Influence of initial components mechanical activation on the properties of protective enamel coating on the cast-iron surface
Автор: Shimanskiy Igor A., Babkin Vladimir G., Frizorger Vladimir K., Goloskin Evgeniy S., Nabiulin Alexey B.
Журнал: Журнал Сибирского федерального университета. Серия: Техника и технологии @technologies-sfu
Статья в выпуске: 1 т.5, 2012 года.
Бесплатный доступ
The influence of initial components mixture mechanical activation on the synthesis processes in enamel mass and on the molten enamel adhesion to the metal was investigated. Its established, that the process of albite formation in the coating material intensifies by components mixture activation. On the base of measured contact angles of cast iron with molten enamel was revealed, that adhesion of the molten enamel to the metal noticeably increases by initial components activation.
Cast iron, gas manifold, skirts, high temperature gas corrosion, protective coatings, mechanical activation
Короткий адрес: https://sciup.org/146114631
IDR: 146114631 | УДК: 666.293.53
Текст научной статьи Influence of initial components mechanical activation on the properties of protective enamel coating on the cast-iron surface
Electrolysis of aluminum from cryolite-alumina melt produces cell gases (СО, СО2, HF, SO2) and bath evaporation products (NaAlF 4 ). Soderberg cells are equipped with gas collection system the most important element of which is the gas manifold consisting of cast iron skirts. The skirts are fixed on the lower part on the anode casing. Together with alumina cover the manifold forms over the cell perimeter a channel to collect and remove gas. At temperatures up to 1000 К the manifold is subject to gas corrosion by oxygen, water steam and cell gases. This deteriorates the gas skirts in cell operation. Disintegration products which are iron compounds get into the bath and then into aluminum. As a result the grade of aluminum produced from the same raw material in a Soderberg cell is lower (and accordingly, its price) than the aluminum made in cells with prebaked anode.
The methods to protect cast-iron castings from gas corrosion by applying protective coatings on its surface, such as painting, tinning, enameling, electrochemical deposition, spraying etc. are known [1]. General disadvantage of these methods is necessity of metal surface preliminary preparation
including degreasing, etching, machining etc. that defines process multistage and requires special equipment.
Earlier, in papers [2,3] the composition of an albite-based protective enamel coating for gas manifold and new one-step method of its application directly in the casting process were proposed.
The aim of present work is investigation of initial components mixture mechanical activation influence on the formation process and properties of protective enamel coating on the cast iron surface.
Research methods
Element and phase composition of materials under study were studied with X-ray analysis. X-ray patterns were made on automated X-ray equipment XRD-6000 (Shimadzu).
Microstructure of samples was studied with optical microscope Axio Observer А1 (Carl Zeiss) with magnification from 200 to 500.
Dilatometric measures were carried out with device DIL 402 C (Netszch).
Thermal properties of coating were measured with LFA 457 Micro Flash (Netzsch).
Mechanical activation of materials was carried out with Rocklabs ring mill.
Determination of the contact angle of cast iron with molten enamel was carried out with lying drop method using vacuum device “Drop” (Giredmet). Preliminary synthesized enamel material granules were placed on the heated to the desired temperature substrate. After melting and reaching desired temperature the drop was photographed.
Results
In paper [2] we had shown that inert with respect to aluminum cell aggressive gas environment material is albite NaAlSi3O8, that confirmed by Gibbs energy change positive value of its reaction with main corrosion source – hydrogenium fluoride.
NaAlSi 3 O 8 + 4HF = NaF + AlF 3 + 3SiO 2 ,
(1) ∆Go 973 K = +7,5, ∆Go 1073 K = +36,46, kJ.
Albite-based protective coating was made by enamel ceramic mass, its composition is given in Table 1.
Broken glass and alumina were the sources of sodium, silicon and aluminum oxides required to form albite. Quartz sand was the filler, argil was the binder. Copper oxide and sodium fluoride acted as surfactants and flux, respectively.
In paper [3] we had offered one-stage method of applying protective anti-corrosion coating on the cast-iron casting surface directly in the casting process. The proposed method of forming corrosionresistant coatings on cast-iron castings is to deposit a layer of enamel ceramic mass on the casting mold surface. Then the casting mold is filled with cast iron melt overheated to the temperature 150-200 К higher than the melting temperature. Ceramic coating layer is heated due to heat conductivity of the solidifying casting to the melting temperature and cools to turn into a compact enamel corrosionresistant coating.
Table 1. Composition of enamel ceramic mass for protective coating
|
Material |
Content, mass % |
|
Broken glass |
60 |
|
Alumina |
15 |
|
Copper oxide |
1 |
|
Sodium fluoride |
3 |
|
Quartz sand |
17 |
|
Argil |
4 |
In this work the influence of enamel mass initial components activation with a view to intensify albite synthesis and melting processes and increase coating material adhesion to the metal was studied.
Thus proceeded with the fact that joint activation of initial components allows to achieve their even distribution in the mix material volume and leads to mechanical intrusion of one substance to the surface layer of another with the process of chemical interaction between them.
Activation length was 1÷7 minutes. Upper limit is caused by the fact that this time length provided maximum dispersion of the system, above which aggregation of particles was observed.
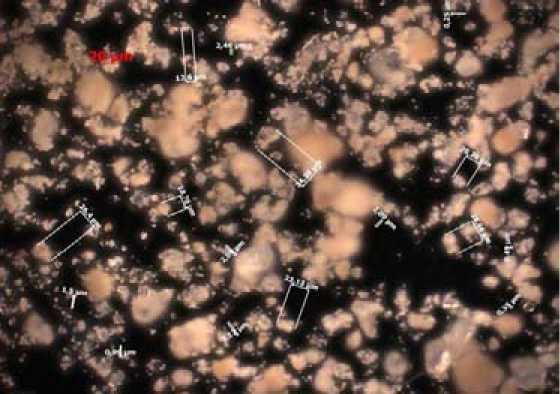
Enamel mass microphotographs in primary and activated conditions are presented on Fig. 1
It’s revealed, that the average particle size in non-activated sample is ~ 90 micron and large particles the size up to 120 micron are present. After components mixture activation during 7 minutes the average particle size decreases to ~ 20 micron and the maximum is ~ 40 micron.
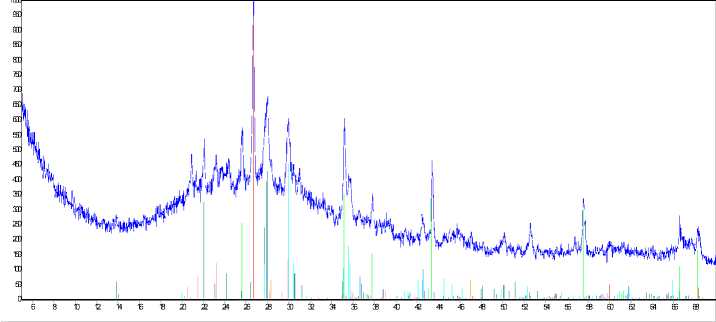
Fig. 2 shows X-ray photograms of activated and non-activated enamel mass samples passed annealing at temperature 1173 K during 40 minutes.
It’s established, that the character of course of phase interactions in investigated system is changed under activation influence. Qualitative difference in the samples composition was not revealed.
But quantitative composition, presented in the Table 2 considerably differs at the same time.
As it follows from experiments results, albite content in the non-activated sample is ~ 35,6 mass %. In the activated sample albite content increasing up to 59,7 mass % was revealed.
So, mechanical activation allows to increase yield of the reaction product during the synthesis under the same conditions significantly, that caused by increasing particles specific surface area and thus increasing their reactivity.
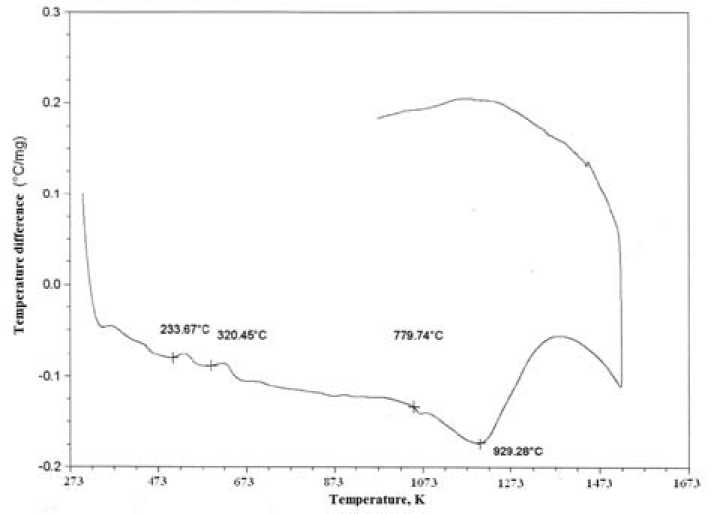
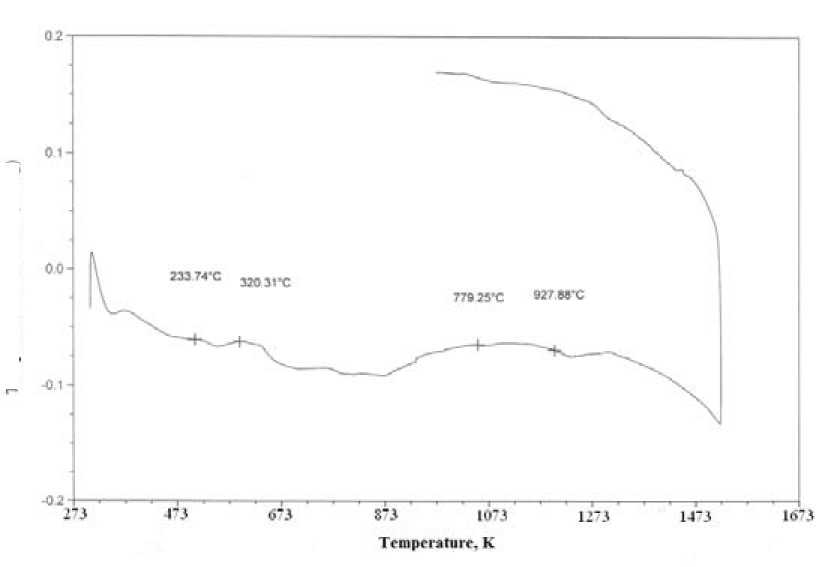
It confirms by the results of differential thermal analysis, presented in Fig. 3, from which follows that the value of the thermal effect at a temperature of 1050 K referred to the albite forming reaction decreases. This fact indicates that the activation energy of chemical transformation in enamel ceramic mass is decreasing.
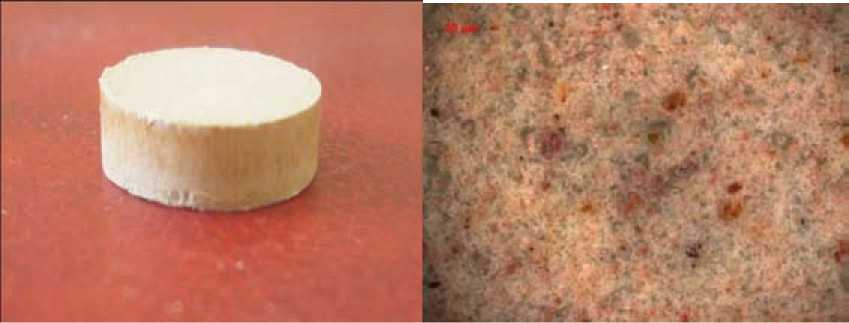
Also it’s revealed that annealing of non-activated components mixture leads to porous and inhomogeneous sample structure. Activation allows to obtain dense and solid samples with more homogeneous structure. Fig.4 shows samples microstructure after annealing.
Samples’ seeming density was defined by hydrostatic weighing method. Its value is 1,75 and 1,95 g/cm3 for non-activated and activated samples respectively.

а)
б)
Fig. 1. microphotographs of powders, ×250: а) non-activated sample; б) sample activated during 7 minutes
Enamel mass mechanical activation may influence not only on the course of phase transformations during coating formation, but it also determines the interaction between enamel melt and cast-iron casting surface, what is possible to judge by adhesion work value.
Adhesion work was calculated with using contact angle measures by Dupre-Young equation:
W a = σ L ∙(1 + сosθ)
where σL – enamel melt surface tension, θ – contact angle.
Enamel melt surface tension was determined by Pavlov-Popel equation:
σ= σ 1 - 2000lg ∑ l k = 1F1x1
а)
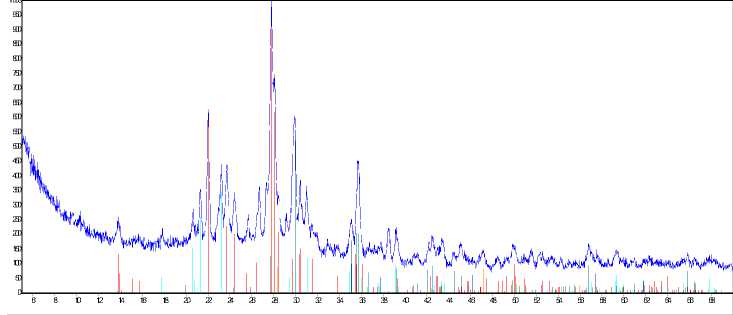
Fig. 2. Results of X-ray phase analysis: а) non-activated sample; б) sample activated during 7 minutes
Table 2. Quantitative composition of samples
|
Main phases |
Content, mass % |
|
|
Non-activated |
Activated during 7 minutes |
|
|
Albite |
35,6 |
59,7 |
|
Diopside |
19,4 |
16,0 |
|
Quartz |
15,8 |
1,84 |
|
Nepheline |
7,76 |
17,5 |
|
Al 2 O 3 |
19,3 |
3,22 |
|
CuO |
0,73 |
0,64 |
а)
Temperature difference Ci'mg
б)
Fig. 3. Results of differential thermal analysis: а) non-activated sample; б) sample activated during 7 minutes

а)
б)
Fig. 4. Samples microstructure: а) non-activated sample; б) sample activated during 7 minutes where σ1 – solvent surface tension (70 mole % SiO2, 30 mole % Na2O – it approximately matches with the content of SiO2 and Na2O in broken glass+quartz sand) which is equal to 282; Fi – the parameter characterizing component’s surface activity; k – quantity of components.
The following enamel composition was accepted in the calculation: (mole %): SiO2 (65), Na2O (10,9), Al 2 O 3 (10,9); CaO (5,7), MgO (2) and others (5,5). Corresponding values of constants F i of enamel melt components are: 1,02; 0,90; 0,65; 0,72; 0,70.
After substitution of σ 1 и F i values equation (3) takes the following form:
σL = 282 – 2000lg (1,02xSiO2 + 0,9xNa2O +
+ 0,65xAl2O3 + 0,72xCaO + 0,70xMgO + ...). (4)
Value of enamel melt σ calculated by equation (4) is 386 mJ/m2. Calculation results are presented in Table 3.
On the base of this data we can conclude that enamel melt forms large contact angles on the cast-iron. It demonstrates rather large value of interfacial tension σSL because of the weak interaction between melt and metal. Decreasing of θ was observed at temperatures upper 1373 K for the melt obtained from – 69 –
Table 3. Mechanical activation influence on the contact angles and adhesion work in the enamel melt-solid cast iron system
Contained in the cast-iron cementite carbon can act like a reducing agent to enamel components placed on the surface layer (Na 2 O, SiO 2 ). Sodium oxide reduction reaction can be presented in following form:
Na 2 O S + Fe 3 C S = 2Na G + 3Fe S + СО G (5)
Balance constant of this reaction depends on substances conditions. Thus the essential role is played by enamel disperse components reducing interaction reaction between melt and metal activation energy. Iron oxidation is possible in the enamel and metal contact area. Iron oxides transfering to enamel melt is also promotes the adhesion work growth.
Conclusion
Enamel mass components mechanical activation leads to intensification of their interaction process with albite formation, to increasing of the enamel melt adhesion to cast-iron casting surface, and to the coating material porosity reduction and density increasing.
Влияние механоактивации исходных компонентовна свойства защитного эмалевого покрытияна поверхности чугунных изделий
И.А. Шиманскийа, В.Г. Бабкина, В.К. Фризоргерб,
Е.С. Голоскинб, А.Б. Набиулина аСибирский федеральный университет, Россия 660041, Красноярск, пр. Свободный 79 бООО «Рус-инжиниринг», Красноярск, Россия,


