Influence of a constant electric field on the adsorption purification of water from iron ions
Автор: Shestakov I. Y., Khilyuk A. V.
Журнал: Siberian Aerospace Journal @vestnik-sibsau-en
Рубрика: Technological processes and material science
Статья в выпуске: 1 vol.21, 2020 года.
Бесплатный доступ
Using electrochemical action (ECA) to treat water was first proposed in UK in 1889. At present, many methods of ECA are known (electro flotation, electro coagulation, electro osmosis, electrophoresis, etc.). In the production of rocket and space technology, galvanic technologies are used, as a result of which waste water is contaminated with metal ions. Known methods of wastewater treatment do not allow to ensure the maximum permissible concentration of metal ions in treated water, or are expensive or difficult to operate in industry. Iron ions are among the most polluting components of wastewater of most industries. So increased control and the development of effective methods of wastewater treatment are necessary. Iron affects the intensity of phytoplankton development and the qualitative composition of microflora in reservoirs. The toxicity of iron compounds in water depends on the hydrogen index of water. The alkaline environment dramatically increases the risk of fish poisoning, as in such conditions, iron hydroxides are formed, which are deposited on the gills, clog and corrode them. In addition, iron compounds bind oxygen dissolved in water, which leads to the mass death of fish and other hydrobionts. The article presents the method of conducting experiments, the methods of sorption, electrochemical and combined water treatment, including electrochemical action and adsorption. The results of studies of these methods of water purification from iron ions are presented. The dependence of the degree of purification on the electric field strength, interelectrode distance and water treatment time is revealed. With an electric field strength of 5.16 V/mm, a temperature of 20–22 °C using quartz sand as an adsorbent and a processing time of 1 minute, the concentration of iron ions decreased from 2.5 to 0.25 mg/l (at MPC = 0.3 mg/l). The proposed combined cleaning method requires inexpensive and affordable materials and is easy to operate.
Electrochemical method, iron, degree of purification, alternating current, direct current, sorbent.
Короткий адрес: https://sciup.org/148321729
IDR: 148321729 | УДК: 621.628 | DOI: 10.31772/2587-6066-2020-21-1-136-141
Текст научной статьи Influence of a constant electric field on the adsorption purification of water from iron ions
Introduction. In the production of space rocket technology, galvanic technologies are used, as a result of which sewage is contaminated with metal ions. Known methods of wastewater treatment do not allow for the maximum permissible concentration of metal ions in treated water, or are expensive or difficult to operate in industry. Iron ions are among the most polluting components of wastewater of most industries. So increased control and the development of effective methods of wastewater treatment are necessary. The development of a method for cleaning industrial wastewater from metals is an actual area of research and assumes the presence of not only a high cleaning result, but also optimal parameters when introduced into the process [1; 2], which includes electricity costs, consumables and processing time.
The methodology of the experiment. The effect of a constant electric field on the adsorption treatment of water from iron ions was studied in a cell using pairs of plate electrodes (fig. 1). The cell is made of a dielectric material in the form of a cylindrical tube in which the anode and cathode are alternately mounted. The electrodes are made of stainless steel 12X18H10T (4 plates 1 mm thick each). The interelectrode distance is 12 and 25 mm. Sorbents (quartz sand or natural zeolites) were poured into the space between the electrodes. The electrodes were connected in parallel to a direct current source. The volume of treated water is 1 liter. A voltage was applied to the terminals of the electrodes and water was passed through in a non-pressure way for 1 minute with stabilization of the current strength of 0.025 A and a voltage at the terminals of the electrodes from 18 to 62 V. Fe (III) salts were dissolved in water at an average ion concentration of 2.5 mg/l. To record the process parameters, standard instruments were used – a voltmeter (accuracy class 0.4), ammeter (0.5).
The degree of purification was determined by the formula, %:
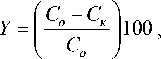
where Со, Ск are the initial and final concentration of the removed metal ion, mg/L.
The specific energy consumption W for purifying a unit volume of water, (kW∙h)/m3, was calculated by the expression:
W = IUr- 10
- 3
V
where I is current, A; U is the voltage at the terminals of the electrodes, V; t tr. – flow interval passing through the treated water, h; V is the volume of filled water, m3; 10–3 is the conversion factor from W to kW.
Research results. Fig. 2 shows the results of purification of water from Fe ions with an initial concentration of 2.5 mg/l at a constant electric current of different tensions with sorbents
In the course of previous experiments at two plants for wastewater treatment [3–5] the main parameters that are most important in water treatment were analyzed: the distance between the electrodes ( L , m ), the voltage at the terminals of the electrodes ( U , V ) and processing time ( T , s ).
Fig. 3 shows the results of the analysis (obtained using the Statgraphics experimental data processing program) of the dependence of the degree of water purification from Fe ions when the time of the action of a constant electric current on quartz sand changes at different voltage on the electrodes.
The temperature of the treated water was in the range from 20 to 22 °C [6; 7] and the dispersion of quartz sand particles was from 0.5–1.2 mm [8–11].
When considering the response surface, it can be seen that the total iron indicators (fig. 3) in purified water are as close as possible to standard values with an increase in processing time [12].
An analysis of the obtained experimental data showed that the efficiency of the electrochemical method using direct electric current and alternating electric current of industrial frequency depends on the interelectrode distance, voltage at the terminals of the electrodes, and processing time. The degree of purification improves with increasing electric field strength [3–5; 13].
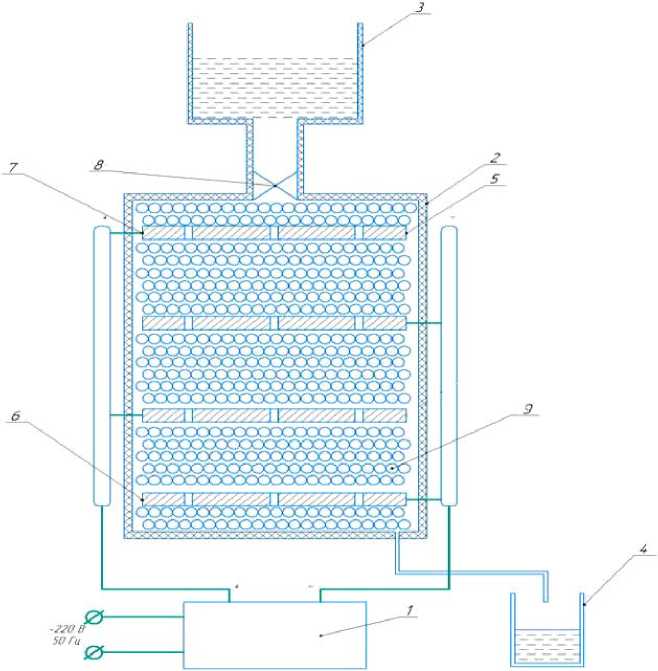
Fig. 1. Experimental setup diagram:
1 – DC power supply with output voltage regulation; 2 – casing cell; 3 – the upper container of water; 4 – purified water tank; 5 – steel electrodes-anodes; 6 – steel electrodes-cathodes; 7 – current leads; 8 – valve (clamp); 9 – adsorbent
Рис. 1. Схема экспериментальной установки:
-
1 – источник постоянного тока с регулированием выходного напряжения; 2 – корпус ячейки; 3 – верхняя ёмкость с водой; 4 – ёмкость для очищенной воды; 5 – стальные электроды-аноды; 6 – стальные электроды-катоды; 7 – токовводы; 8 – вентиль (зажим); 9 – адсорбент
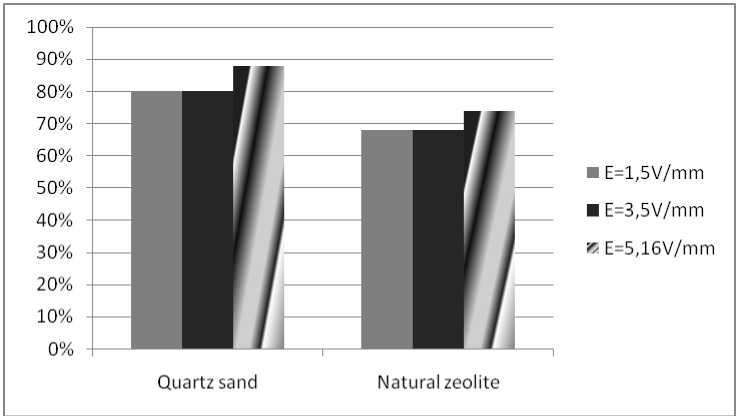
Fig. 2. Results of influence of input parameters of the process on the degree of purification in a plant with direct current and sorbents
Рис. 2. Результаты влияния входных параметров процесса на степень очистки в установке с постоянным током и сорбентами
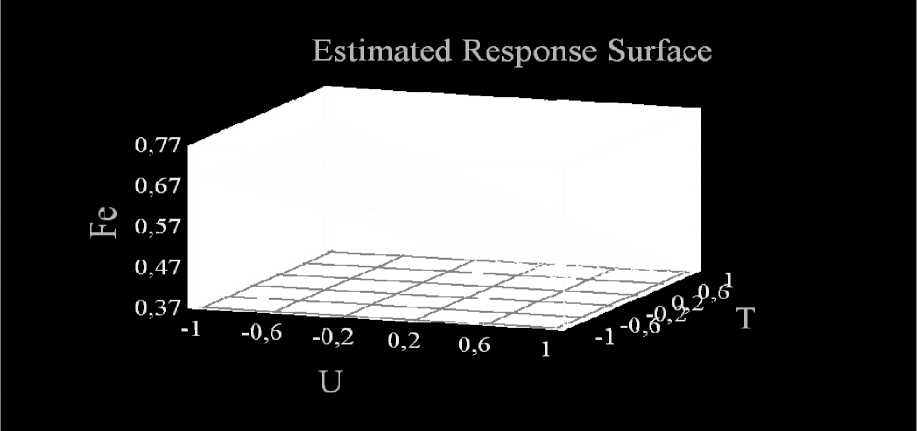
Fig. 3. Dependence of the degree of water purification from Fe ions when changing the time of exposure to direct electric current on quartz sand at different voltages on the electrodes
Рис. 3. Зависимость степени очистки воды от ионов Fe при изменении времени воздействия постоянного электрического тока на кварцевый песок при различном напряжении на электродах
■ L=0,025m □ L=0,012m
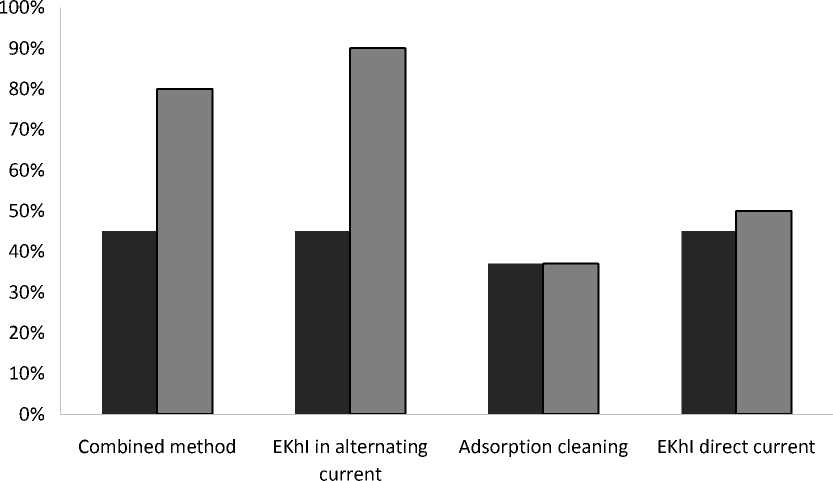
Fig. 4. Results of research on water treatment methods
Рис. 4. Результаты исследований методов очистки воды
At the same voltage ( U = 18 V) the cleaning technology with alternating electric current of industrial frequency turned out to be more effective.
The specific energy consumption and the degree of purification of the electrochemical method based on the use of direct electric current were:
W = 1,5 - 18 ■ 0,0167 10 - 3 = 4,5 . 3
E 0,001
Y = | 2,5 - 0,5 j ioo = 80 % _ at и = 18 v
-
1 I 2,5 J
The results of studies of water treatment methods at a voltage of U = 18 V, processing time t proc. = 60 s, and the initial ion concentration Fe = 2.5 mg/m3 are presented in fig. 4.
Conclusion. The specific energy consumption and the degree of purification of the electrochemical method using alternating electric current of industrial frequency: W = 1.8 (kW∙h)/m3, Y = 99 % [4]. However, this method is based on the introduction of a coagulant after electrochemical exposure and settling of water for eight hours, which reduces productivity and requires additional settling tanks. In the electrochemical process, the formation of iron hydroxide occurred in the volume of water without sorbents, which led to an increase in the turbidity of the water. To extract iron hydroxide, water settling is necessary [4.14].
The combination of electrochemical action and the use of adsorbents made it possible to raise the degree of purification to 80 %, while the specific energy consumption is 4.5 (kW∙h)/m3. An increase in the electric field strength to 5.16 V/mm will allow purifying water from iron ions to the MPC level (0.3 mg/l).
Список литературы Influence of a constant electric field on the adsorption purification of water from iron ions
- Strokach P. P., Electrochem. Ind. Process. Bio. 1975, Vol. 55, P. 375.
- Micka K., Kimla A. Rousar. Electrochemical engineering. 1986. Part I. 337 p.
- Shestakov I. Ya., Raeva O. V. Patent RF 2519383. Sposob ochistki vody i vodnykh rastvorov ot anionov I kationov [A method for purifying water and aqueous solutions from anions and cations]. 2014, 3 p.
- Shestakov I. Ya., Raeva O. V., Nikiforova E. M., Eromasov R. G. Issledovanie ochistki vody elektrokhimicheskim sposobom v nestatsionarnom elektricheskom pole s posleduyushchey koagulyatsiey [The study of water purification by the electrochemical method in an unsteady electric field with subsequent coagulation]. Available at: www.science-education.ru/107-8154 (accessed: 10.03.2014).
- Khilyuk A. V., Rogov V. A., Prusakova V. A. [The effect of the electrostatic field on adsorption during the purification of natural water]. Vestnik KrasGAU. 2013, No. 12, P. 134–137 (In Russ.).
- Kengerli A. D. [Influence of the temperature of the treated water on the process of flocculation]. Tekhn. Tereggi ugrunda. 1972, No. 4, P. 39–40 (In Russ.).
- Kowal A. L., Mackiewicz J. The effect of water temperature on the course of alum coagulation of colloidal particles in water-Environ. Prot. Eng. (PRL). 1975, Vol. l, No. l, P. 63–70.
- GOST R 51641–2000 Materialy fil'truyushchie zernistye. Obshchie tekhnicheskie usloviya [State Standard R 51641–2000 Granular filtering materials. General specifications]. Moscow, IVS URALTEST Publ., 2000.
- Bratilova M. M., Grechushkin A. N. [Investigation of the properties of filter media for water purification from iron]. Universum: Khimiya i biologiya: elektron. nauchn. Zhurn. 2015, No. 6 (14). Available at: http:/7universum.com/ru/natur/archive/item/2185 (accessed: 29.12.2019).
- Tagibaev D. D. [Filtering characteristics of granular filter materials]. Innovatsionnaya nauka. 2017, No. 1-2, P. 90–92 (In Russ.).
- Kuznetsov L. K., Gabitov A. I. [Filtering technology in the physicochemical processes of water treatment]. Bash. khim. zh., 2009, No. 2, P. 84–92.
- GOST 4011–72 Voda pit'evaya. Metody izmereniya massovoy kontsentratsii obshchego zheleza [State Standard 4011–72 Drinking water. Methods for measuring the mass concentration of total iron.]. Moscow, IPK Izdatel'stvo standartov Publ., 1974.
- Khilyuk A. V. [The study of the influence of pollutants and electroactivated water on aquatic organisms]. Reshetnevskie chteniya: materialy XXII Mezhdunar. nauch.-prakt. Konf. [Reshetnev readings: materials of the XXII Intern. scientific-practical Conf.]. Krasnoyarsk, 2018, Vol. 2, P. 66–69 (In Russ.).


