Induction of somatic embryo and plantlet regeneration from immature inflorescence culture in kodo millet (Paspalum scrobiculatum L.) under salinity stress conditions
Автор: Roselin Roobavathi M., Vikrant
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 3 т.20, 2024 года.
Бесплатный доступ
Salinity stress is a major abiotic stress factor that affects plant growth, physiological activities and developmental processes. This study involves establishing efficient somatic embryogenesis and plantlet regeneration system using immature inflorescence of kodo millet ( Paspalum scrobiculatum L. cv. TNAU86) under NaCl-salinity stress conditions. To begin with, the immature inflorescence (0.5cm) of kodo millet was excised from the 40-45 days old field growing plants followed by surface sterilization and inoculation in Murashige and Skoog (MS) medium supplemented with the various concentrations of NaCl (10mM, 25mM, 50mM, 75mM, 100mM, 150mM, and 200mM) salt along with 2,4-Dichlorophenoxyacetic acid (2,4-D) (1.5 mg/L). The results reveal that the maximum mean frequency (89.3±0.3%) of somatic embryogenesis was obtained from embryogenic callus that was growing with medium added with NaCl (50mM) while it was found to be the least mean frequency (12±4.1%) with 150mM of NaCl-salt treatments. Moreover, the highest concentration of NaCl (200mM) salinity was found to be lethal and explants were observed to get gradually necrosed. Later, embryogenic calli showing differentiation of somatic embryos were sub-cultured on basal medium supplemented with 0.5mg/L of 6-Benzylaminopurine (BAP) along with respective concentrations of NaCl-salt for the germination of somatic embryos into plantlets. Significantly, 100mM of NaCl-treatment was proved to show strong inhibitions and thus minimum salt tolerant plantlets regeneration (4±2.21%) was recorded. Further, in vitro grown salinity stress tolerant plantlets were transferred to plastic cups and gradually acclimatized under greenhouse conditions.
Immature inflorescence, kodo millet, regeneration, salinity stress, somatic embryogenesis
Короткий адрес: https://sciup.org/143182812
IDR: 143182812
Текст научной статьи Induction of somatic embryo and plantlet regeneration from immature inflorescence culture in kodo millet (Paspalum scrobiculatum L.) under salinity stress conditions
Millets are C 4 plants found in arid and semi-arid regions (O’Kennedy et al. , 2006) having the capacity to fight against diabetic and main source for the calories and proteins. Several millets such as, pearl millet, kodo millet, finger millet, proso millet, little millet and foxtail millet have phenol compounds (Rao et al. , 2011), which induce the anti-proliferative property that helps to prevent cancer (Chandrasekara and Shahidi, 2011).
Paspalum scrobiculatum L. is traditionally used to treat diabetes mellitus, the particular reason to increase the serum insulin level significantly and also causes fall in fasting blood glucose (Murthy and Subramanyam, 1989; Jain et al. , 2010; Kiran et al. , 2014). Also, it provides the gluten free protein; however, it has less protein than other millets. Moreover, it consists of vitamins and minerals, especially B-complex vitamins, B6, niacin and folic acid, Fe, Ca, Mg, K, Zn and also rich in essential amino acids (Ravindran, 1992; Parvathy and Thayumanavan, 1995; Anthony et al. , 1996; Gopalan and Shastri, 2009).
India is the largest millet producer in the world. Significantly, in order to create awareness about nutritional food to the people globally; United Nations has declared 2023 as International Year of Millets (IYOM-2023).
Due to the recent climatic changes and environmental stresses, the productivity of the crops was affected. According to Abdel et al. , (2003), the salinity and drought stress are the two major abiotic stresses that affect the crop productivity. The environmental stresses like presence of high salinity in the soil affect the crop productivity in terms of quality and also quantity (Vikrant, 2015).
Salinity stress mainly affecting the places in arid and semi-arid areas (Hernández, 2019). The ability of water utilization of plants reduces due to salinity stress and retard the plant growth rate. Also, it changes the metabolism of the plants (Munns, 2002). In order to resolve the difficulties, in vitro plant tissue culture has been practiced especially having the capacity of enhancing the tolerance of the plant against abiotic factor like salinity, drought, heavy metal stresses, the major responsibility of reducing the productivity of the crops (Abdel-Qader et al., 2003).
Also, the techniques in in vitro tissue culture considered as most efficient method and also having its own values by selection of the valuable crop cultivars. Moreover, in vitro propagation of millets helps to achieve the improvements among millets because of growth and productivity was severely affected by environmental stresses. Moreover, it is obligate necessary to build the well-structured establishment of somatic embryogenesis and plantlet regeneration for the recovery of transgenic millet crops (Ceasar and Ignacimuthu, 2009).
Embryogenic callus and somatic embryogenesis from immature tissues have been achieved in different millets. Paspalum scrobiculatum has been described by Nayak and Sen (1989) and Vikrant and Rashid (2001), P. dilatatum (Akashi and Adachi, 1992), P. notatum (Bovo and Mroginski, 1986 and 1989), P. vaginatum (Cardona and Duncan, 1997), Likewise, Eleusine coracana (George and Eapen, 1990), Panicum maximum (Lu and Vasil, 1982) and Setaria italica (Vishnoi and Kothari, 1996).
Moreover, it is documented that the immature inflorescence and tissues have been proved to be the best type of explants because of being capable to regenerate the plantlets in high frequency compared to the mature embryos or seeds, especially in recalcitrant crops like monocotyledons (Jha et al. , 2009).The objective of the study was to analyze the induction of somatic embryo under salinity stress conditions followed by regeneration of stress tolerant plantlet from immature inflorescence of kodo millet as explant.
MATERIALS AND METHODS
Collection of Plant Material
Seeds of Paspalum scrobiculatum L. cv. TNAU86 were collected from Centre of Excellence in Millets, Thiruvannamalai, Tamil Nadu (India). The kodo plants were cultivated in APJ Abdul Kalam Garden, KMGIPSR, Puducherry. The Immature inflorescences (0.5cm) were excised from 40–45-day-old field grown plants and were used as explants.
The explants were surface sterilized using ‘tween -20’ followed by 70% (v/v) ethanol for 15 seconds.
Further, explants were then treated with 0.1% HgCl 2 about 5 minutes followed by the sterilized distilled water (4-5 times) wash in Laminar Air Flow chamber. The sterilized explants were subjected to nutrient medium further.
Nutrient Medium for Induction of Callus and Somatic Embryogenesis
To regulate the protocol, the immature inflorescence cultured in various concentrations of auxin (2,4-D) along with or without cytokinins (BAP, kinetin, and zeatin). Initially, the explants were cultured on the nutrient medium consisting of MS salts and vitamins (Murashige and Skoog, 1962) supplemented with 2% sucrose (w/v) alongwith different concentrations and combinations of plant growth regulator (TABLE 1).
The cultures were incubated at 25±2°C in the dark for callus induction. The cultures were subsequently sub-cultured in respective fresh medium every two weeks routinely.
Nutrient Medium for Plantlets Regeneration
After six weeks, the embryogenic callus was subcultured into regeneration media. The MS medium supplemented with different concentrations of phytohormones (TABLE 2). These cultures were incubated at 25±2°C with 16/8h (light/dark) photoperiod for 14 days. The germination of somatic embryos and regeneration of plantlets were observed after 10 days of sub-culture.
The regenerated plantlets were gradually acclimatized and transferred to pot containing mixture of soil: sand: vermicompost (1:1:1) in the greenhouse condition.
Salinity Stress Treatments
To determine the effect of salinity stress, the explants were inoculated in MS medium containing 2, 4-D (1.5 mg/L) along with various concentrations (10, 25, 50, 75, 100, 150, and 200mM) of NaCl (v/v) salt solutions. The MS medium supplemented with 2,4-D (1.5 mg/L) and without NaCl is considered as control. The cultures were maintained at optimum temperature (25±2°C) and light condition for 2 weeks interval of time.
Once the callus formation was observed, it subsequently sub-cultured in regular interval of time for somatic embryo induction. The embryogenic calluses were sub-cultured into regeneration medium along with respective concentrations of NaCl (TABLE 3) for plantlet germination from somatic embryos. The germinated plantlets were further acclimatized into pot.
STATISTICAL ANALYSIS
Each experiment was repeated three times. The mean percentage of somatic embryogenesis was calculated after 8 weeks (No. of callus showing Somatic embryogenesis / Total no. of Callus X 100). The mean number of shoots regenerated form the somatic embryos were calculated after 20 days of somatic embryo germination. The data were presented as student ‘t’ test to compare its significance.
RESULTS
In order to establish embryogenic callus formation followed by somatic embryogenesis in kodo millet, immature inflorescence explants were treated with various concentrations of auxins and cytokinins were used in present study (Table 1).
Callus induction and Somatic Embryogenesis
In basal medium, explants were found to be non-responsive in terms of callus induction even after 10-days of culture initiation. Moreover, such explants were found to be necrosed with slight proliferation in explants tissues (Fig. 1A) . In contrast, the explants that were treated with 2,4-D concentration (1.5mg/L) were found to exhibit good amount of nodular and compact callus induction (Fig. 1C) .
The frequency of callus induction found in 2,4-D with cytokinins (BAP, kinetin and zeatin) was observed to be relatively less when compared to the frequency of callus induction found in 2,4-D alone supplemented culture (Table 1) . Furthermore, the higher concentration (3.0mg/L) of 2,4-D supplemented culture exhibit non-embryogenic callus results with browning of callus in course of time.
Calli that were growing with 2,4-D containing nutrient media were further sub-cultured into same nutrient media after 2 weeks of culture initiation. Small white colored, globular somatic embryos were observed in the embryogenic callus after 1-2 weeks of sub-culture (Fig. 1D-F).
The yellowish compact embryogenic callus showing highest frequency of somatic embryogenesis (87.8±0.5%) was recorded with the explants that were treated with the lowest concentration of 2,4-D (1.5mg/L). However, explants that were treated with combination of 2,4-D (3.0mg/L) with zeatin (0.1mg/L) show the least frequency (31.6±1.6) of callus induction in comparison to the other combination of plant growth regulators (Table 1).
Plantlet Regeneration
The embryogenic callus with mature somatic embryos was sub-cultured into various concentrations of regeneration medium (Table 2). The regeneration frequency was found to be maximum at 0.5mg/L of BAP (78.8±0.63%) (Fig. 2A-B) , followed by basal medium the germination frequency found to be relatively less (64.9±0.37%). While the germination frequency was found to be the minimum (11.7±0.87%) on the IBA (1.0mg/L) and kinetin (0.5mg/L) combination.
Effects of Salt Stress on Somatic Embryogenesis
The difference between the control as well as the salt treated callus was significant. The culture was observed a primary callus (FIG.1G) in lower concentration of NaCl stress (50mM). Later, it formed the embryogenic callus after two weeks of incubation. Perhaps, the salt treated explants show good embryogenic callus and formed the milky white somatic embryos after 15 days of incubation (Fig.1 J&K) .
The minimum concentrations (50mM) of NaCl could promote the somatic embryogenesis up to maximum frequency (89.3±0.3%). However, the higher concentration (150mM) of NaCl shows the least mean frequency percentage (12.0±4.1%) for somatic embryogenesis and proved to be little toxic (Table 3). Significantly, further increase in NaCl concentration (200mM) observed that the callus was necrosed (Fig. 1L) thus it was proved to be lethal concentration of NaCl for any morphogenic response.
The independent ‘t’- test to compare the embryogenesis in control and salinity stress treated culture. There was a significant difference between the above study (t = 7.3, p<0.05). The mean frequency of somatic embryogenesis under salinity condition (50mM) (89.3±0.3%) higher than the mean frequency of somatic embryogenesis in control condition (87.0±0.5%).
Plantlets Regeneration under Salinity Stress
The embryogenic callus with somatic embryos was sub-cultured into nutrient medium with or without 0.5mg/L of BAP along with the respective NaCl concentrations (Table 4). The control (Fig. 2B) medium shows the highest frequency (78±0.63%) of plantlets regeneration. The plantlet germination from somatic embryos was recorded as the maximum frequency (69.2±0.77%) with 25mM of NaCl (Fig. 2C&D) followed by 50mM of NaCl stress (54.6±0.92%) while the least frequency (04.7±2.21%) of plantlet regeneration was obtained with 100mM of NaCl treatment. The in vitro plantlet that grown under salinity stress was considered as salt-tolerant and was transferred to plastic cups for further acclimatization process under greenhouse condition (Fig. 2H).
Table 1 : Paspalum scrobiculatum L., effect of auxin either alone or along with cytokinin on callus induction and somatic embryogenesis in immature inflorescence culture.
|
Concentration of Auxin (mg/L) |
Concentration of Cytokinins (mg/L) |
Percentage of Embryogenic Callus Mean ± SD |
Mean Number of Somatic Embryos/Callus (Mean ± SD) |
||
|
2, 4- D |
1.0 |
BAP |
0 |
76.5±1.4 |
48.2±0.9 |
|
1.5 |
87.8±0.5 |
58.4±0.2 |
|||
|
2.0 |
71.3±3.0 |
43.2±0.3 |
|||
|
3.0 |
41.7±1.5 |
14.8±0.9 |
|||
|
1.0 |
0.5 |
48.0±1.6 |
30.3±1.3 |
||
|
2.0 |
63.9±9.2 |
27.9±0.5 |
|||
|
3.0 |
53.8±2.6 |
19.4±0.4 |
|||
|
1.0 |
Kn |
0.5 |
68.3±2.1 |
34.5±0.9 |
|
|
2.0 |
58.1±1.5 |
25.8±1.3 |
|||
|
3.0 |
35.3±2.5 |
9.5±1.7 |
|||
|
1.0 |
Zn |
0.1 |
65.2±3.5 |
30.8±0.6 |
|
|
2.0 |
57.5±2.0 |
21.5±0.8 |
|||
|
3.0 |
31.6±1.6 |
11.9±0.2 |
|||
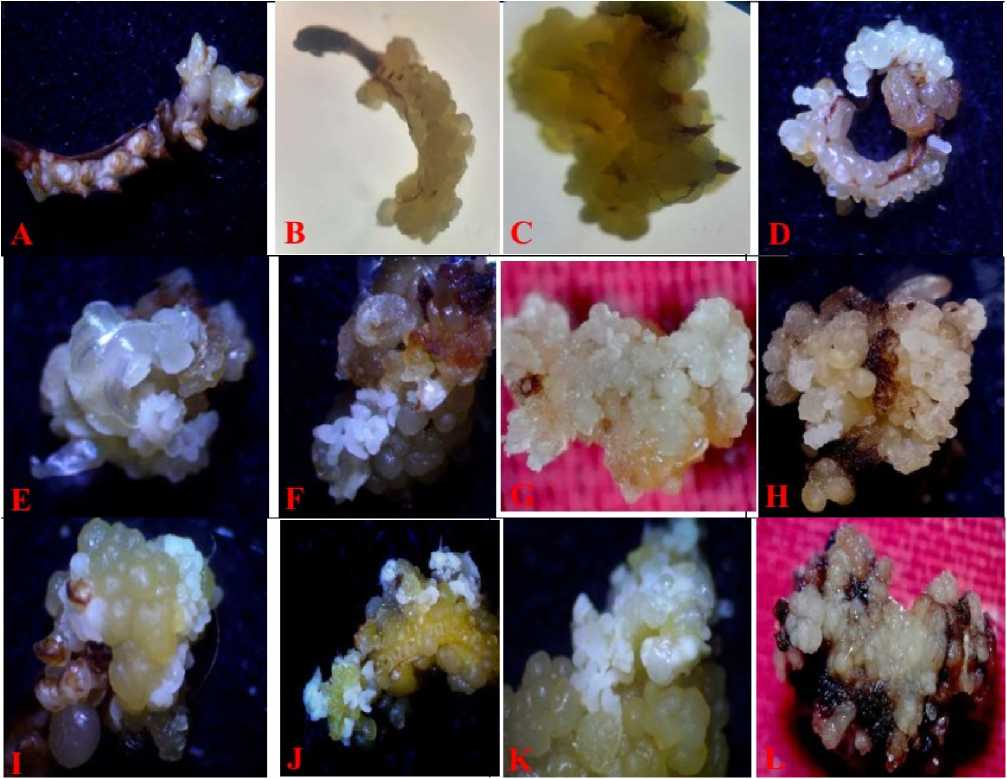
Figure 1. Paspalum scrobiculatum L. Immature Inflorescence culture on MS- medium supplemented;
(A) Basal medium; (B) callus formation under 1.0 mg/L of 2,4-D; (C) callus formation under 1.5mg/L of 2,4-D; (D) somatic embryogenesis on 1.0mg/L of 2,4-D; (E) somatic embryogenesis on 1.5mg/L of 2,4-D; (F) somatic embryogenesis on 2.0mg/L of 2,4-D; (G) callus formation on 1.5mg/L 2,4-D with 50mM NaCl; (H) callus formation on 1.5mg/L of 2,4-D with 100mM of NaCl; (I) somatic embryogenesis on 1.5mg/L of 2,4-D with 25mM of NaCl; (J-K) somatic embryogenesis on 1.5mg/L of 2,4-D with 50mM of NaCl; (L) callus formation on 1.5mg/L of 2,4-D with 200mM of NaCl.
Table 2: Paspalum scrobiculatum L., effect of auxin and cytokinin on regeneration of plantlets in immature inflorescence culture
|
Concentration of Auxins (mg/L) |
Concentration of Cytokinins (mg/L) |
Percentage of embryogenic callus showing plantlets regeneration (Mean±SD) |
No. of Plantlets regeneration / Embryogenic callus (Mean±SD) |
||
|
2, 4- D |
0 |
BAP |
0 |
64.9±0.37 |
22.8±0.79 |
|
0 |
0.5 |
78.8±0.63 |
31.3±0.20 |
||
|
0.5 |
37.5±0.94 |
19.5±1.53 |
|||
|
1.0 |
23.3±0.97 |
9.1±0.71 |
|||
|
1.5 |
15.1±1.40 |
8.3±1.63 |
|||
|
0 |
Kn |
0.5 |
29.4±1.81 |
11.2±1.01 |
|
|
0.5 |
14.5±0.32 |
5.3±0.80 |
|||
|
1.0 |
20.2±0.97 |
3.5±1.41 |
|||
|
IBA |
0.5 |
BAP |
0.5 |
24.1±0.60 |
9.9±0.81 |
|
1.0 |
18.6±0.53 |
5.9±0.93 |
|||
|
0.5 |
Kn |
0.5 |
13.4±0.72 |
7.4±0.62 |
|
|
1.0 |
11.7±0.87 |
6.3±0.87 |
|||
Table 3: Paspalum scrobiculatum L., effect of NaCl-salinity stress on somatic embryogenesis in immature inflorescence culture
|
Concentration of NaCl (mM) |
Concentration of 2,4-D (mg/L) |
Percentage of Somatic Embryogenesis (Mean±SD) |
Mean Number of Somatic Embryos/Callus (Mean ± SD) |
|
0 |
1.5 |
87.0±0.5 |
58.1±0.20 |
|
10 |
1.5 |
83.9±1.6 |
49.7±0.41 |
|
25 |
1.5 |
85.3±0.4 |
52.4±0.76 |
|
50 |
1.5 |
89.3±0.3 |
64.7±0.82 |
|
75 |
1.5 |
68.9±0.2 |
48.3±0.82 |
|
100 |
1.5 |
31.3±0.5 |
16.3±0.98 |
|
150 |
1.5 |
12.0±4.1 |
08.6±1.23 |
|
200 |
1.5 |
0 |
0 |

Figure 2. Salinity Stress- Paspalum scrobiculatum L. Immature Inflorescence culture showing plantlet regeneration;
(A-B) Control (0.5mg/L of BAP); (C-D) Plantlets regeneration on 0.5mg/L of BAP with 25mM NaCl; (E) Plantlets regeneration on 0.5mg/L of BAP with 50mM of NaCl; (F) Plantlets regeneration on 0.5mg/L of BAP with 100mM of NaCl (G) Salinity tolerant plantlet; (H) Potted plantlet.
Table 4: Paspalum scrobiculatum L., effect of NaCl-salinity stress on plantlets regeneration in immature inflorescence culture
|
Concentration of NaCl (mM) |
Concentration of BAP (mg/L) |
Percentage of embryogenic callus showing plantlets regeneration (Mean%±SD) |
No. of Plantlets Regeneration/ Embryogenic Callus (Mean±SD) |
|
0 |
0.5 |
78.8±0.63 |
31.9±0.20 |
|
25 |
0.5 |
69.2±0.77 |
23.6±0.78 |
|
50 |
0.5 |
54.6±0.92 |
11.6±1.58 |
|
100 |
0.5 |
04.7±2.21 |
1.3±1.87 |
DISCUSSION
Abiotic stress in general and salinity in particular has been proved to be a big challenge for our agriculture yields. However, one of the important strategies has been adopted to overcome salinity is to exploit the in vitro regeneration procedures under salinity stress conditions to identify and achieve salinity tolerant genotype that may sustain a reasonable yield on salt affected soils (Ashraf et al. , 2006).
Establishment of Somatic Embryogenesis in Millets
The immature tissues, such as immature and mature zygotic embryos (Vikrant and Rashid, 2002a), immature inflorescence (Nayak and Sen, 1989; Vikrant and Rashid, 2001; Kaur and Kothari, 2004), leaves and roots (Kaur and Kothari, 2003; Hoque and Mansfield, 2004), mesocotyl and leaf segments (Vikrant and Rashid, 2003) of kodo millets were used as explants in order to establish the induction of somatic embryogenesis and plantlet regeneration. According to the literature, the immature inflorescence shows greatest embryogenesis and regenerative capacity rather than the other explants such as, mature seeds and shoots tips (Jha et al. , 2009).
The present study involves the both somatic embryogenesis and regeneration from immature inflorescence as explants in Paspalum scrobiculatum L. to induce the salinity stress tolerant plantlets in efficient way in major cereals and millet crops. 2,4-D was used in order to induce callus on millets and other crops (Mohanty et al. , 1985; Chandra and Kothari, 1995; Kothari-chajer et al. , 2008; Sonia Plaza-Wüthrich and Zerihun Tadele, 2012).
To induce embryogenic callus, the present study involves auxins (2,4-D) tested alone or with combinations of BAP, kn and zeatin. The medium containing 2,4-D alone observed that increased frequency percentage of somatic embryogenesis (1.5mg/L). Recently, Kothari-chajer et al., 2008 used 8.6µM 2,4-D alone in the micronutrient-modified MS medium for somatic embryogenesis of the kodo millet genotype GUPK3.
It has been reported that the higher concentrations of auxin found to inhibit the induction of embryogenic calli. In contrast, the lower concentration of auxin is proved to be effective in cereals (Lu et al. , 1983; Bi et al. , 2007) and other millets like, sorghum (Amali et al. , 2014).
However, in present study, 2,4-D alone could be proved more effective in terms of induction of somatic embryos than the combination of cytokinin with 2,4-D. Moreover, in contrast, the combination of 2,4-D along with BA, kinetin and zeatin increases the percentage of embryogenic callus induction in Eleusine coracana (Ceasar and Ignacimuthu, 2008), Sorghum bicolor (Wernicke and Brettell, 1980; Belide et al. , 2017; Espinoza-Sánchez et al. , 2018).
Germination of Somatic Embryo and Plantlets Regeneration
Historically, a direct organogenesis from immature inflorescence has been observed in millets (Kavi Kishore et al. , 1992). However, the combinations of BA and NAA were used to promote the regeneration from somatic embryos in Paspalum scrobiculatum L. (Nayak and Sen, 1989; Arokiyasamy et al. , 2001; Kaur and Kothari, 2004), P. vaginatum (Cardona and Duncan, 1997).
Moreover, an efficient regeneration protocol from shoot apex (Arokiyasamy et al., 2001) and immature inflorescence (Vikrant and Rashid, 2001; Kothari-chajer et al., 2008) could be possible by modifying medium micronutrient in P. scrobiculatum. Apart from auxins, the cytokinins (kinetin and BA) also have been used for plantlet regeneration from somatic embryos in pearl millet (Mythili et al., 2001; Srivastav and Kothari, 2002), finger millet (Ceasar and Ignacimuthu, 2008) and foxtail millet (Xu et al., 1984).
Hence, in the present study, the BAP (0.5mg/L) was proved to be helpful to promote the plantlet germination frequency (78.8±0.63%) in comparison with kinetin (0.5mg/L supplemented medium) (29.4±1.81%). Whereas, the combination of kinetin (0.5mg/L) with IBA (1.0mg/L) observed to be least germination frequency (11.7±0.87%) of somatic embryos. The plantlet regeneration from embrogenic callus was highly boosted by cytokinins than auxins (Girgi et al. , 2002) in finger millet (Sankhla et al. , 1992; Yemets et al. , 2003; Latha et al. , 2005; Ceasar and Ignacimuthu, 2008; Nethra et al. , 2009), kodo millet (Ceasar and Ignacimuthu, 2010) and pearl millet (Mythili et al. , 1997; Goldman et al. , 2003; Satyavathi et al. , 2016).
Somatic Embryogenesis under Salinity Stress
The present study reveals that, the frequency of callus induction as well the somatic embryogenesis was found to be higher (89.3±0.3%) when it is exposed to saline stress (50mM) as it compared to control (87.0±0.5%). In literature, the increase in saline concentration leads to the reduction of callus formation as well as somatic embryogenesis (Vikrant, 2015).
There were many reports stated that, some genotypes were producing high germination frequency, high yield and biomass, when they treated with salt condition (Ashraf et al. , 2006; Krishnamurthy et al. , 2007). In some cases, the germination rate found to be reduced because, the reduction in water potential; hence, it affects the water absorption due to, toxic effect of saline condition (Jamil et al. , 2006). The increase in NaCl salt concentration (150mM) decreases the callus induction and affects the somatic embryo formation in 2, 4-D supplemented medium (12.0±4.1%).
Plantlet regeneration under Salt Stress
The establishment of in vitro regeneration from somatic embryogenesis under saline stress condition has been reported in many plants. The plantlet germination was found to be inhibited while the increase in the concentrations of NaCl treatments. The control medium (0.5mg/L of BAP) shows the highest frequency of somatic embryo germination (78.8±0.63%). The lowest concentration of NaCl (25mM) shows moreover, mention equally high number of plantlets regeneration (69.2±0.77%) as compared to control. In contrast, the maximum shoot length was produced by some genotypes when they were exposed to NaCl stress. Even they show high mean frequency of shoot length under salinity stress condition for saline tolerant genotypes (Hakim et al., 2010; Bashir et al., 2011).
The rate of somatic embryo germination found to be reduced in salt-tolerant cultivar in finger millet (Mukami et al. , 2020). Likewise, increase in NaCl concentration (50mM and 100mM) the plantlet germination frequency found to be deceasing (54.6±0.92% and 4.7±2.21%) respectively. The higher concentration (150mM) of NaCl proves to be the lethal for plantlets regeneration.
CONCLUSION
The present study produces the protocol for efficient somatic embryogenesis and plantlet regeneration from the explant immature inflorescence of kodo millet under salinity stress. Interestingly, NaCl (50mM) proves to support somatic embryogenesis instead of inhibiting the process, however, in contrast the same concentration of NaCl (50mM) was found to be inhibitory for the plantlet germination. Also, the plantlet withstands the saline condition when it was acclimatized. This study further could be useful in production of genetic transformation of millet crops in order to withstand the salinity stress and produce higher yields.
Список литературы Induction of somatic embryo and plantlet regeneration from immature inflorescence culture in kodo millet (Paspalum scrobiculatum L.) under salinity stress conditions
- Abdel-Qader, I., Abudayyeh, O. and Kelly, M.E. (2003) Analysis of Edge-Detection Techniques for Crack Identification in Bridges. Journal of Computing in Civil Engineering, 17(4): 255-263.
- Akashi, R. and Adachi, T. (1992) Somatic embryogenesis and plant regeneration from cultured immature inflorescences of apomictic Dallisgrass (Paspalum dilatatum Poir.). Plant Sci., 82: 213-218.
- Amali, Kingsley and Ignacimuthu (2014) High frequency callus induction and plant regeneration from shoot tip explants of Sorghum bicolor L. Moench original article. Int. J. Pharm. Pharm. Sci., Vol 6, Issue 6, 213-216.
- Anthony, U., Sripriya, G. and Chandra, T.S. (1996) Effect of fermentation on the primary nutrients in finger millet (Eleucine coracana). Journal of Agriculture and Food Chemistry, 44: 2616-2618.
- Arockiasamy, S., Prakash, S. and Ignacimuthu, S. (2001) High regenerative nature of Paspalum scrobiculatum L., an important millet crop. Curr. Sci., 80:496-498.
- Ashraf, M.Y., Akhtar, K., Hussain, F. and Iqbal, J. (2006) Screening of different accession of three potential grass species from Cholistan desert for salt tolerance. Pakisthan Journal of Botany, 38: 15891597.
- Bashir, F., Ali, M., Hussain, K., Majeed, A. and Nawaj, K. (2011) Morphological variations in sorghum (Sorghum bicolor L.) under different levels of Na2SO4 salinity. Botany Research International, 4(1): 01-03.
- Belide, S., Vanhercke, T., Petrie, J.R. and Singh, S.P. (2017) Robust genetic transformation of sorghum (Sorghum bicolor L.) using differentiating embryogenic callus induced from immature embryos. Plant Methods, 13: 109.
- Bi, R., Kou, M.M., Chen, L.G., Mao, S.R. and Wang, H.G. (2007) Plant regeneration through callus initiation from mature embryo of Triticum. Plant Breed, 126.
- Bovo, O.A. and Mroginski, L.A. (1986) Tissue culture in Paspalum (Gramineae) plant regeneration from cultured inflorescences. J. Plant Physiol., 124: 481-492.
- Bovo, O.A. and Mroginski, L.A. (1989) Somatic embryogenesis and plant regeneration from cultured mature and immature embryos of Paspalum notatum (Gramineae). Plant Sci., 65: 217-223.
- Cardona, C.A. and Duncan, R.R. (1997) Callus induction and high frequency plant regeneration via somatic embryogenesis in Paspalum. Crop Sci., 37: 12971302.
- Ceasar, S.A. and Ignacimuthu, S, (2010) Effects of cytokinins, carbohydrates and amino acids on induction and maturation of somatic embryos in kodo millet (Paspalum scorbiculatum Linn.). Plant Cell Tiss. Organ. Cult., 102: 153-162.
- Ceasar, S.A. and Ignacimuthu, S. (2008) Efficient somatic embryogenesis and plant regeneration from shoot apex explant of different Indian genotypes of finger millet (Eleucine coracana (L.) Gaertn). In vitro Cell. Dev. Biol. Plant, 44: 427-435.
- Ceasar, S.A. and Ignacimuthu, S. (2009) Genetic engineering of millets: current status and future prospects. Biotechnol. Lett., 31:799-788.
- Chandra, N. and Kothari, S.L. (1995) Advances in tissue culture and genetic transformation of cereals. J. Indian Bot. Soc.,74: 323-342.
- Chandrasekara, A. and Shahidi, F. (2011) Antiproliferative potential and DNA scission inhibitory activity of phenolics from whole millet grains. J. Funct. Foods, 3: 159-170.
- Espinoza-Sánchez, E.A., Sánchez-Peña, Y.A., Torres-Castillo, J.A., García-Zambrano, E.A., Ramírez, J.T., Zavala-García, F. and Sinagawa-García, S.R. (2018) Somatic embryogenesis induction from immature embryos of Sorghum bicolor L. (Moench). Phyton-Int. J. Exp. Bot, 87: 105-112.
- George, L. and Eapen, S. (1990) High frequency plant regeneration through direct shoot development and somatic embryogenesis from immature inflorescence cultures of finger millet (Eleusine coracana Gaertn.). Euphytica, 48: 269-274.
- Girgi, M., O'Kennedy, M.M., Morgenstern, A., Mayer, G., Lorz, H. and Oldach, K.H. (2002) Transgenic and herbicide resistant pearl millet (Pennisetum glaucum L.) R.Br. via microprojectile bombardment of scutellar tissue. Mol. Breed, 10: 243-252.
- Goldman, J.J., Hanna, W.W., Fleming, G. and Ozias-Akins (2003) Fertile transgenic pearl millet [Pennisetum glacum (L.) R. Br.] plants recovered through microprojectile bombardment and phosphinothricin selection of apical meristem, inflorescence, and immature embryo-derived embryogenic tissues. Plant Cell Rep., 21: 9991009; DOI 10.1007/s00299-003-0615-8
- Gopalan, C. and Shastri, B. (2009) Nurtitive Value of Indian Foods, Nutritional Institute of Nutrition, Indian Council of Medical Research, Hydrabad, India. 99.
- Hakim, M.A., Juraimi, Begum, M., Hanafi, M.M., Ismail, M.R. and Selamat, A. (2010) Effect of salt stress on germination and early seedling growth of rice (Oryza sativa L.). African journal of biotechnology, 9(13): 1911-1918.
- Hernández, J. A. (2019) Salinity Tolerance in Plants: Trends and Perspectives. International Journal of Molecular Sciences, 20(10): Article 10.
- Hoque, E.H. and Mansfield, J.W. (2004) Effect of genotype and explant age on callus induction and subsequent plant regeneration from root derived callus of Indica rice genotypes. Plant Cell Tiss. Org. Cult., 78: 217- 223.
- Jain, S., Bhatia, G., Barik, R., Kumar, P., Jain, A. and Dixit, V.K. (2010) Anti-diabetic activity of Paspalum scrobiculatum Linn. in alloxan induced diabetic rats. J. Ethnopharmacol ., 127(2): 325-8.
- Jamil, M., Lee, D.B., Jung, K.Y. Ashraf, M., Lee, S.C. and Rha, E.S. (2006) Effect of salt (NaCl) stress on germination and early seedling growth of four vegetables species. Journal of central European agriculture, 7(2): 273-282.
- Jha, P., Yadav, C.B., Anjaiah, V. and Bhat, V. (2009) In vitro plant regeneration through somatic embryogenesis and direct shoot organogenesis in Pennisetum glaucum (L.) R, Br. In vitro Cell. Dev. Biol. Plant, 45: 145-154.
- Kaur, P. and Kothari, S.L. (2003) Embryogenic callus induction and efficient plant regeneration from root cultures of kodo millet. Phytomorphology, 53:4956.
- Kaur, P. and Kothari, S.L. (2004) In vitro culture of kodo millet: influence of 2,4-D and picloram in combination with kinetin on callus initiation and regeneration. Plant Cell Tiss. Org. Cult., 77: 73-79.
- Kavi Kishore, P.B., Rao, A.M., Dha, A.C. and Naidu, K.R. (1992) Plant regeneration in tissue culture of some millets. Ind. J. Exp. Biol., 30:729-733.
- Kiran, P., Denni, M. and Daniel, M. (2014) Anti-diabetic Principles, Phospholipids and Fixed Oil of Kodo Millet (Paspalum scrobiculatum Linn.). Indian Jr. of Appl. Res., 4 (2): 13-15.
- Kothari-Chajer, A., Sharma, M., Kachwaha, S. and Kothari, S.L. (2008) Micronutrient optimization results into highly improved in vitro plant regeneration in kodo (Paspalum srobiculatumL.) and finger (Eleucine coracana (L.) Gaertn) millets. Plant Cell Tiss. Org. Cult., 94: 105-112.
- Krishnamoorthy, L., Serraj, R., Hash, A.J. and Reddy, B.V. (2007) Screening sorghum genotypes for salinity tolerant biomass production. Euphytica,156:15-24.
- Latha, A.M., Rao, K.V. and Reddy, V.D. (2005) Production of transgenic plants resistant to leaf blast disease in finger millet (Eleusine coracana (L.) Gaertn.). Plant Sci., 169: 657-667.
- Lu, C and Vasil, I.K. (1982) Somatic embryogenesis and plant regeneration in tissue cultures of Panicum maximum Jacq. Am.J. Bot., 69: 77.
- Lu, C., Vasil, V. and Vasil, I.K. (1983) Improved efficiency of somtic embryogenesis and plant regeneration in tissue cultures of maize (Zea mays L.). Theoretical and applied genetics, 66(3-4):285-9.
- Mohanty, B.D., Gupta, S.D. and Ghosh, P.D. (1985) Callus initiation and plant regeneration in ragi (Eleusine coracana (L.) Garetn). Plant Cell Tiss. Org. Cult., 5: 147-150.
- Mukami, A., Ng'etich, A., Syombua, E., Oduor, R. and Mbinda, W. (2020) Varietal differences in Physiological and biochemical responses to salinity stress in six finger millet plants. Physiol. Mol. Biol. Plants, 26: 1569-1582; DOI: 10.1007/s12298-020-00853-8
- Munns, R.C. ( 2002) Comparative physiology of salt and water stress. Plant Cell Environment, 25: 239-250.
- Murashige, T. and Skoog, F. (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant, 15: 473-497.
- Murthy, A.V.S. and Subramanyam, N.S.A. (1989) Textbook of Economic Botany. (Wiley Eastern Limited), New Delhi.
- Mythili, P., Madhavi, A., Reddy, V.D. and Seetharam, N. (2001) Efficient regeneration of pearl millet Pennisetum glacum (L.) from shoots tip cultures. Ind J Exp Biol., 39:1274-12.
- Mythili, P.K., Satyavathi, V., Pavankumar, G., Rao, M.V.S. and Manga, V. (1997) Genetic analysis of short term callus culture and morphogenesis in pearl millet, Pennisetum glaucum, Plant Cell. Tiss. Organ. Cult, 50: 171-178.
- Nayak, P. and Sen, S.K. (1989) Plant regeneration through somatic embryogenesis from suspension cultures of a minor millet, Paspalum scrobiculatum L. Plant Cell Rep., 8: 296-299.
- Nethra, N., Gowda, R. and Gowda, P.H.R. (2009) Influence of culture medium on callus proliferation and morphogenesis in finger millet. In: Tadele Z. (ed) New approaches to plant breeding of orphan crops in Africa. Proceedings of an International Conference, 19-21. Bern, Switzerland. Univ. Bern., pp. 167-178.
- O'Kennedy, M.M., Grootboom, A. and Shewry, R.R. (2006) Harnessing sorghum and millet biotechnology for food and health. J.Cereal Sci., 44: 224-235.
- Parvathy, K. and Thayumanavan, B. (1995) Homologies between prolamins of different minor millets. Plant Foods Hum. Nutr., 48:119-126.
- Rao, B.R., Nagasampige, M.H. and Ravikiran, M. (2011) Evaluation of nutraceutical properties of selected small millets. J. Pharm. Bioallied. Sci., 3:277-279.
- Ravindran, G. (1992) Seed proteins of millets: amino acid composition, proteinase inhibitors and in vitro digestibility. Food Chemistry, 44(1): 13-17.
- Sankhla, A., Davis, T.D., Sankhla, D., Sankhla, N., Upadhyaya, A. and Joshi, S. (1992) Influence of growth regulators on somatic embryogenesis, plantlet regeneration, and post-transplant survival of Echinochloa frumentacea. Plant Cell Rep., 11: 368-371.
- Sathyavathi, V.V., Manga, V., Muktinutalapati, V., Subba Rao, Malladi Chittibabu (2016) Genetic analysis of reciprocal differences in the inheritance of in vitro characters in pearl millet. Genetics and molecular biology, 39: 54-61.
- Sonia Plaza-Wüthrich and Zerihun Tadele (2012) Millet improvement through regeneration and transformation. Biotechnology and Molecular Biology Review, Vol. 7(2): pp. 48-61.
- Srivastav, S. and Kothari, S.L. (2002) Embryogenic callus induction and high frequency plant regeneration in pearl millet. Cer. Res. Commun., 30: 69-74.
- Vikrant (2015). Induction of Somatic Embryos from Mature Embryo Culture under Abiotic Stress and Estimation of Proline Status in a Millet Crop, Paspalum scrobiculatum L. International Journal of Advanced Biotechnology and Research (IJBR); ISSN 0976-2612, Online ISSN 2278-599X,Vol 6: Issue1, pp96-109://www.http bipublication.com
- Vikrant and Rashid (2001) Direct as well as indirect somatic embryogenesis from immature (unemerged) inflorescences of a minor millet Paspalum scrobiculatum L. Euphytica, 120: 167172.
- Vikrant and Rashid (2002a) Somatic embryogenesis from immature and mature embryos of a minor millet Paspalum scrobiculatum L. Plant Cell Tiss. Org. Cult., 69: 71-77.
- Vikrant and Rashid (2003) Somatic embryogenesis from mesocotyl and leaf base segments of Paspalum scrobiculatum L. minor miller. In Vitro Cell Dev. Biol. Rep., 39:485-489.
- Vishnoi, R.K and Kothari, S.L. (1996) Somatic embryogenesis and efficient plant regeneration in immature inflorescence culture of Setaria italica (L.) Beauv. Cereal Res. Commun.,24: 291-297.
- Wernicke, W. and Brettell, R. (1980) Somatic embryogenesis from Sorghum bicolor leaves. Nature, 287: 138-139.
- Xu, Z., Wang, D., Yang, L. and Wei, Z. (1984) Somatic embryogenesis and plant regeneration in callus cultured immature inflorescence of Setaria italica. Plant Cell Rep., 3: 144-150.
- Yemets, A.I., Klimkina, L.A., Tarassenko, L.V. and Blume, Y.B. (2003) Efficient callus formation and plant regeneration of goosegrass (Eleusine indica (L.) Gaertn.). Plant Cell. Rep., 21: 503-510.


