochemical figures of the various treatment groups stained with specific antibodies.
Reverse-Transcriptase-PCR of AANAT:
AANAT is the rate limiting enzyme of melatonin biosynthesis and the expression level of AANAT gene in melatonin synthesising cell directly reflects the melatonin biosynthesis process. The sets of enzyme required for melatonin biosynthesis are also present in lymphoid organs and immune cells. Melatonin from extra pineal sources shows localized effects. The reverse-transcriptase-PCR of AANAT mRNA of the spleen tissue of second experimental subset showed increase in expression pattern along with the rise of temperature of thermally stressed groups (Fig.15A). 430C exposed mice group showed significant (P<0.01) increased expression than the 410C exposed mice and control mice group (Fig.15C).
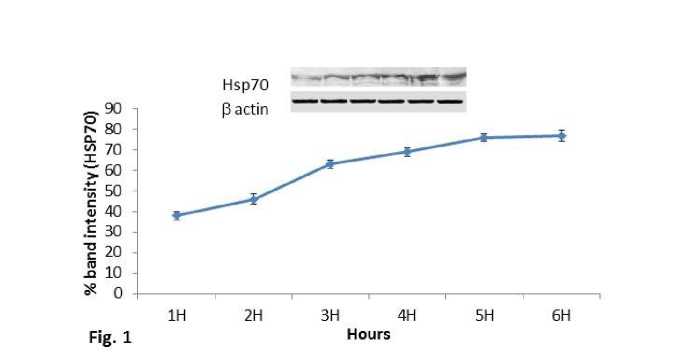
Figure 1. Line graph showing western blot analysis of inducible heat shock protein 70 (Hsp70). β-actin was used as loading control.
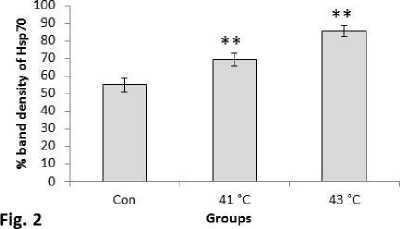
Figure 2. Western blot analysis of inducible heat shock protein 70 (Hsp70). β-actin was used as loading control. Lower panel shows % expression of protein following Scion Image analysis. Histogram represents Mean + SEM. The mean differences were considered significant when p< 0.05.
**p<0.01: Con vs. Group 410C; Con vs. Group 430C; Group 410C vs Group 430C
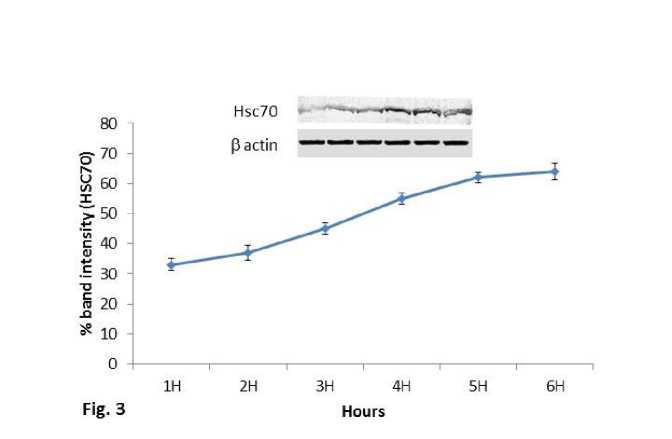
Figure 3. Line graph showing western blot analysis of heat shock cognate protein 70 (Hsc70). β-actin was used as loading control.
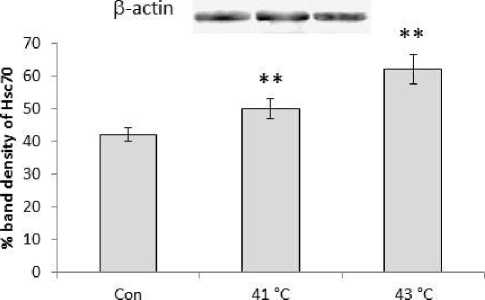
Figure 4. Western blot analysis of heat shock cognate protein 70 (Hsc70). β-actin was used as loading control. Lower panel shows % expression of protein following Scion Image analysis. Histogram represents Mean + SEM. The mean differences were considered significant when p< 0.05.
**p<0.01: Con vs. Group 410C; Con vs. Group 430C; Group 410C vs Group 430C
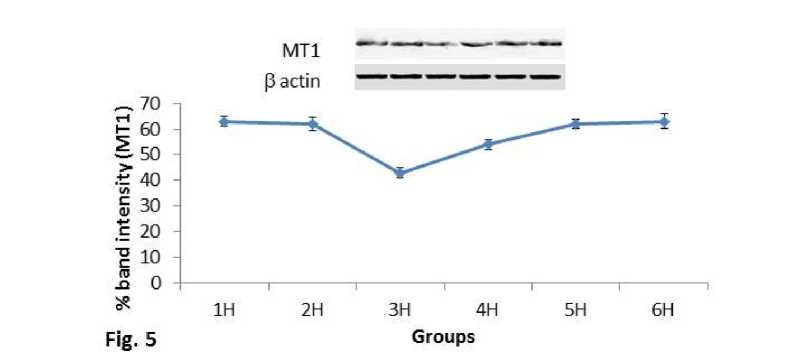
Figure 5. Line graph showing western blot analysis of MT1 melatonin receptor. β-actin was used as loading control.
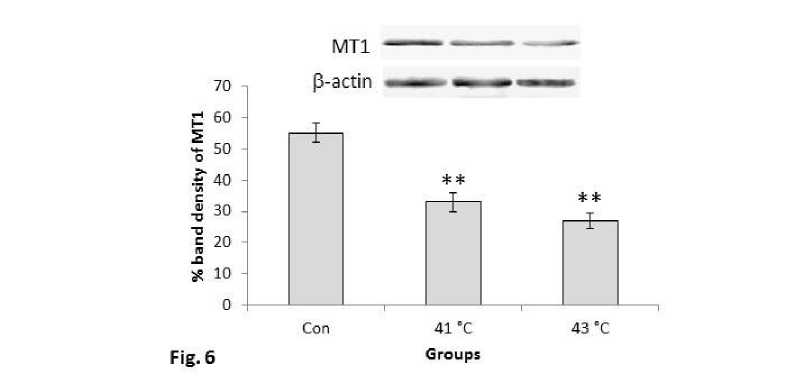
Figure 6. Western blots analysis of MT1 receptor proteins. β-actin was used as loading control. Lower panel shows % expression of protein following Scion Image analysis. Histogram represents Mean + SEM. The mean differences were considered significant when p< 0.05.
**p<0.01: Con vs. Group 410C; Con vs. Group 430C; Group 410C vs Group 430C
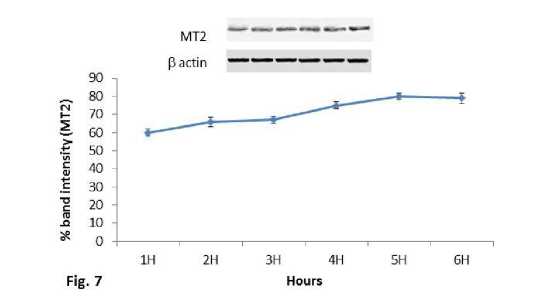
Figure 7. Line graph showing western blot analysis of MT2 melatonin receptor. β-actin was used as loading control.
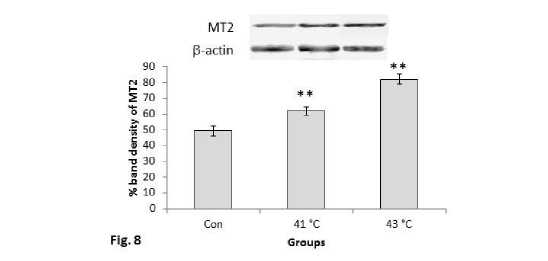
Figure 8. Western blots analysis of MT2 receptor proteins. β-actin was used as loading control. Lower panel shows % expression of protein following Scion Image analysis. Histogram represents Mean + SEM. The mean differences were considered significant when p< 0.05.
**p<0.01: Con vs. Group 410C; Con vs. Group 430C; Group 410C vs Group 430C
8$$
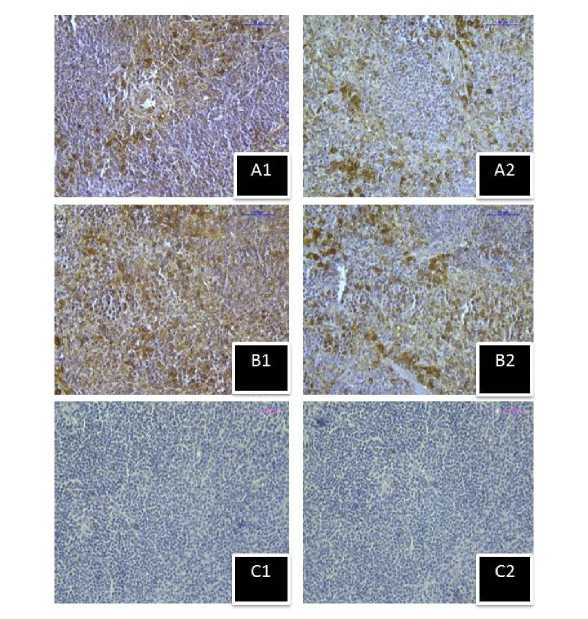
Figure 9. Immunostaining of inducible heat shock protein 70 (Hsp70) in spleen of stress exposed mice group sacrificed after (A1) 1hour, (B1 ) 5hour respectively, and (C1) negative control section. Immunostaining of heat shock cognate protein 70 (Hsc70) in spleen of stress exposed mice group sacrificed after (A2) 1hour, (B2) 5hour respectively, and (C2) negative control section.(Magnification bars=50µm).
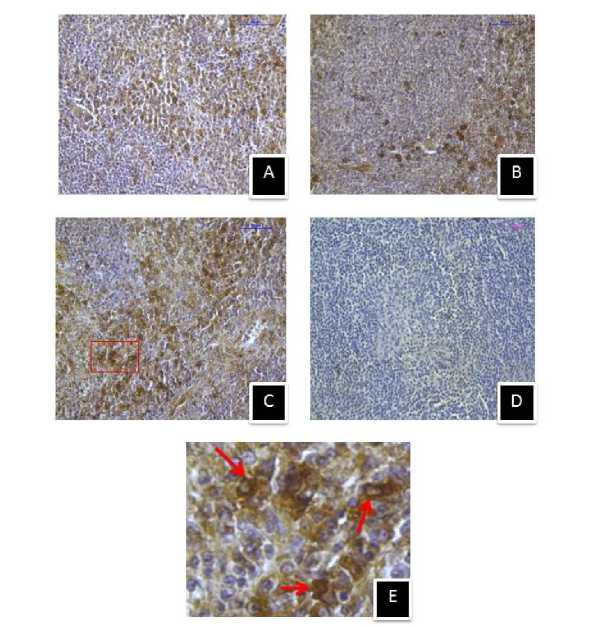
Figure 10. Immunostaining of inducible heat shock protein 70 (Hsp70) in spleen of (A) control, (B) 410C heat stressed, (C) 430C heat stressed mice, and (D) negative control section. (E) Enlarged view of selected area showing extracellular and intra cellular Hsp70 immunostaining. (Magnification bars=50µm).
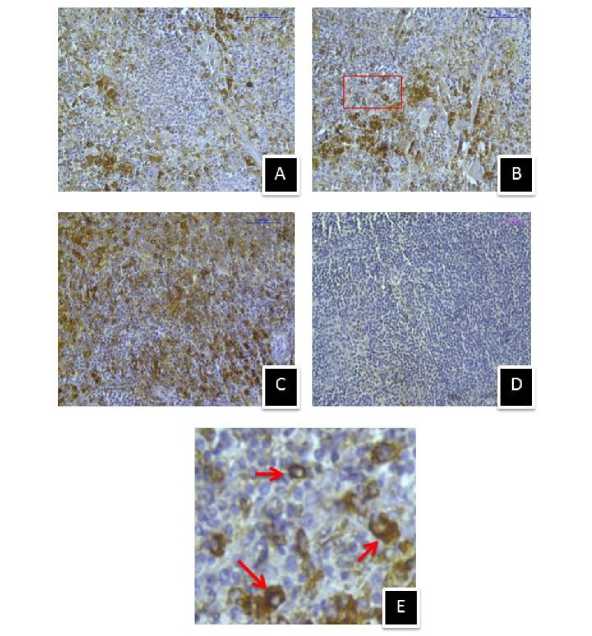
Figure 11. Immunostaining of heat shock cognate protein 70 (Hsc70) in spleen of (A) control, (B) 410C heat stressed, (C) 430C heat stressed mice, and (D) negative control section. (E) Enlarged view of selected area showing extracellular and intracellular Hsc70 immunostaining. (Magnification bars=50µm).
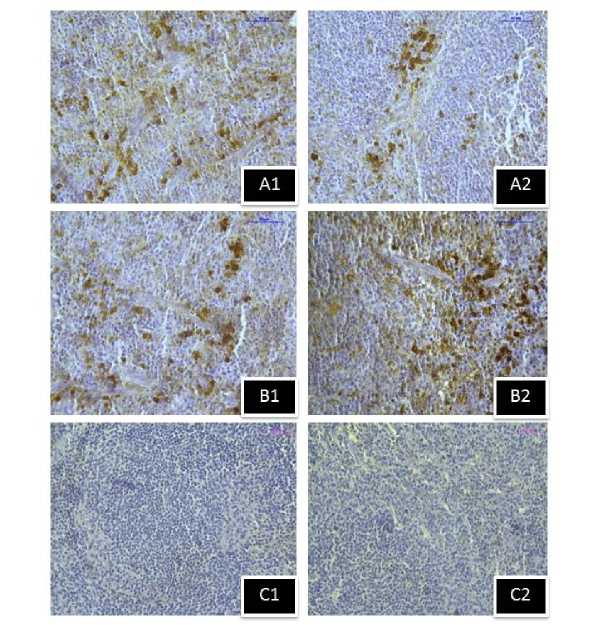
Figure 12. Immunostaining of MT1 melatonin receptor in spleen of stress exposed mice group sacrificed after (A1) 1hour, (B1) 5hour respectively, and (C1) negative control section. Immunostaining of MT2 melatonin receptor in spleen of stress exposed mice group sacrificed after (A2) 1hour, (B2) 5hour respectively, and (C2) negative control section.(Magnification bars=50µm).
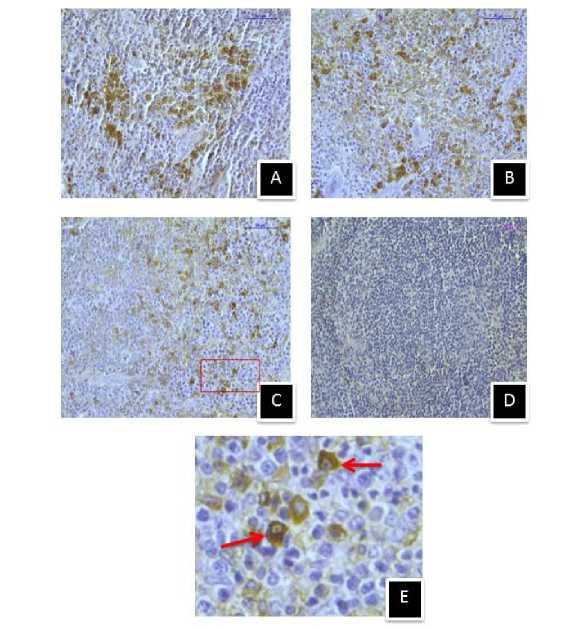
Figure 13. Immunostaining of MT1 melatonin receptor in spleen of (A) control, (B) 410C heat stressed, (C) 430C heat stressed mice, and (D) negative control section, (E) Enlarged view of selected area showing membrane specific MT1 immunostaining. (Magnification bars=50µm).
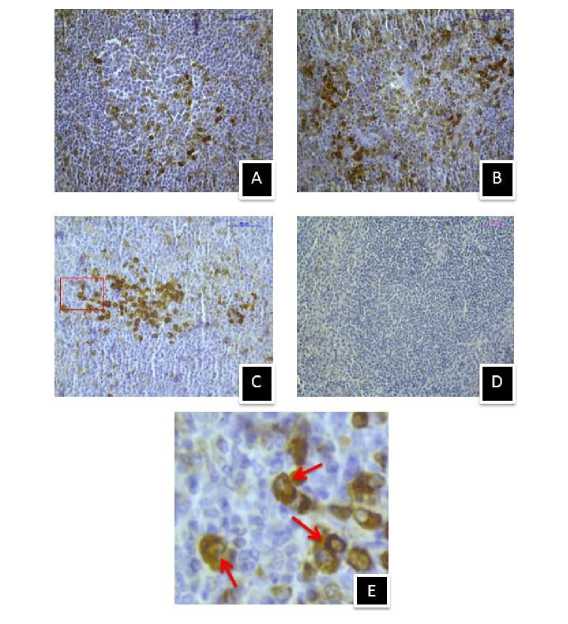
Figure 14. Immunostaining of MT2 melatonin receptor in spleen of (A) control, (B) 410C heat stressed, (C) 430C heat stressed mice, and (D) negative control section, (E) Enlarged view of selected area showing membrane specific MT2 immunostaining. (Magnification bars=50µm).
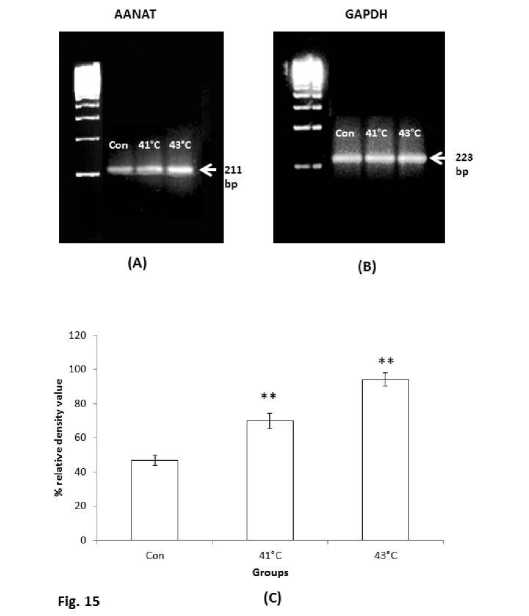
Figure 15. Reverse-transcriptase-PCR of (A) AANAT mRNA and (B) GAPDH mRNA in the spleen of thermally stressed mice groups (410C and 430C temperature exposed mice groups). (C) Lower panel shows the histogram after the densitometry analysis. The values were expressed as Mean + SEM. The mean differences were considered significant when p< 0.05.
Table 1. Semi quantitative analysis of immunohistochemistry: ‘+’ denotes less immunoreactivity; ‘+ +’ denotes medium immunoreactivity ; ‘+ + +’ denotes high immunoreactivity of the splenic tissue by specific antibodies.
|
Temperature dependent study |
Time dependent study |
|||||
|
Treatment Groups |
Control group |
Thermal stress at 41°C |
Thermal stress at 43°C |
Mice sacrificed after 1 hour thermal exposure |
Mice sacrificed after 5 hour thermal exposure |
|
|
Antibody Immunoreactivity in immunohistochemistry |
Hsp70 immunoreactivity |
+ |
+ + |
+ + + |
+ |
+ + |
|
Hsc70 immunoreactivity |
+ |
+ + |
+ + + |
+ / + + |
+ + /+ + + |
|
|
MT1 immunoreactivity |
+ + + |
+ + |
+ |
+ / + + |
+ / + + |
|
|
MT2 immunoreactivity |
+ |
+ +/ + + + |
++/+++ |
+ |
+ + + |
|
DISCUSSION
Thermal stress is an important cue for the production of heat shock protein in relation to cellular protection. Therefore, heat shock protein expression is considered as a sensitive biomarker for identifying challenging conditions of environment (Mukhopadhyay et al., 2003). The thermal sensitivity and tolerance ability in the diverse animal groups could be assessed via the estimation of these heat shock protein expression level (Webster et al., 2013; Lencioni et al., 2013; Sorensen et al., 2013; Tutar et al., 2013; Sharma et al., 2013). Expression and characterization of these proteins were also reported in various vertebrate tissues like intestine, kidney, spleen, gut, gills, thymus and brain (Stolte et al., 2009; Ozacmak et al., 2009; Dang et al., 2010; Lollo et al., 2013). Further, the temperature dependent time delay matter was considered for improvement of accuracy in Arrhenius model of cell death assay (Pearce, 2015). Therefore, differential temperature dependent expression of heat shock protein (Hsp70/Hsc70) is an important aspect, because sometimes extreme hyperthermia might cause degradation of cellular proteins rather than refolding them into their native structure and conformation by the help of heat shock proteins assembly. Recently, a study on pond snail showed that the thermal stress increases Hsp70 and Hsp40 gene expression at transcriptional level in a time dependent manner. The same report also documented that after thermal stress the Hsp40 gene expression increases 40 folds and returns to control level within 8 hours of heat exposure, whereas Hsp70 gene expression rises to 100 folds and not returned to control level within 8 hours (Foster et al., 2015). Therefore, the time dependent expression of heat shock protein after hyperthermic stress might be different in different animal groups and it is also an important query which should be understood in the studied mice strain, which is considered as an important model in the biological research. Therefore, temperature and time dependent expression pattern of heat shock proteins along with melatonin receptors in the concerned experimental mice strain was investigated in the present study. Our immunohistochemical studies showed strong extra and intra cellular Hsp70 and Hsc70 immunoreactivities in the spleen of 410C and 430C heat stressed mice as well as mice group sacrificed after 5 hours of heat exposure. Earlier reports suggested that cytosolic Hsp70 associates with the antigenic peptide and mediates their translocation and processing (Ishii et al., 1999); whereas extra cellular Hsp70 stimulates dendritic cells through TLR-4 (Chen et al., 2009; Asea et al., 2002). Our western blot analysis showed increased expression of Hsp70 and Hsc70 along with the increase in temperature as well as increase in time after hyperthermic stress in the splenic tissue of experimental groups sacrificed after 1hr, 2hr, 3hr, 4hr, 5hr and 6hr respectively. Semi-quantitative analysis of immunohistochemistry within the splenic tissue of the concerned groups also supported the expression pattern observed during western blot analysis. Lovell and his coworkers (2007) suggested temperature dependent Hsp70 response during in vitro heat shock treatment on peripheral blood mononuclear cells. Another report showed increase in body temperature during exercise induces increased expression of Hsp70 in immune cells (Fehrenbach and Northoff, 2001). The time dependent increasing trend in the expression pattern of Hsp70 and Hsc70 was maximized and became in plateu after 5hr. Similar kind of report was suggested by Kiang and Tsokos (1998) where maximum heat shock proteins expression was noticed within 3-5 hours of thermal exposure. The study of Lovell et al., (2007) also showed that during in vitro heat shock treatment on peripheral blood mononuclear cells the time profile of Hsp70 response shows temperature dependent manner, which increases up-to four hour and then again returned to baseline by the sixth hour of post heat shock treatment. Our study showed that in vivo heat shock treatment to mice also shows temperature dependent and time dependent increase in expression of the Hsp70 and Hsc70 response in the splenic tissue of mice. In the study, it was also observed that 430C temperature was optimum for this mice strain for induction of thermal stress, as above this temperature the mortality of the subject was increased. It could be also resolved from the study that the time gap of 5 hour between the heat stress and animal sacrifice was optimum for expression of heat shock protein. Increased levels of both Hsp70 and Hsc70 in the present study indicate the involvement of heat shock proteins in thermal acclimation process.
Several studies showed that melatonin is involved in nocturnal thermoregulation (Aschoff, 1983; Smolander et al., 1993; Krauchi et al., 1997, 2000). Reports also suggested that melatonin ingestion causes fall in internal temperature (Cagnacci et al., 1998; Gilbert et al., 1999; Harris et al., 2001). Day time exogenous melatonin administration also reduces internal core temperature both under control as well as heat stressed environment (Aoki et al., 2006). Melatonin mediates most of its activities through membrane receptors MT1 and MT2 in mammals ( lotos et al., 2014). In the present study, thermal stress caused a change in the expression pattern of heat shock protein along with melatonin receptors which indicate their possible involvement in thermoregulation process. The study showed temperature and time dependent decreased and increased pattern of expression of MT1 and MT2 receptors respectively. MT2 receptor is responding in all experimental conditions of temperature dependent as well as time dependent manner corresponding to changes of Hsp70 and Hsc70 expression. Increased AANAT gene expression along with the rise of temperature suggested the induction of melatonin synthesis in the spleen of thermally stressed mice. Earlier reports suggested that heat stress causes increase of melatonin secretion (Abbas et al., 2007; Sejian et al., 2008) and the circulatory increase of melatonin favours the increase in AANAT activity in lymphoid tissues (Gupta et al., 2015). Our study intensely suggested that heat stress generated such melatonin, as a signal molecule might be upregulating the activity of the stress responsive genes in splenic tissue which ultimately results the increase of Hsp70/Hsc70 proteins, whereas the simultaneous increased expression of MT2 receptors shows its possible involvement during such mechanism. Reports on exogenous melatonin mediated upregulation of the gene expression of heat shock proteins like: Hsp60, Hsp70 and Hsp90 in PBMC of thermally stressed goat (Sharma et al., 2013), and in AR42J cells (Bonior et al., 2005) are present. Cabrera and his co-workers (2003) also suggested that melatonin through its MT2 receptors showed anti-apoptotic activity in the heat shocked HL-60 cells, where simultaneously induced expression of Hsp27 was observed. Earlier reports are also suggesting that melatonin regulates differentially its own receptors in different tissues and organs in mammals (Masana et al., 2003) and mediates most of its immunoenhancing activity through MT2 receptors present in immune organs (Guerrero and Reiter, 2002; Dubocovich and Markowska, 2005).
CONCLUSION
Inducible heat shock protein 70 (Hsp70) and heat shock cognate protein 70 (Hsc70) levels showed proportional increase with the rise of temperature as well as along with the increase in time after exposure to hyperthermic stress. The expression of these heat shock proteins were maximised at 5-6 hours of heat exposure and might protect the splenic cells from hostile effects of stress. In studied mice, 430C was optimum temperature for induction of thermal stress for conduction of such experiments. The increased expression of heat shock proteins in the splenic tissue after hyperthermic stress might be preparatory phenomenon of spleen cells for the protection against the adverse effects of stress. At the same time, melatonin MT1 and MT2 receptors are expressed differentially on the spleen cells in response to heat stress. MT1 receptors and MT2 receptors showed reduced and increased expression in the splenic tissue respectively along with the increase of temperature and increase of time after thermally stressed condition. MT2 receptor showed similar pattern of increased response along with heat shock protein (Hsp70 and Hsc70) might be responsible for acclimatization under hyperthermic stressed conditions. Further, increased AANAT gene expression suggested the heat induced synthesis of melatonin which might upregulated the heat response genes and resulted in the increased heat shock proteins expression. Moreover, possible extended efforts to evaluate the signalling mechanism involved behind such phenomenon, may bring new information in the melatonin mediated regulation process of stress responses.
ACKNOWLEDGEMENTS:
Financial support from State Biotech Hub, Tripura University funded by DBT, New Delhi, India are gratefully acknowledged. The authors are also thankful for the help from CSIR, New Delhi, India, Grant No. 37(1514)/11 EMR-II and UGC, New Delhi, India, Grant No. 39-652/2010 (SR).
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interest.
Список литературы In-vivo thermal stress induces melatonin receptors and heat shock proteins expression in the spleen of mice in a time and temperature dependent manner
- Abbas, O.A., Gehad A.E., Hendricks G.L., Gharib H.B.A., Mashaly M.M. (2007) The effect of lighting programme and melatonin on the alleviation of the negative impact of heat stress on the immune response in broiler chickens. Int. J. of Poultry Sci., 6, 651-660.
- Ahmad, R., Haldar, C. (2010) Photoperiodic regulation of MT1 and MT2 melatonin receptor expression in spleen and thymus of a tropical rodent Funambulus pennanti during reproductively active and inactive phases. Chronobiol. Int., 27, 446-462.
- Aoki, K., Stephens, D.P., Zhao, K., Kosiba, W.A., Johnson, J.M. (2006) Modification of cutaneous vasodilator response to heat stress by daytime exogenous melatonin administration. Am. J. Physiol. Regul. Integr. Comp. Physiol., 291, R619-R624.
- Aschoff, J. (1983) Circadian control of body temperature. J. Therm. Biol., 8, 143-147.
- Asea, A., Rehli, M., Kabingu, E., Boch, J.A., Bare, O., Auron, P.E., Stevenson, M.A., Calderwood, S.K. (2002) Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J. Biol. Chem., 277, 1502815034.
- Berra, B., Rizzo, A.M. (2009) Melatonin: circadian rhythm regulator, chronobiotic, antioxidant and beyond. Clin. Dermatol., 27, 202-209.
- Bonior, J., Jaworek, J., Konturek, S.J., Pawlik, W.W. (2005) Increase of heat shock protein gene expression by melatonin in AR42J cells. J Physiol Pharmacol., 56, 471-481.
- Brotto, L.A., Gorzalka, B.B., La Marre, A.K. (2001) Melatonin protects against the effects of chronic stress on sexual behaviour in male rats. Neuroreport., 12, 3465-3469.
- Browning, C., Beresford, I., Fraser, N., Giles, H. (2000) Pharmacological characterization of human recombinant melatonin mt1 and MT2 receptors. Br. J. Pharmacol., 129, 877-886.
- Cabrera, J., Quintana, J., Reiter, R.J., Loro, J., Cabrera, F., Estevez, F. (2003) Melatonin prevents apoptosis and enhances HSP27 mRNA expression induced by heat shock in HL-60 cells: possible involvement of the MT2 receptor. J. Pineal Res., 35, 231-238.
- Cagnacci, A., Arangino, S., Angiolucci, M., Maschio, E., Melis, G.B. (1998) Influences of melatonin administration on the circulation of women. Am. J. Physiol. Regul. Integr. Comp. Physiol., 274, R335-R338.
- Chen, T., Guo, J., Han, C., Yang, M., Cao, X. (2009) Heat shock protein 70, released from heat-stressed tumor cells, initiates antitumor immunity by inducing tumor cell chemokine production and activating dendritic cells via TLR4 pathway. J. Immunol., 182, 1449-1459.
- Dang, W., Hu, Y.H., Zhang, M., Sun, L. (2010) Identification and molecular analysis of a stress inducible Hsp70 from Sciaenops ocellatus. Fish Shellfish Immunol., 29, 600-607.
- Dawson, D., Gibbon, S., Singh, P. (1996) The hypothermic effect of melatonin on core body temperature: is better?_J. Pineal Res., 20, 192-197.
- Dubocovich, M.L. (1995) Melatonin receptors: are there multiple subtypes? Trends Pharmacol Sci, 16, 5056.
- Dubocovich, M.L., Markowska, M. (2005) Functional MT1 and MT2 melatonin receptors in mammals. Endocrine., 27,101-110.
- Fehrenbach, E., Northoff, H. (2001) Free radicals exercise apoptosis and heat shock proteins. Immunol Rev., 7, 66-89.
- Foster, N.L., Lukowiak, K., Henry, T.B. (2015) Time-related expression profiles for heat shock protein gene transcripts (HSP40, HSP70) in the central nervous system of Lymnaea stagnalis exposed to thermal stress. Commun. Intergr. Biol., Article ID 8:e1040954. Doi: 10.1080/19420889.2015.1040954.
- Gabai, V.L., Meriin, A.B., Mosser, D.D., Caron, A.W., Rits, S., Shifrin, V.I., Sherman, M.Y. (1997) Hsp70 prevents activation of stress kinases. A novel pathway of cellular thermotolerence. J. Biol. Chem., 272, 18033- 18037.
- Garcia, J.J., Lopez-Pingarron, L., Almeida-Souza, P., Tres, A., Escudero, P., Garcia- Gil, F.A., Tan, D.X., Reiter, R.J., Bernal-Perez, M. (2014) Protective effect of melatonin in reducing oxidative stress and in preserving the fluidity of biological memebranes: a review. J. Pineal Res., 56, 225-237.
- Gharib, H.B.A., Desoky, A.A., EI-Menawey, M.A., Abbas, A.O., Hendricks, G.L., Mashaly, M.M. (2008) The role of photoperiod and melatonin on alleviation of the negative impact of heat stress on broilers. Int. J. Poult. Sci., 7, 749-756.
- Gilbert, S.S., Vanden Heuvel, C.J., Dawson, D. (1999) Daytime melatonin and temazepam in young adult humans: equivalent effects on sleep latency and body temperature. J. Physiol., 514, 905-914.
- Guerrero, J.M., Reiter, R.J. (2002) Melatonin-immune system relationships. Curr. Top. Med. Chem., 2, 167-179.
- Gupta, S., Haldar, C., Ahmad, A. (2015) Photoperiodic regulation of nuclear melatonin receptor ROR ' in lymphoid organs of a tropical rodent Funambulus pennant: Role in seasonal oxidative stress. J. Photochem. Photobiol. B: Biology., 142, 141-153.
- Hardeland, R., Fuhrberg, B. (1996) Ubiquitous melatonin - presence and effects in unicells, plants and animals. Trends Comp. Biochem. Physiol., 2, 2545.
- Harris, A.S., Burgess, H.J., Dawson, D. (2001) The effect of day-time exogenous melatonin administration on cardiac autonomic activity. J. Pineal Res., 31, 199 -205.
- Ishii, T., Udono, H., Yamano, T., Ohta, H., Uenaka, A., Ono, T., Hizuta, A., Tanaka, N., Srivastava, P.K., Nakayama, E. (1999) Isolation of MHC class I-restricted tumor antigen peptide and its precursors associated with heat shock proteins hsp70, hsp90, and gp96. J. Immunol., 162, 1303-1309.
- Kalmar, B., Greensmith, L. (2009) Induction of heat shock proteins for protection against oxidative stress. Adv. Drug Deliv. Rev., 61, 310-318.
- Khan, V.R., Brown, I.R. (2002) The effect of hyperthermia on the induction of cell death in brain, testis, and thymus of the adult and developing rat. Cell Stress Chaperones., 7, 73-90.
- Kiang, J.G., Tsokos, G.C. (1998) Heat shock protein 70 KDa: molecular biology, biochemistry and physiology. Pharmacol Ther., 80, 183-201.
- Kondo, T., Matsuda, T., Tashima, M., Umehara, H., Domae, N., Yokoyama, K., Uchiyama, T., Okazaki, T. (2000) Suppression of heat shockprotein-70 by ceramide in heat shock-induced HL60 cell apoptosis. J. Biol. Chem., 275, 8872-8879.
- Krauchi, K., Cajochen, C., Werth, E., Wirz-Justice, A. (2000) Functional link between distal vasodilation and sleep-onset latency? Am. J. Physiol. Regul. Integr. Comp. Physiol., 278, R741-R748.
- Krauchi, K., Cajochen, C., Wirz-Justice, A. (1997) A relationship between heat loss and sleepiness: effects of postural change and melatonin administration. J. Appl. Physiol., 83, 134-139.
- Lencioni, V., Bernabo, P., Cesari, M., Rebecchi, L., Cesari, M. (2013) Thermal stress induces HSP70 proteins synthesis in larvae of the cold stream non-biting midge Diamesacinerella meigen. Arch. Insect Biochem. Physiol., 83, 1-14.
- Li, C., Lee, J., Ko, Y., Kim, J., Seo, J. (2000) Heats hock protein 70 inhibits apoptosis downstream of cytochome c release and upstream of caspase-3 activation. J. Biol. Chem., 275, 25665-25671.
- Liberek, K., Lewandowska, A., Zietkiewicz, S. (2008) Chaperones in control of protein disaggregation. EMBO J., 27, 328-335.
- Lochner, A., Huisamen, B., Nduhirabandi, F. (2013) Cardioprotective effect of melatonin against ischaemia/reperfusion damage. Front Biosci (Elite Ed)., 5, 305-315.
- Lollo, P.C., Moura, C.S., Morato, P.N., Amaya-Farfan, J. (2013) Differential response of heat shock proteins to uphill and downhill exercise in heart, skeletal muscle, lung and kidney tissues. J. Sports Sci. Med., 12, 461-466.
- Lovell, R., Madden, L., Carroll, S., Mc Naughton, L. (2007) The time-profile of the PBMC HSP70 response to in vitro heat shock appears temperature-dependent. Amino Acids., 33, 137144.
- Lowry, O.H., Rosebrough, N.J., Farr, A.L., Randall. R.J. (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem., 193, 265-275.
- Maestroni, G.J., Conti, A. (1991a) Immuno-derived opioids as mediators of the immune-enhancing and anti-stress action of melatonin. Acta Neurol (Napoli)., 13, 356-360.
- Maestroni, G.J., Conti, A. (1991b) Anti-stress role the melatonin-immuno-opioid network: evidence for a physiological mechanism involving T cell-derived, immunoreactive beta-endorphin and MET-enkephalin binding to thymic opioid receptors. Int. J. Neurosci., 61, 289-298.
- Maestroni, G.J. (1993) The immunoneuroendocrine role of melatonin. J. Pineal Res., 14, 1-10.
- Masana, M.I., Witt-Enderby, P.A., Dubocovich, M.L. (2003) Melatonin differentially modulates the expression and function of the hMT1 and hMT2 melatonin receptors upon prolonged withdrawal. Biochem. Pharmacol., 65, 731-739.
- McLellan, T.M., Gannon, G.A., Zamecnik, J., Gil, V., Brown, G.M. (1999) Low doses of melatonin and diurnal effects on thermoregulation and tolerance to uncompensable heat stress. J. Appl. Physiol (1985)., 87, 308-316.
- Mukhopadhyay, I., Nazir, A., Saxena, D.K., Chowdhuri, D.K. (2003) Heat shock response: hsp70 in environmental monitoring. J. Biochem. Mol. Toxicol., 17, 249-254.
- Ozacmak, V.H., Barut, F., Ozacmak, H.S. (2009) Melatonin provides neuroprotection by reducing oxidative stress and HSP70 expression during chronic cerebral hypoperfusion in ovariectomized rats. J. Pineal Res., 47, 156-163.
- Paulis, L., Simko, F., Laudon, M. (2012) Cardiovascular effects of melatonin receptor agonists. Expert Opin. Investig. Drugs., 21, 1661-1678.
- Pearce, J.A. (2015) Improving Accuracy in Arrhenius Models of Cell Death: Adding a Temperature-Depandent Time Delay. J. Biomech. Eng., Artcle ID 137. Doi: 10.1115/1.4031851.
- Reiter, R.J., Tan, D.X., Manchester, L.C., Qi, W. (2001) Biochemical reactivity of melatonin with reactive oxygen and nitrogen species: a review of the evidence. Cell Biochem. Biophys., 34, 247-256.
- Reiter, R.J. (1991) Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr. Rev., 12, 151-180.
- Reppert, S.M. (1997) Melatonin receptors: molecular biology of a new family of G-protein-coupled receptors. J. Biol. Rhythm., 12, 528-531.
- Rezzani, R., Buffoli, B., Rodella, L., Stacchiotti, A., Binachi, R. (2005) Protective role of melatonin in cyclosporine A- induced oxidative stress in rat liver. Internation. Immunopharmacol., 5, 1397-1405.
- Savaskan, E., Wirz-Justice, A., Olivieri, G., Pache, M., Krauchi, K., Brydon, L., Jockers, R., Muller-Spahn, F., Meyer, P. (2002) Distribution of melatonin MT1 receptor immunoreactivity in human retina. J. Histochem. Cytochem., 50, 519-526.
- Pineal-adrenal relationship: modulating effect of glucocorticoids on pineal function to ameliorate thermal-stress in goats. Asian-Aust. J. Animal. Sci., 21, 988-994.
- Sharma, S., Ramesh, K., Hyder, I., Uniyal, S., Yadav, V.P., Panda, R.P., Maurya, V.P., Singh, G., Kumar, P., Mitra, A., Sarkar, M. (2013) Effect of melatonin administration on thyroid hormones, cortisol and expression profile of heat shock proteins in goats (Capra hircus) exposed to heat stress. Small Ruminant Res., 112, 216-223.
- Identification of human erythrocyte cytosolic proteins associated with plasma membrane during thermal stress. J. Membr. Biol., 246, 591-607.
- Smolander, J., Harma, M., Lindqvist, A., Kolari, P., Laitinen, L.A. (1993) Circadian variation in peripheral blood flow in relation to core temperature at rest. Eur. J. Appl. Physiol., 67, 192-196.
- Sorensen, J.G., Loeschcke, V., Kristensen, T.N. (2013) Cellular damage as induced by high temperature is dependent on rate of temperature change - investigating consequences of ramping rates on molecular and organismal phenotypes in Drosophila melanogaster. J. Exp. Biol., 216, 809814.
- Stolte, E.H., Chadzinska, M., Przybylska, D., Flik, G., Savelkowl, H.F., Verburg-van Kemenade, B.M. (2009) The immune response differentially regulates Hsp70 and glucocorticoid receptor expression in vitro and in vivo in common carp (Cyprinus carpio L.). Fish Shellfish Immunol., 27, 916.
- Tan, D.X., Manchester, L.C., Hardeland, R., Lopez-Burillo, S., Mayo, J.C., Sainz, R.M., Reiter RJ. (2003) Melatonin: a hormone, a tissue factor, an autocoid, a paracoid and an antioxidant vitamin. J. Pineal Res., 34, 75-78.
- Torigoe, T., Tamura Y., Sato, N. (2009) Heat shock proteins and immunity: application of hyperthermia for immunemodulation. Int. J. Hyperthermia., 258, 610-616.
- Treeck, O., Haldar, C., Ortmann, O. (2006) Antiestrogens modulate MT1 melatonin receptor expression in breast and ovarian cancer cell lines. Onclo. Rep., 15, 231-235.
- Tutar, Y., Coskun, K. A., Tutar, L. (2013) Hsp70 from Cyprinion macrostomus macrostomus and Garrarufa obtuse: stability and stability dependent activity. Biochemistry (Mosc)., 78, 531-535.
- Webster, N., Pantile, R., Botte, E., Abdo, D., Andreakis, N., Whalan, S. (2013) A complex life cycle in a warming planet: gene expression in thermally stressed sponges. Mol. Ecol., 22, 1854-1868.
- Zlotos, D.P., Jockers, R., Cecon, E., Rivara, S., WittEnderby, P.A. (2014). MT1 and MT2 melatonin receptors: ligands, models, oligomers, and therapeutic potential. J. Med. Chem., 57, 31613185.
- Sejian, V., Srivastava, R.S., Varshney, V.P. (2008)
- Sharma, S., Zingde, S.M., Gokhale, S.M. (2013)


