Induction of oxidative stress by hydrogen peroxide treatment in rice genotypes to study the osmolyte accumulation pattern and antioxidant capacity
Автор: Vijayalakshmi D., Srividhya S., Muthulakshmi S., Satishraj R.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 3 т.10, 2014 года.
Бесплатный доступ
The aim of the study was to compare the rice genotypes for oxidative stress tolerance. Induction of oxidative stress, by in vivo treatment with hydrogen peroxide (H 2O 2) in rice genotypes to study the osmolyte accumulation pattern and antioxidant capacity was investigated. Leaf strips of uniform size from rice genotypes FL 478, IR 29,Co 43 and FR13A were subjected to various concentrations of H 2O 2 (0, 0.05, 0.1, 0.15 and 0.2 mM). All the four rice genotypes exhibited varied responses to proline accumulation. FL 478 and Co 43 exhibited an increase in the accumulation of proline contents initially with low concentrations of H 2O 2, and thereafter showed a sharp decline in proline contents with higher concentrations. Degradation of protein contents in rice leaves was observed in all the varieties and the protein contents decreased with increase in concentration of hydrogen peroxide treatment. A gradual increase in the activities of catalase and peroxidase were recorded under H 2O 2 treatments. Significant upregulation of antioxidant enzyme systems and slow degradation of protein contents in the tolerant genotypes (FR 13A and FL 478) play important roles in stress protection.
Catalase, peroxidase, proline, oxidative stress, rice, soluble protein
Короткий адрес: https://sciup.org/14323897
IDR: 14323897
Текст научной статьи Induction of oxidative stress by hydrogen peroxide treatment in rice genotypes to study the osmolyte accumulation pattern and antioxidant capacity
Stress often leads to the production of Reactive Oxygen Species (ROS) such as O 2 – and H 2 O 2 in plant tissues (Desikan et al ., 2004). H 2 O 2 is produced and accumulates, leading to oxidative stress in plants.
High salinity and submergence stress induces oxidative stress by accumulation of H2O2 (Hernandez et al., 2000; Gosset et al., 1996; Gomez et al., 1999; Savoure et al., 1999). Hydrogen peroxide (H2O2) is a versatile molecule that is involved in several cell processes under normal and stress conditions (Quan et al., 2008). H2O2 are highly reactive to membrane lipids, protein and DNA; they are believed to be the major contributing factors to stress injuries and to cause rapid cellular damage (Hariyadi and Parkin, 1993; O’Kane et al., 1996; Prasad, 1996).
Plants have evolved complex regulatory mechanisms in adapting to various environmental stresses. Recently, H 2 O 2 , in addition to being a toxicant, has been regarded as a signalling molecule (Hung et al., 2005). Therefore, the control of H 2 O 2 concentration is critical for cell homeostasis. Plants require biochemical and molecular strategies to survive the problem of salinity. Biochemical strategies used to enhance oxidative stress tolerance in plants include synthesis of osmotic regulators and induction of oxidative enzymes and certain hormones (Nakamura et al., 2002). Under physiological steadystate conditions, there is a balance between the production and scavenging of ROS (Skopelitis et al ., 2006). Enzymes, including superoxide dismutase (SOD), catalase (CAT), peroxidase (POX), ascorbate peroxidase (APX) and glutathione reductase (GR) (Zhang et al., 1995; Lee and Lee, 2000), and nonenzymatic antioxidants such as tocopherols, ascorbic acid (AsA), and glutathione (GSH) (Wingsle and Hallgren, 1993; Kocsy et al., 1996; Noctor et al., 1998) work in concert to detoxify ROS.
The other way by which plants adjust to any environmental stresses is by increasing their tissue osmotic potential. Thus the cells tend to accumulate osmoprotective compounds which increases the osmotic potential of the cell. Proline is the major compound that protects cells by stabilizing proteins and cellular membranes (Kumar et al., 2003;Martinez et al., 2003).
Rice genotypes tolerant/ susceptible to salinity and flooding stress show differential H 2 O 2 accumulation, physiological response and antioxidant activity (Lee et al ., 2001; Khan and Panda., 2008; Stanisavljevic et al., 2011; Blokhina et al., 2001). H 2 O 2 induced an increase in membrane permeability, chlorophyll damage, lipid peroxidation (Lin and Kao, 1998; Patra and Panda, 1998). However, how a plant perceives environmental changes and how it subsequently triggers signals to activate the physiological response are yet to be explored. Hence, the present study was aimed to study the effect of various H 2 O 2 concentrations on oxidative stress, osmolyte accumulation and antioxidant activity in a salt tolerant (FL 478); salt susceptible (IR29); flooding tolerant (FR 13A) and flooding susceptible (Co 43) rice genotypes.
MATERIALS AND METHODS
Rice genotypes (Oryza sativa L.) cvs. FL 478 (salt tolerant); IR29 (salt susceptible); FR 13A (flooding tolerant) and Co 43 (flooding susceptible) were planted in earthen pots (medium size) filled with 10 kg mixture of tank silt and farm yard manure in 5:1 ratio. Each pot was fertilized with N, P, K corresponding to 150, 50, 50 kg/ha, respectively. Three seedlings were maintained in each pot. A total of sixty pots were maintained with three pots for each treatment in a variety. Plants were watered regularly. Samples for various assays/estimations were taken on 30-35 days after sowing. Assays were performed in the first fully expanded leaves. Samples collected in ice bucket were washed with tap water and then with double distilled water. Leaf strips of uniform size were submerged in about 150 cm3 of various concentrations of H2O2 (0, 0.05, 0.1, 0.15 and 0.2 mM) in 0.1M potassium phosphate buffer, pH 7.5 contained in 250 cm3 beakers and incubated for 6 h in dark at 25o C. Samples incubated in phosphate buffer served as control. After incubation the samples were twice washed with double distilled water and soaked dry, and processed for various observations.
The soluble protein content of the leaves was determined by measuring the colour developed by the reduction of Folin-Ciocalteau reagent by the amino acids like tyrosine and tryptophan of protein, following the method of Lowry et al . (1951) and expressed in mg g-1 FW. Proline was estimated by selective extraction with three per cent aqueous sulphosalicylic acid after removing the interfering proteins. The chromophore developed, while reacting with acid ninhydrin, was estimated spectrophotometrically adopting the procedure of Bates et al . (1973) and expressed in mg g-1 FW.
Catalase activity was determined following the method of Luck (1974). One gram of the sample was macerated and extracted in 0.067 M phosphate buffer (pH 7.0). A known volume of the extract was added to the experimental cuvette containing three ml H2O2 – PO4 buffer. The time taken for per cent change in absorbance (Δt) at 240 nm was recorded for calculating the enzyme activity and expressed as enzyme units g-1 tissue. All the operations were carried out at 0 – 5oC. Peroxidase activity was determined by adopting the method of Malik and Singh (1980). One gram of leaf was macerated and extracted in 0.1 M phosphate buffer (pH 7.0). A known volume of the extract was added to an experimental cuvette containing three ml phosphate buffer and 0.05 ml guaiacol reagent and then 0.03 ml of H2O2 solution was added rapidly and the increase in absorbance at 436 nm was recorded. This Δt in minutes was used to calculate the enzyme activity. The enzyme activity was expressed as enzyme units per litre. All the operations were carried out at 0 – 5oC.
RESULTS
Four rice genotypes differing in their tolerance behavior to salinity (FL 478 and IR 29) and submergence (FR 13A and Co 43) were subjected to oxidative stress by exposing them to various H2O2 concentrations to study the osmolyte accumulation pattern, protein degradation and antioxidant activity. The results revealed that the genotypes FL 478 and Co 43 exhibited an increase in the accumulation of proline contents with 0.5 mM H2O2 and 1 mM H2O2, and thereafter showed a sharp decline in proline contents when exposed to 0.15mM H2O2 and 0.20mM H2O2. The genotypes IR 29 and FR 13A showed a decline in proline contents with exposure to increasing concentrations of H2O2 (Table 1). Among the genotypes taken for the study, FR 13A recorded a higher content of proline (1000 µg/g) under control conditions and was also able to maintain a higher proline content (840 µg/g) compared to other genotypes even after exposure of 0.20mM H2O2. Statistically significant changes were observed in the accumulation of proline contents within the genotypes and treatments.
With respect to the soluble protein contents, hydrogen peroxide treatment showed pronounced and adverse effects on soluble protein contents irrespective of the varieties taken for the study. Hydrogen peroxide treatment resulted in decrease in protein contents and the decrease increased with increase in concentration of hydrogen peroxide treatment (Fig.1). In genotypes FL 478 and FR 13A the protein content gradual decreased with H 2 O 2 treatments (FL 478: 20.15 to 16.12 mg/g; FR 13A: 20.15 to 16.53 mg/g) while in varieties IR 29 and Co 43 the protein contents gradually decreased upto 0. 1mM H 2 O 2 and thereafter there was a steep decline in the protein contents at
0.15mM and 0.2mM H 2 O 2 treatments.
The antioxidant activity was studied by observing the ROS scavenging enzymes namely catalase and peroxidase. Activities of catalase and peroxidase showed increasing trends with increasing H 2 O 2 treatments in all the varieties. FR 13A manifested higher activity of catalase and peroxidase than the other genotypes at all concentrations of H 2 O 2 treatments. In this genotype the catalase activity ranged from 0.455 µmoles H 2 O 2 /g/s under control to 1.526 µmoles H 2 O 2 /g/s at 0.2mM H 2 O 2 treatments (Table 2). Similar pattern was also observed with peroxidase activity where FR 13A recorded 110 enzyme units/g under control and increased upto 192 enzyme units/g at 0.2mM H 2 O 2 treatments (Table 3).
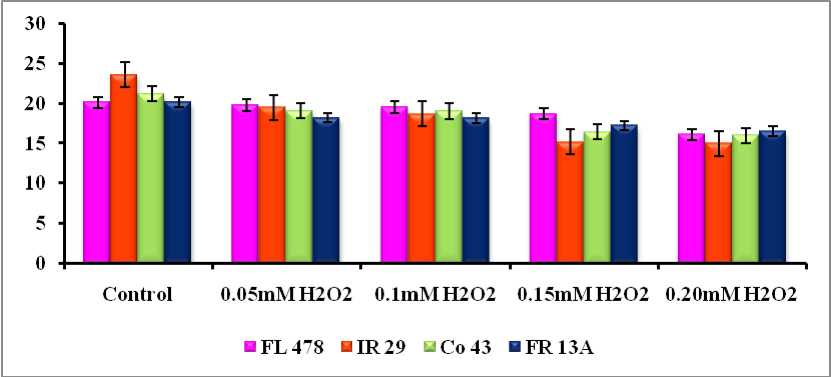
Figure 1 . Effect of Hydrogen peroxide treatment on the soluble protein contents (mg/g) in rice genotypes
Table 1 Effect of Hydrogen peroxide treatment on the proline contents (µg/g) in rice genotypes
|
Treatments |
Rice genotypes |
|||
|
FL 478 |
IR 29 |
Co 43 |
FR 13A |
|
|
Control |
955±18.75 |
892±17.51 |
908±17.82 |
1000±19.63 |
|
0.05mM H 2 O 2 |
986±19.36 |
875±17.18 |
926±18.18 |
978±19.20 |
|
0.1mM H 2 O 2 |
942±18.49 |
855±16.78 |
922±18.10 |
972±19.08 |
|
0.15mM H 2 O 2 |
915±17.96 |
842±16.53 |
911±17.88 |
940±18.45 |
|
0.20mM H 2 O 2 |
802±15.74 |
692±13.58 |
799±15.68 |
840±16.49 |
|
SEd. |
25.75 |
|||
|
CD (P=0.05) |
52.036* |
|||
Table 2 Effect of Hydrogen peroxide treatment on the Catalase activity (µmoles H 2O2/g/s) in rice genotypes
|
Treatments |
Rice genotypes |
|||
|
FL 478 |
IR 29 |
Co 43 |
FR 13A |
|
|
Control |
0.422±0.01 |
0.399±0.01 |
0.402±0.01 |
0.455±0.01 |
|
0.05mM H 2 O 2 |
0.578±0.01 |
0.485±0.01 |
0.499±0.01 |
0.502±0.01 |
|
0.1mM H 2 O 2 |
0.644±0.01 |
0.594±0.01 |
0.591±0.01 |
0.679±0.01 |
|
0.15mM H 2 O 2 |
0.754±0.01 |
0.667±0.01 |
0.753±0.01 |
0.825±0.02 |
|
0.20mM H 2 O 2 |
1.322±0.03 |
0.699±0.01 |
0.984±0.02 |
1.526±0.03 |
|
SEd. |
0.021 |
|||
|
CD (P=0.01) |
0.056** |
|||
Table 3 Effect of Hydrogen peroxide treatment on the Peroxidase activity (units/g) in rice genotypes
|
Treatments |
Rice genotypes |
|||
|
FL 478 |
IR 29 |
Co 43 |
FR 13A |
|
|
Control |
80±1.57 |
98±1.92 |
85±1.67 |
110±2.16 |
|
0.05mM H 2 O 2 |
92±1.81 |
104±2.04 |
112±2.20 |
125±2.45 |
|
0.1mM H 2 O 2 |
130±2.55 |
122±2.39 |
135±2.65 |
140±2.75 |
|
0.15mM H 2 O 2 |
154±3.02 |
136±2.67 |
142±2.79 |
174±3.42 |
|
0.20mM H 2 O 2 |
175±3.44 |
140±2.75 |
160±3.14 |
192±3.77 |
|
SEd. |
202.88 |
|||
|
CD (P=0.01) |
410.04 ns |
|||
DISCUSSION
Hydrogen peroxide is a potent cytotoxic compound produced during salinity, drought, high and low temperature stresses (Sairam and Srivastava, 2000). In order to understand the physiological mechanisms underlying salinity and flooding stress tolerance in rice, genotypes exhibiting contrasting tolerance behaviour to these stresses were taken for the study. The study clearly indicated that the genotypes varied significantly in the proline accumulation pattern and there was no uniform trends observed with the proline accumulation. The slight increase in proline, an osmoprotectant in H 2 O 2 (0.5 and 1.0 mM) in FL 478 and Co 43 may be attributed to the free radical scavenging function of proline as reported (Smirnoff, 1993; Sairam and Srivastava, 2000; Matysik, 2002).
Enhanced accumulation of proline in FL 478 might be the basis for its salinity tolerance. These findings are in line with Yazici et al., (2007) who has reported improved salt tolerance of Portulaca oleracea L.with proline accumulation. Proline accumulation and stress tolerance correlation have been reported in different studies, and it has been observed that proline concentrations are higher in stress-tolerant plants than in stress-sensitive plants (Misra and Gupta, 2005). Although a positive correlation between abiotic stress tolerance and free proline accumulation has been reported (Martinez et al., 2003), a negative correlation between proline accumulation and submergence tolerant line (FR 13A) was observed in the present study. FR 13A showed a decline in proline contents with exposure to increasing concentrations of H 2 O 2 .
It is known that water, salt, metal toxicity and other stress factors induced endogenous H 2 O 2 accumulation (Upadhyay et al., 2007). H 2 O 2 treatment of primary rice leaves induced an increase in chlorophyll, carotenoid and protein degradation in senescing leaves as observed also for other abiotic stresses (Sairam et al., 1997; Panda et al., 2002). In this study also it was found that in all the varieties taken for the study, hydrogen peroxide treatment resulted in decrease in protein contents and the decrease increased with increase in concentration of hydrogen peroxide treatment. In the stress tolerant lines (FR 13A and FL 478) the protein content gradually decreased, while in the other varieties (Co 43 and IR 29) there was a steep degradation of protein contents at 0.15mM and 0.2mM H 2 O 2 treatments. The protein degradation in senescing leaves may be due to a cytotoxic effect of H 2 O 2 (Mukherhee and Choudhuri, 1983; Menconi et al., 1995; Khan and Panda, 2002).
POX, CAT, SOD are the three major antioxidant enzymes responsible for scavenging, the reactive oxygen species generated via different mechanisms in plant cells (Winston, 1990). This formed the basis to study the activities of two major antioxidant enzymes POX and CAT. Activities of catalase and peroxidase showed increasing trends with increasing H2O2 treatments in all the varieties. The increased CAT and POX activities point to a signalling role of H2O2 in the induction of H2O2 synthesis detoxifying enzymes in rice leaves, as reported for other abiotic stresses (Guo et al., 1997; Sairam and Srisvastava, 2000; Lee et al., 2001; Mittova et al., 2002). The study clearly stated that under conditions of oxidative stress, such as exposure to H2O2 the antioxidant levels increase in plant tissues and was much higher in the tolerant genotypes. Similar findings has been reported by Smith et al., 1990 with exposures to 03, SO2, heat shock or drought stresses.
From the study it is clear that induction of oxidative stress, by in vivo treatment with hydrogen peroxide in rice genotypes varying in their tolerance behaviour to different abiotic stresses (Submergence and salt stress) gave a clear understanding of the signalling role of H 2 O 2. There was no uniform pattern in the accumulation of the osmolytes but significant upregulation of antioxidant enzyme systems and slow degradation of protein contents in the tolerant genotypes (FR 13A and FL 478) could explain the physiological basis of tolerance and play important roles in stress protection.
Список литературы Induction of oxidative stress by hydrogen peroxide treatment in rice genotypes to study the osmolyte accumulation pattern and antioxidant capacity
- Bates, L.S., Waldeen, R.P. and Teare, I.D. (1973) Rapid determination of free proline for water stress studies. Plant Soil, 39: 205
- Blokhina, O.B., Chirkova, T.V. and Fagerstedt, K.V. (2001) Anoxic stress leads to hydrogen peroxide formation in plant cells. J. Exp. Bot., 52, 1179-1190
- Desikan, R., Cheung, M.K., Bright, J., Henson, D., Hancock, J.T. and Neill, S.J. (2004) ABA, hydrogen peroxide and nitric oxide signalling in stomatal guard cells. J. Exp. Bot., 55, 205-212
- Góemez, J.M., Hernández, J.A., Jiménez, A., del Río, L.A. and Sevill, F. (1999) Differential response of antioxidative enzymes of chloroplasts and mitochondria to long-term NaCl stress of pea plants [J]. Free Radic Res., 31, S11-S18
- Gosset, D.R., Banks, SW., Millhollon, E.P. and Lucas, M.C. (1996) Antioxidant response to NaCl stress in a control and an NaCl-tolerant cotton cell line grown in the presence of paraquat, but lionine sulfoximine and exogenous glutathione [J]. Plant Physiol., 112, 803-809
- Hariyadi, P. and Parkin, K.L. (1993) Chilling-induced oxidative stress in cucumber (Cucumis sativus L. cv. calypso) seedlings. J. Plant Physiol., 141, 733-738
- Hernandez, J.A., Mullineaux, P. and Sevilla, F. (2000) Tolerance of pea (Pisum sativum L.) to long term stress is associated with induction of antioxidant defences. Plant Cell Environ., 23, 853-862
- Hung, C.C., Warnken, K.W. and Santschi, P.H. (2005) A seasonal survey of carbohydrates and uronic acids in the Trinity River, Texas; Org. Geochem., 36, 463-474
- Khan, M.H. and Panda, S.K. (2008) Alterations in root lipid peroxidation and antioxidative responses in two rice cultivars under NaCl-salinity stress. Acta Physyol. Plant., 30, 91-89
- Kocsy, G., Brunner, M., Ruegsegger, A., Stamp, P. and Brunold, C. (1996) Glutathione synthesis in maize genotypes with different sensitivity to chilling. Planta, 198, 365-370
- Kumar, S.G., Reddy, A.M. and Sudhakar, C. (2003) NaCl effects on proline metabolism in two high yielding genotypes of mulberry (Morus alba L.) with contrasting salt tolerance. Plant Sci., 165, 1245-1251
- Lee, D.H. and Lee, C.B. (2000). Chilling stress-induced changes of antioxidant enzymes in the leaves of cucumber: in gel enzymes activity assays. Plant Sci., 159, 75-85
- Lee, D.H., Kim, Y.S. and Lee, C.B. (2001). The inductive response of the antioxidant enzymes by salt stress in the rice (Oryza sativa L.). J. Plant Physiol., 158, 737 -745
- Lin, J.N. and Kao, C.H. (1998). Effect of oxidative stress caused by hydrogen peroxide on senescence of rice leaves. Bot. Bull. Acad. Sin., 39, 161 -165
- Lowry, O.H., Rose, N.T., Brough, N.T., Farr, L.A. and Randall, R.J. (1951) Protein measurement with folin phenol reagent. J. Biol. Chem., 193, 265-275
- Luck, H. (1974) In: Methods in Enzymatic Analysis, Bergmeyer (ed.). Academic Press, New York. p.885
- Malik, C.P. and Singh, M.B. (1980) In: Plant Enzymology and Histoenzymology. Kalyani Publishers, New Delhi. p.53
- Martinez, C.A., Maestri, M. and Lani, E.G. (2003) In vitro salt tolerance and proline accumulation in Andean potato (Solanum spp.) differing in frost resistance. Plant Sci., 116, 117-184
- Misra, N. and Gupta, A.K. (2005) Effect of salt stress on proline metabolism in two high yielding genotypes of green gram. Plant Sci., 169, 331-339
- Nakamura, I., Murayama, S. and Tobita, S. (2002) Effect of NaCl on the photosynthesis, water relations, and free proline accumulation in the wild Oryza species. Plant Prod. Sci., 5, 305-310
- Nicholas, J.C., Harper, J.E. and Hageman, R.H. (1976) Nitrate reductase activity in soyabeans. I. Effects of light and temperature. Plant Physiol., 58, 731-735
- Noctor, G., Arisi, A.C.M., Jouanin, L., Kunert, K.J., Rennenberg, H. and Foyer, C.H. (1998) Glutathione: biosynthesis, metabolism and relationship to stress tolerance explored in transformed plants. J. Exp. Bot., 49, 623-647
- O’Kane, D., Gill, V., Boyd., P. and Burdon, R. (1996). Chilling, oxidative stress and antioxidant responses in Arabidopsis thaliana callus. Planta, 198, 371-377
- Ozden, M., Demirel, U., Kahraman and Smith, K. (1990). Glutathione. In: Alscher,R.G. and Cumming,J.R.(Eds.). Stress Responses in Plants: Adaptation and Acclimation Mechanisms. Wiley-Liss New York. 205-215
- Patra, J. and Panda, B.B. (1998). A comparison of biochemical responses to oxidative metal stress in seedlings of barley (Hordeum vulgare L.). Environ Pollut., 101, 99-105
- Prasad, T.K. (1996). Mechanisms of chilling-induced oxidative stress injury and tolerance in developing maize seedlings: changes in antioxidant system, oxidation of proteins and lipids, and protease activities. Plant J., 10, 1017-1026
- Quan, L.J., Zhang, B., Shi, W.W. and Li, H.H. (2008). Hydrogen peroxide in plants: a versatile molecule of the reactive oxygen species network. J. Integr. Plant Biol., 50, 2-18
- Sairam, R.K. and Srivastava, G.C. (2000). Induction of oxidative stress and antioxidant activity by hydrogen peroxide treatment in tolerant and susceptible wheat genotypes, Biol. Plant., 43, 381-386
- Savouré, A., Thorin, D., Davey, M., Hua, X.J., Mauro, S., Van Montagu, M., Inzé, D. and Verbruggen, N. (1999). NaCl and CuZnSO4 treatments trigger distinct oxidative defence mechanism in Nicotiana plumbaginifolia L. [J]. Plant Cell Environ., 22, 387-396
- Skopelitis, D.S., Paranychianakis, N.V. and Paschalidis, K.A. (2006) Abiotic stress generates ROS that signal expression of anionic glutamate dehydrogenases to form glutamate for proline synthesis in tobacco and grapevine. Plant Cell, 18, 2767-2781
- Stanisavljević, N.S., Nikolić, D.B., Jovanović, Ž.S., Samardžić, J.T., Radović, S.R. and Maksimović, V.R. (2011). Antioxidative enzymes in the response of buckwheat (Fagopyrum esculentum Moench) to complete submergence. Arc. Biolo Sci., 63, 399-405
- Wingsle, G. and. Hallgren, J.E. (1993). Influence of SO2 and NO2 exposure on glutathione, superoxide dismutase and glutathione reductase activities in Scots pine needles. J. Exp. Bot., 44, 463-470
- Yazici, I., Turkan, I. and Sekmen, A.H. (2007) Salinity tolerance of purslane (Portulaca oleracea L.) is achieved by enhanced antioxidative system, lower level of lipid peroxidation, and proline accumulation. Environ. Exp. Bot., 61, 49-57
- Zhang, J., Cui, S., Li, J., Wei, J. and Kirkham, M.B. (1995). Protoplasmic factors, antioxidants responses, and chilling resistance in maize. Plant Physiol. Biochem., 33, 567-575


