Influence of sucrose and cytokinin stress on shoot regeneration in cotyledon culture of oilseed crop black sesame (Sesamum indicum L.)
Автор: Abirami K., Vikrant
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 1 т.20, 2024 года.
Бесплатный доступ
This study involves the evaluation of in vitro shoot regeneration during cotyledon culture in black sesame ( Sesamum indicum L. cv. TMV3) under various concentrations of sucrose and cytokinin (BAP) in Murashige and Skoog (MS) nutrient medium. Cotyledon explants were cultured on MS basal medium supplemented with various concentrations of sucrose (2%, 4%, 6%, and 8%) and combinations of BAP (1.0mg/L, 2.5mg/L, 3.5mg/L, 4.5mg/L, 5.5mg/L, 6.5mg/L, and 7.5mg/L) and IAA (1.0mg/L). The multiple-shoot regeneration was achieved after 5-weeks of culture initiation. Significantly, the maximum mean percentage of shoot regeneration (75±4.3%) and number of shoots/explants (1.3±0.8) was recorded with explants that were treated with MS medium supplemented with sucrose (4%) including BAP (3.5mg/L) and IAA (1.0mg/L) while the high concentration (8%) of sucrose was proved to be completely ineffective to induce shoot regeneration on same nutrient medium (BAP+IAA). Furthermore, results indicate that sucrose (8%) could be observed slightly effective for shoot regeneration with higher concentrations of BAP (6.5mg/L and 7.5mg/L) in presence of IAA (1.0mg/L). Significantly, cotyledon explants that were growing with low concentration of sucrose (2%) were failed to show regeneration if BAP concentration exceeds to (5.5mg/L). Moreover, optimal frequency of shoot regeneration (64.4±1.4%) could be recorded with 2% of sucrose in presence of lower concentration of BAP (2.5mg/L) and IAA (1.0mg/L). The regenerated shoots were further transferred to half strength of MS medium supplemented with various concentrations of IBA (0.5, 1.0, and 1.5mg/L) for induction of roots and complete plantlets formation. Significantly, optimal mean percentage of shoots showing root formation (90±2.8%) was obtained in shoots regenerated with 4% of sucrose in presence of IBA (1.0mg/L) and shoot-root length ratio was recorded to be the maximum (4.2±0.11cm/2.81±0.12cm). However, in contrast, shoots that were regenerated on medium containing high sucrose (8%) with high BAP (7.5mg/L) + IAA (1.0mg/L) were found to exhibit lack of root formation irrespective of IBA concentrations. Present study reports that sucrose and BAP contents of the medium influences the sesame cotyledon explants regeneration. In comparison to control (2%) of sucrose, increase in sucrose concentration (4%) with BAP (3.5mg/L) and IAA (1.0mg/L) proved to be the optimal combination for shoot regeneration and moreover, regenerated shoots could induce the maximum root length in MS1/2 medium supplemented with IBA (1.0mg/L). In contrast, the IBA medium containing very high concentration of sucrose (8%) was turned out to be completely inhibitory for root regeneration. Study indicates that concentration of sucrose and BAP cytokinin in nutrient medium influences considerably the regeneration potential of explants tissues in black sesame oil crop. Moreover, the regenerated plantlets were further gradually acclimatized and transferred to plastic cup soil under the greenhouse conditions.
Cotyledon, cytokinin, oil crop, regeneration, sesame, sucrose
Короткий адрес: https://sciup.org/143182399
IDR: 143182399
Текст научной статьи Influence of sucrose and cytokinin stress on shoot regeneration in cotyledon culture of oilseed crop black sesame (Sesamum indicum L.)
Sesamum indicum L. is an herbaceous annual plant belonging to the pedaliaceae family. Internationally known as sesame, is one of the oldest oilseed crops and is used for its nutritional, medicinal and industrial purposes (Amoo et al ., 2017). The sesame plant, Sesamum spp., is spread throughout the tropical and subtropical areas in Asia, Africa and South America. Sesame seed is known as queen of oil seeds with about 50% oil content and 20% of protein (Das et al ., 2015).
During the last few years, this crop has been in much attention due to its unique lignans such as sesamin, sesamolin, sesamol and tocopherols that are known to have tremendous potential to cure hypertension, obesity and cancer (Zimik and Arumugam, 2017). Although sesame is in cultivation practice for a long time, but the productivity of sesame crop remains restricted caused by biotic and abiotic stresses (Pathak et al ., 2014).
Interestingly, establishment of a suitable regeneration protocol is a primary requirement to carry out genetic transformation study in Sesamum indicum L. (Debnath and Gangopadhyay, 2018). Due to lack of a proper regeneration system, genetic engineering of the crop has yielded no tangible result so far (Taskin et al ., 1999; Al-Shafeay et al ., 2011; Chowdhary et al ., 2014; Yadav et al ., 2010; Zimik and Arumugam, 2017). Moreover, lack of efficient regeneration protocol further limits genetic engineering of this crop for developing improved lines (Zimik and Arumugam, 2017).
Significantly, previous efforts have been also made to regenerate sesame under in vitro conditions using explants such as hypocotyls (Younghee, 2001; Baskaran and Jayabalan, 2006; Were et al ., 2006) and cotyledons (Kim et al ., 2001; Were et al ., 2006; Yadav et al ., 2010; Malaghan et al ., 2013; Chowdhary et al ., 2014; Zimik and Arumugam, 2017; Anandan et al ., 2018). Simultaneously, remarkable success has also been seen for plant regeneration through somatic embryogenesis using hypocotyl or cotyledon as explants (Mary and Jayabalan, 1997; Younghee, 2001; Honnale and Rao, 2013).
Although during previous studies, regeneration protocols have been established from different parts of seedlings or explants in sesame crop but sesame tissues have been proved to be relatively recalcitrant to in vitro regeneration and thus, restricting the use of established regeneration protocols for its genetic improvement (Zimik and Arumugam, 2017).
As per literature, protocol for shoot regeneration from the cotyledon explant was established by using different genotypes of sesame shoot regeneration but so far developed regeneration protocol has been proved to be unsuitable for transformation of any of the Indian varieties of sesame. It is known that the genotype of a crop strongly influences shoot regeneration efficiencies (Mary and Jayabalan, 1997).
Moreover, carbohydrates play various essential roles in plants by controlling developmental processes (Gibson, 2000; Smeekens, 2000). Plant cell, tissue or organ culture normally requires the incorporation of a carbon source to the culture medium (Karhu, 1997). The nutritional importance of an adequate carbon source in a culture medium is widely known. However, the addition of medium components, especially macronutrients and carbon sources, represent considerable decrease in the medium osmotic potential (George, 1993; Deljou et al ., 2007).
The use of sucrose and the concentration of sucrose is an important factor during in vitro propagation studies. It was previously explained that the plants cultivated display different regeneration characteristics depending on the sucrose concentrations (Vinterhalter and Vinterhalter, 1999; Gabryszewska, 2015; Dogan, 2019, 2020).
During present study on black sesame ( Sesamum indicum L.), various concentrations of sucrose and BAP cytokinin were employed to evaluate the stress response of sucrose contents and BAP in the nutrient medium that affects the cotyledon explants to show morphogenic response under In Vitro conditions. Present study further reveals that the composition of the culture media modified with the contents of sucrose and IBA helps in enhancing the root regeneration frequency.
MATERIALS AND METHODS
Seed Collection and Sterilization
The mature seeds of black sesame (Sesamum indicum L. cv.TMV3) were collected from Tamil Nadu Agriculture University, Coimbatore (India) and were used as source of explants. The healthy seeds were selected and washed with running tap water for 3-4 times, followed by soaking in tween-20 for 10 minutes and then continuously rinsed with distilled water for 5-6 times.
Furthermore, washed seeds were treated with 70% ethanol for one minute followed by washing with sterile distilled water (SDW) for three times. These seeds were further treated with mercuric chloride (0.1%) for 10 minutes and again repeatedly washed 4-5 times with sterile distilled water.
The sterilized seeds were then kept under soaking conditions in sterile distilled water for 3h. Further, de-embryonated cotyledons were excised from soaked seeds and used as explants inoculated in 15ml culture vial containing 5-10ml of nutrient medium.
Nutrient Medium Formulations
In order to evaluate the stress response of sucrose and BAP contents in the nutrient medium for regenerations from cotyledon explants, MS (Murashige and Skoog, 1962) basal medium was supplemented with various concentrations of BAP (1.0mg/L, 2.5mg/L, 3.5mg/L, 4.5mg/L, 5.5mg/L, 6.5mg/L, and 7.5mg/L) and IAA (1.0mg/L) as growth hormones and sucrose (2%, 4%, 6%, and 8%) as source of carbohydrate. After 3-4 weeks of culture initiation, explants were sub-cultured to MS nutrient medium with same composition of BAP (1.0mg/L - 7.5mg/L) along with IAA (1.0mg/L) and respective sucrose concentrations (2%-8%).
Furthermore, MS basal medium was supplemented with agar (0.8%) and the pH of the nutrient medium was adjusted to 5.5–5.8 with 1N (NaOH or HCl). The nutrient media were further autoclaved at 1210C for 20mins. All the cultures were maintained in 16/8 (light/dark) hrs photoperiod for 40-45 days.
Rooting and Transplantation
The regenerated shoots formed during cotyledon cultures were transferred to MS ½-nutrient medium supplemented with various concentrations of IBA (0.5mg/L, 1.0mg/L, and 1.5mg/L) and supplemented with respective concentration of sucrose for the root initiation. These plantlets with roots were gradually acclimatized in plastic cups containing sterilized mixture of red soil (20%), vermiculite (40%), and compost (40%) for hardening under green house conditions.
Statistical Analysis
At the end of sixth weeks, frequencies of the shoot and root regeneration and length of roots/shoots were recorded and calculated. A minimum of 25-30 explants were used for the treatments and each experiment was repeated at three times. The mean frequency of shoot regeneration and percentage of shoots showing root formation was recorded. The data were analyzed based on SPSS software.
Number of Shoot forming Cultures Frequency of Shoot Regeneration (%) = ---------------------- x 100
Total number of explants cultured
Number of Root forming Shoots Frequency of Root Regeneration (%) = -----------------------x 100
Total number of
Regenerated Shoots
Root length: Length of the root of all regenerated shoots from each replication was measured and from these values, the mean was calculated as the root length (cm).
Shoot length: Shoot lengths of all the regenerated shoots were calculated. Moreover, from these values, the mean was taken as the shoot length (cm).
RESULTS
Results indicate that the frequency of shoot regeneration from cotyledon explants was strongly influenced by increased concentrations of sucrose as sucrose stress and combination of increased concentrations of BAP as cytokinin stress in the MS-nutrient medium.
Effect of Sucrose Stress on Shoot Regeneration
Within a week of culture, the white cotyledons started turning green and became swollen all through, with some of them showing more proliferative activities at the cut ends ( FIG. 1A & B ). By the end of third week, the explants showed appearance of leafy structures and young shoot-buds developing from the proximal cut end of the cotyledons ( FIG. 1C ).
Moreover, cotyledon explants that were growing with 2% of sucrose started to show shoot regeneration at the end of 3rd week of culture initiation while with very high concentration of sucrose (8%), explants could show merely proliferation in explants tissues within one week of culture ( FIG. 1B) . However, at the end of 3rd week of culture initiation, such explants could show slight regeneration potential ( FIG. 1F).
In contrast, sucrose concentration (4%) was found to be very effective to support shoot regeneration and significantly maximum potential of multiple shoot regeneration could be observed from cotyledon explants ( FIG. 1D ) while further increase in sucrose concentration (6%) was turned out to be inhibitory and at the end of 3rd week of culture initiation, number of shoots regenerating from cotyledon was obtained relatively low ( FIG. 1E ).
Thus, results indicate that once sucrose concentration increases in nutrient medium, potential of shoot regeneration from cotyledon tissues also increases and therefore (4%) of sucrose proved to be the optimal concentration for multiple shoot regeneration and further increase in sucrose concentrations (6% and 8%) were proved to be gradually inhibitory in comparison to control sucrose concentration (2%).
Significantly, cotyledon explants at an early stage of organogenesis revealed that the leafy structures develop from around the periphery of the cut end of the cotyledon. Furthermore, at the end of 3 weeks of culture initiation, these structures developed into leafy shoots ( FIG. 1D-F ). Among the various treatments of sucrose concentrations applied, 4% sucrose was found to be the optimal concentration of sucrose for inducing shoot regeneration in comparison to other sucrose concentrations (2%, 6%, and 8%).
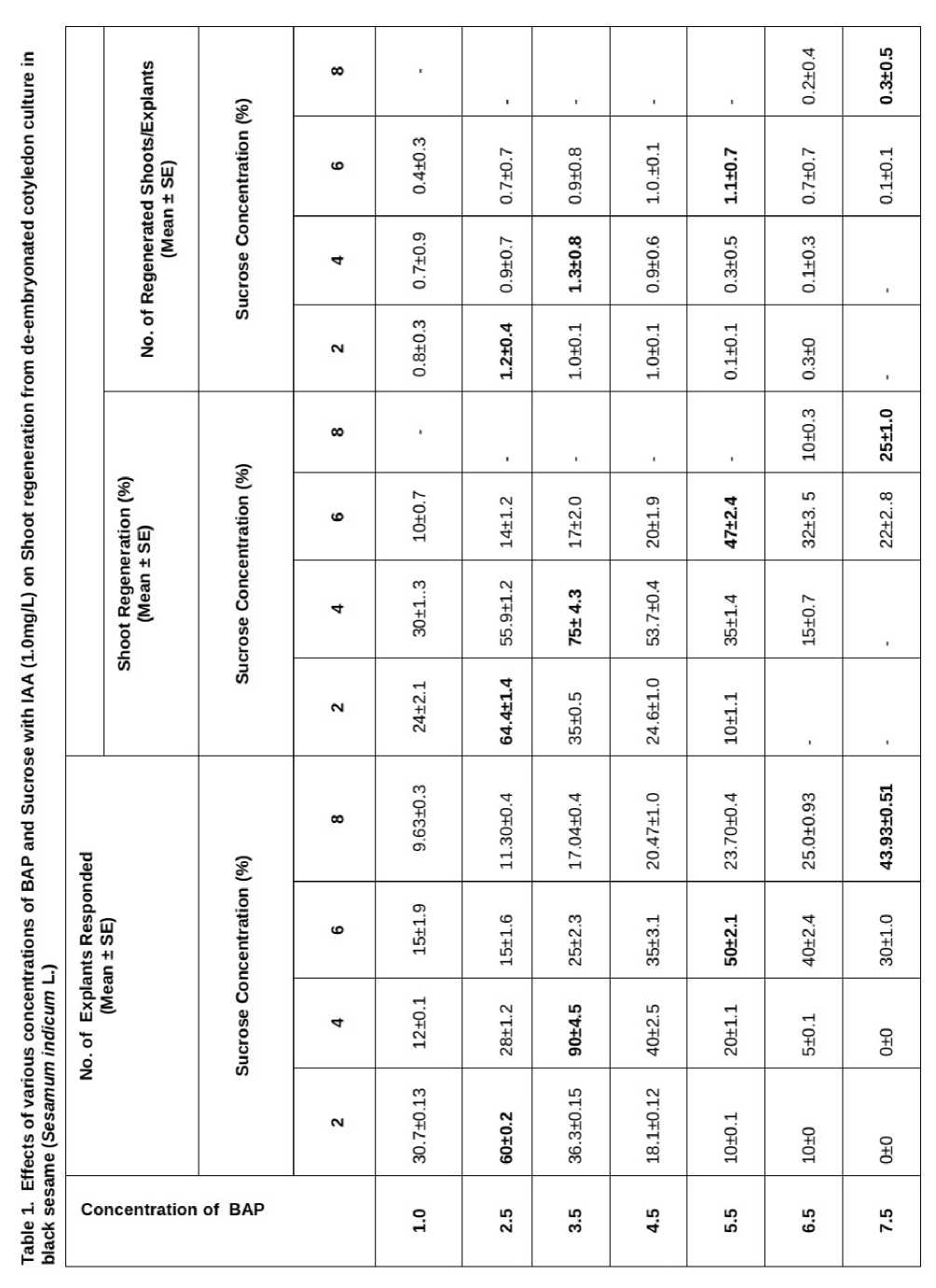
It was observed that different concentration of sucrose (2%, 4%, 6%, and 8%) with respect to the shoot regeneration frequency. Moreover, with the increase in sucrose concentration (2% and 4%) was recorded to be variable and obtained as (64.4±1.4% and 75±4.3%) respectively. However, further increase in sucrose concentration (6%) was turned out to be slightly inhibitory and therefore, shoot-regeneration frequency was recorded as (47±2.4%).
Significantly, continuous maintenance of culture on 8% of sucrose resulted in browning of cotyledons, however, 8% sucrose could show poor frequency (25±1.0%) of shoot regeneration if explants were treated with high concentration of BAP (7.5mg/L) in combination with IAA (1.0mg/L). Moreover, maximum number of shoots per explants (1.3±0.8) was recorded with 4% sucrose treatment followed by 2% of sucrose (1.2±0.4) ( Table 1 ).
Hence, results reveal that continuous presence of high concentration of sucrose (8%) was proved to be strongly inhibitory for shoot regeneration from cotyledon explants. Moreover, low frequency of shoot regeneration with 8% of sucrose could be observed but with high concentration of BAP (6.5mg/L and 7.5mg/L). In contrast, high concentrations of BAP (6.5mg/L and 7.5mg/L) were found to be strongly inhibitory shoot regeneration was found to be completely lacking in presence of low concentration of sucrose (2%).
Effects of BAP Stress on Shoot Regeneration
The cotyledon explants were started to turning greenish and swollen although, with some of them showing more proliferation after 2nd week with low concentration (2.5mg/L) of BAP along with IAA (1.0mg/L) ( FIG. 1A ). At the end of the third weeks of culture initiation showing shoot buds developing from the cotyledon explants ( FIG. 1C-F ). These shoot buds later developed into leafy shoot by the fourth week ( FIG. 2A-C ). Significantly, in terms of shoot elongations, the lower concentrations of BAP (2.5mg/l and 3.5mg/L) along with IAA (1.0mg/L) were proved to be more effective and exhibited elongated shoot regeneration ( FIG. 2A & B ) in comparison to the shoots ( FIG. 2C ) that were growing with higher concentrations of BAP (5.5mg/L and 6.5mg/L). Moreover, very high concentration of BAP (7.5mg/L) along with IAA (1.0mg/L) was turned out to be inhibitory for shoot regeneration and shoot elongation both and therefore, regenerated shoot was observed to be suppressed growth ( FIG. 2D).

Figure 1: Effect of sucrose and BAP cytokinin on shoot regeneration from cotyledon culture in Sesamum indicum L.: (A) Sucrose (2%) + BAP (2.5mg/L) + IAA (1.0mg/L) (B) Sucrose (8%) + BAP (7.5mg/L) + IAA (1.0mg/L) after 1st week of culture initiation (C) Sucrose (2%) + BAP (2.5mg/L) + IAA (1.0mg/L) (D) Sucrose (4%) + BAP (3.5mg/L) + IAA (1.0mg/L) (E) Sucrose (6%) + BAP (5.5mg/L) + IAA (1.0mg/L) (F) Sucrose (8%) + BAP (7.5mg/L) + IAA (1.0mg/L) – cotyledon explants show slight callusing with multiple shoot regeneration in 3-week-old cultures.

Figure 2. Effect of sucrose and BAP cytokinin on plantlet formation from cotyledon culture in Sesamum indicum L.: (A) Sucrose (2%) + BAP (2.5mg/L) + IAA (1.0mg/L) (B) Sucrose (4%) + BAP (3.5mg/L) + IAA (1.0mg/L) (C) Sucrose (6%) + BAP (5.5mg/L) + IAA (1.0mg/L) (D) Sucrose (8%) + BAP (7.5mg/L) + IAA (1.0mg/L) - after 4th week of culture initiation showing elongation of regenerated shoots (E) Plantlet with root initiation after 6th week of culture (F) Regenerated plantlet on transfer to plastic cup soil condition.

|
£ С о ? с ф о с о о я о со |
со |
оо О +1 о |
с 5 с |
о +1 см см о |
||||
|
€ и a +i C c — О O -С с/) |
о |
см о +1 о |
О CD О см |
см 00 о СП о |
||||
|
^ |
см со о й о |
+1 см ^ |
см 00 о 9 |
|||||
|
см |
о с 4 С |
) 5 э |
о о +1 со |
О +1 со |
||||
|
£ о Е с о о о ф о о 3 ел |
со |
О |
о |
о |
||||
|
со |
со о +1 СМ CD |
о с 8 V |
о 00 00 о |
|||||
|
ф а |
’2 S 5 |
'Г |
00 О 6 |
О с + а см |
со о +1 05 о |
|||
|
см |
со о +1 |
см о |
^ о •н см |
|||||
|
ев |
£ С о 2 С ф с о О ф z О О я |
со |
о |
о |
о |
|||
|
О о IX о |
СП 2 |
(О |
а с* 4 С* |
к 3 |
't-со я со 'Г |
см о см см |
||
|
't |
И -н 05 |
со см +1 о О) |
00 +1 о CD |
|||||
|
о |
см |
+1 'Т р |
5 с ст |
ю |
д^ |
|||
|
< га “ Е -1 |
из О |
о |
in |
|||||
|
wi 2 О |
«ч |
см |
со |
|||||
Furthermore, BAP concentrations (2.5mg/L and 3.5mg/L) were emerged as the optimal concentrations for the regeneration of shoots from cotyledon explants.
Moreover, maximum mean percentage (75±4.3% and 64.4±1.4%) of shoot regeneration was recorded with (3.5mg/L and 2.5mg/L) of BAP-treatments respectively
( Table 1 ). Significantly, highest frequency of shoot regeneration (75±4.3%) and maximum number of shoots per explants (1.3±0.8) could be possible with application of BAP (3.5mg/L) along with IAA (1.0mg/L) at 4% of sucrose as carbohydrate source. Frequency of shoot regeneration declined when the BAP concentration was higher than 3.5 mg/L in the presence of IAA.
The result indicated that concentration of BAP (3.5mg/L) with IAA (1.0mg/L) in presence of sucrose (4%) is suitable for shoot regeneration and proved to be the most effective treatment both in terms of percentage of explants showing shoot regeneration as well as number of shoots per cotyledon explant.
Effect of IBA on Induction of Root formation
During present study, regenerated shoots from cotyledon cultures were transferred to the rooting medium after fifth week of culture initiation. To begin with, regenerated shoots formed on regeneration medium were separated into individual shoots. These shoots regenerated on various concentrations (2%, 4%, 6%, and 8%) of sucrose and BAP-IAA combinations were transferred to half-strength MS medium supplemented with various concentrations (0.5mg/l, 1.0mg/L, and 1.5mg/L) of IBA and sucrose (2%, 4%, 6%, and 8%) could show the root induction within a week of culture ( FIG. 2E ).
Of the three concentrations (0.5mg/L,1.0mg/L, and 1.5mg/L) of IBA tested for rooting, maximal response in terms of root forming shoots (90±2.8%) was observed with 4% of sucrose and 1.0mg/L of IBA treatments ( Table 2 ). Moreover, this medium was also proved to be highly effective for plantlets heights and root-shoot length ratio (2.81±0.12cm/4.2±0.11cm) was recorded to be the maximum.
Acclimatization and Transplantation to Soil
The rooted plants were successfully transferred to disposable plastic cup soil through a step of gradual hardening ( FIG. 2F ). These plantlets with roots and shoots were gradually acclimatized in disposable plastic cups containing sterilized mixture of soil (20%), vermiculite (40%), and compost (40%) for hardening under green house conditions.
DISCUSSION
In vitro study on Sesame regeneration has been recorded for almost past three decades, however, a suitable regeneration protocol is lacking even today. During previous study, among several explants tested, shoot tip has been found the most popular one used for regeneration with a maximum of 70% response and with an intensity of 14 shoots/explants (George et al ., 1987). However, the most preferred mode of shoot regeneration suitable for genetic engineering of a crop is the formation of shoots de novo from the explant rather than from the pre-existing shoot tips or shoot buds (Zimik and Arumugam, 2017).
Juvenile explants are found to be the most totipotent explants tissue for induction of de novo shoot regeneration. In sesame seed, cotyledons are the only visible juvenile portion that could be dissected and cultured. Therefore in the previous studies, only cotyledons directly excised from the sterilized and soaked seeds were used as explants. De novo shoot regeneration has been observed when de-embryonated cotyledon was used as explant. But the regeneration frequency was so low that they fail to meet the requirement of genetic engineering of the crop for agronomic traits (Chowdhary et al ., 2014; Seo et al ., 2007; Were et al ., 2006; Younghee, 2001; Zimik and Arumugam, 2017).
Effect of Sucrose Stress on Shoot Regeneration
In general, sucrose is considered as an important carbon and energy source in plant tissue culture as it is the most common carbohydrate found in phloem sap and involved in controlling various development processes (Gibson, 2000). Moreover, supply of sucrose is necessary because the in vitro culture condition restricts the synthesis of carbohydrate necessary for growth and development of plants. Sucrose concentration of 3.0% is the most commonly used carbohydrate source in tissue culture (Baque et al ., 2011).
It is further suggested that starch grains in tissues accumulate during early shoot-meristem formations which are later utilized during shoot differentiation process (Ross et al., 1973). In contrast, higher concentration of sucrose might result in higher uptake of sucrose in explants which favours the formation of shoots (Seo et al., 2007; Zimik and Arumugam, 2017).
During previous study in Bacopa monnieri (L.), nodal and leaf explants were cultured to a culture medium with sucrose added at a level of 0.5-10% and determined the maximum number of shoots in nodal explants as 22.6 ± 0.31 shoots/explant in cultures with 5% sucrose and 20.6 ± 0.35 shoots/explant in cultures with 3% sucrose in leaf explants (Srivastava et al . 2017).
In addition, it was reported that there was no shoot exodus in cultures containing the highest level of sucrose (10%). In contrast of it, the effect of different sucrose concentrations (0-6%) on shoot regeneration of the B. monnieri plant from leaf explants and reached the highest number of shoots (79.00±2.30 shoots/explant) at 2% of sucrose concentration (Naik et al ., 2010). They recorded the least number of shoots in the control group (6.66±0.24) and in application of 1% of sucrose (22.25±0.13).
Furthermore, it is reported that the application of 3% sucrose was the most suitable concentration for the production of Asparagus densiflorus (Kunth) Jessop cv Sprengeri (Rasheed and Yaseeen, 2013). These results revealed that the sucrose concentration in nutrient media was important for the regeneration properties of explants and may show different results depending on the plant species (Dogan, 2020).
During present study, sucrose concentration in the nutrient medium was proved to be highly effective to influence the cotyledon regeneration in terms of percentage of explants showing multiple shootregeneration and number of shoots per explants in sesame oil crop.
In control experiment with sucrose concentration (2%), frequency of shoot regeneration (64.4±1.4%) was obtained while the shoot regeneration percentage was recorded to be the maximum (75±4.3%) with higher concentration (4%) of sucrose in the nutrition medium. Significantly, the number of shoots per explants was also found to be the highest (1.3±0.8) with 4% of sucrose treatment in comparison to control (1.2±0.4) with 2% of sucrose concentration.
However, results in present study reveal that very high concentration of sucrose (8%) was turned out to be strongly inhibitory and shoot regeneration from cotyledon culture was completely lacking. Similar inhibitory response of high concentration (10%) of sucrose treatment in Bacopa monnieri (L.) was observed on shoot regeneration in nodal and leaf explants cultures (Srivastava et al . 2017).
In another study, young stem parts of Lilium longiflorum Thunb. were cultured in a nutrient medium containing different sucrose concentrations (10-30g/L) and achieved a high number of shoots and best regeneration in cultures with 30g/L sucrose (8.9±0.2 shoots/explant). The least number of shoots were detected in 10g/L sucrose application (2.2±0.1 shoots/explant) (Nhut et al ., 2001).
It has been observed that sugar concentration affects the formation of somatic embryos in culture medium (Kamada et al., 1989; Gray et al., 1993; Lou and Kako, 1995; Lou et al., 1996; Nakagawa et al., 2001). Among the many available carbon sources, sucrose has been the most tested carbon source and osmoticum for somatic embryogenesis in plant species (Fuentes et al. , 2000).
Moreover, during anther culture, a higher concentrations of sucrose (4-6%) has been used as a carbon source in callus induction medium to regulate the osmotic pressure, stimulate dedifferentiation during growth induction and to prevent callus formation from somatic anther tissue with respect to the number of embryo-like structures (Cristea et al ., 2012; Dewi et al ., 2017, 2020).
Effect of BAP Cytokinin Stress on Shoot Regeneration
It is established fact that cytokinins in general favour shoot formation. However, the response of an explant to externally supplied hormones depends on the endogenous level of hormone present in the explants. During this study, we report that the BAP cytokinin and IAA combinations and concentrations determine the hormone equilibrium to favour optimal shoot regeneration in black sesame oil crop.
In previous studies, explants cultured on MS media enriched with 2.0mg/L BAP and 0.5mg/L NAA give the high frequency of shoot per explant, as compared to the MS supplemented with BAP (1.0mg/L) and 0.05mg/L of NAA (Sayem et al., 2010). Similarly, the optimum combination of PGRs for adventitious shoot regeneration of sesame was reported in MS with BAP + IAA (Were et al., 2006; Seo et al., 2007; Al-Shafeay et al., 2011). A very recent study reported that the maximum number of young shoots appeared on MS medium with (0.5mg/L) of BAP (Asad et al., 2020).
Furthermore, node and internodes of the blackberry ( Rubus spp . L.) were cultured in a nutrient medium containing 1.0mg/L of BAP and 10-40 g/L of sucrose and obtained the best regeneration values at a concentration of 20g/L sucrose (Ayub et al. , 2019; Dogan, 2020).
Both set of cultures are getting lowest frequency 10% of shoot regeneration with equal concentration 1.0mg/L of BAP and 1.0mg/L of IAA. Similarly the best shoot number was observed on wild species of Sesamum indicum L., cultured on higher concentration of hormones (Dasharath et al ., 2007).
In another study, calli from a medium with 6.5% sucrose increase green plant regeneration in all genotypes that did not produce any green plant at 6% (Control) and 7% sucrose. Another interesting finding was recorded 6.5% sucrose in callus induction medium also minimized the frequency of albino plants from anther-derived calli compared to control (6.0% sucrose) and 7% sucrose (Dewi et al ., 2020).
In this study results reveal efficacy of sucrose concentrations in nutrient media from cotyledon derived into direct regeneration of sesame ( Sesamum indicum L.). The highest frequency of shoot regeneration (75± 4.3%) on 4% sucrose with 3.5mg/L of BAP along with IAA (1.0mg/L) while BAP (6.5mg/L and 7.5mg/L) with IAA (1.0mg/L) were found to be ineffective for shoot regeneration with 2% of sucrose.
However, same combinations of BAP and IAA were seen to support shoot regeneration with 8% of sucrose indicating that high concentration BAP could be little effective to induce shoot regeneration if sucrose content in the nutrient medium is high (8%). Similarly, the maximum number of shoot regeneration (1.3±0.8) was observed with 4% of sucrose treatment along with 3.5mg/L of BAP and IAA (1.0mg/L).
Root Regeneration and Transplantation
Regenerated adventitious shoots formed in BAP and IAA supplemented medium with various concentrations of sucrose added medium were separated into individual shoots. The optimal mean number of shoots showing root formation (90±2.8%) was observed in medium containing IBA (1.0mg/L) with sucrose (4%).
Significantly, the maximum length of root and shoot was obtained with IBA (1.0mg/L) with sucrose (4%), thus, root-shoot length ratio was obtained as (2.81±0.12cm/4.2±0.11cm) while other lower and higher concentrations of IBA was recorded to be little effective for root formation. Interestingly, shoots that were regenerated with 8% of sucrose added with high BAP (6.5-7.5mg/L) were found to fail to form root initiation.
Shoot regenerated plants were later transferred into root induction media to induce rooting in regenerated young shoots. These plantlets with roots and shoots were gradually acclimatized in disposable plastic cups containing sterilized mixture of soil (20%), vermiculite (40%), and compost (40%) for hardening under green house conditions. In present study, we therefore, established a regeneration system through adventitious shoot formation from excised cotyledons of black sesame and were able to produce normal healthy plantlets. This protocol can be suitably further used in several gene transformation studies for black sesame crop improvement.
CONCLUSION
Sucrose as carbon source and BAP cytokinin as plant growth regulators are vital factors for in vitro regeneration of black sesame ( Sesamum indicum L.). The highest regeneration potential of cotyledon explants was achieved with the application of 4% sucrose added with BAP (3.5mg/L) and IAA (1.0mg/L), which was higher than the control with sucrose (2%). The results showed that sucrose doses were important for multiple in vitro productions of plants. This study also showed that high concentration of sucrose (8%) was proved to be completely ineffective to support shoot regeneration.
Additionally, regenerated shoots supported maximum root length on transfer to MS medium added with IBA (1.0mg/L) and sucrose (4%). Moreover, the shoot and root lengths were measured and recorded, 4% of sucrose with IBA (1.0mg/L) showed the maximum shoot/root length. Furthermore, regenerated plantlets were gradually acclimatized and transferred to plastic cup under green house conditions. Hence, results indicate that 4% of sucrose could be helpful for best shoot regeneration from the cotyledon explants in comparison to 2% of sucrose in sesame.
ACKNOWLEDGEMENTS
Список литературы Influence of sucrose and cytokinin stress on shoot regeneration in cotyledon culture of oilseed crop black sesame (Sesamum indicum L.)
- Al-Shafeay, A.F., Ibrahim, A.S., Nesiem, M.R., and Tawfik, M.S. (2011). Establishment of regeneration and transformation system in Egyptian sesame (Sesamum indicum L.) cv Sohag 1. GM Crops, 2, 182-192.
- Amoo, S.O. and Venter, S.L. (2017). In medicinal spieces and vegetables from Africa. (549-579) ELSEVIER chapter 20.
- Anandan, R, Prakash, M., Deenadhayalan, T., Nivetha, R., and Sumanth Kumar, N. (2018). Efficient in vitro plant regeneration from cotyledon-derived callus cultures of sesame (Sesamum indicum L.) and genetic analysis of True-to-Type regenerants using RAPD and SSR markers. South African Journal of Botany, 119, 244-251.
- Asad, M., Ahmed, N., Sohail, A., Burni, T., Hadi, F., Ali, R.....& Muhammad, A. (2020). 35. In vitro shoots multiplication from nodal explants of Sesame (Sesamum indicum L.). Pure and Applied Biology (PAB), 9(1), 303-308.
- Ayub, R.A., Santos, J.N.D., Zanlorensi Junior, L.A., Silva, D.M.D., Carvalho, T.C.D., and Grimaldi, F. (2019). Sucrose concentration and volume of liquid medium on the in vitro growth and development of blackberry cv. Tupy in temporary immersion systems. Ciência e Agrotecnologia, 43. e007219.
- Baque, M. A., Shin, Y. K., Elshmari,T., Lee, E. J., and Paek, K. Y. (2011). Effect of light quality, sucrose and coconut water concentration on the microporpagation of Calanthe hybrids ('Bukduseong' x 'Hyesung' and 'Chunkwang' x 'Hyesung'). Aust. J. Crop Sci, 5 (10), 1247-1254.
- Baskaran, P. and Jayabalan, N. (2006). In vitro mass propagation and diverse callus orientiation on Sesamum indicum L. an important oil plants. Journal of Agricultural Technology, Vol. 2, 259-269.
- Chowdhary, S., Basu, A., and Kundu, S. (2014). A new high-frequency Agrobacterium-mediated transformation technique for Sesamum indicum L. using de-embryonated cotyledon as explant. Protoplasma, 251 (5), 1175-1190.
- Cristea, T. O., Leonte, C., Prisecaru, M., Brezeanu, C., and Brezeanu, M. (2012). Lucrari §tiin\ifice seria Agronomie, 55, 169.
- Das, R. and Bhattacharjee, C. (2015). In processing and impact on active components in Food. Science Direct, Chapter 46, 385-394.
- Dasharath, K., Sridevi, O., Salimath, P., and Ramesh (2007). Production of in terspecific hybrids in sesame through embryo rescue. Indian Journal of Crop Science, Vol. 2, 193-195.
- Debnath, A.J., Gangopadhyay, G., Basu, D., and Sikdar, S.R. (2018). An efficient protocol for in vitro direct shoot organogenesis of Sesamum indicum L. using cotyledon as explant, 3 Biotech, Vol. 8, p. 146.
- Deljou, A., Karami, O., and Ahmadi, O.P. (2007). Effect of sucrose concentration on somatic embryogenesis in carnation (Dianthus caryophyllus L.). Journal of Applied Horticulture, 9(1), 77-80.
- Dewi, T., Safitri, H., and Purwoko, B.S. (2020). Effect of sucrose on callus induction and green plantlet regeneration in anther culture of Indica x Indica rice. IOP Conf. Series: Earth and Environmental Science, 484, 012023
- Dogan, M. (2019). In vitro shoot regeneration performance of Pogostemon erectus (Dalzell) Kuntze in culture medium containing different sucrose concentrations. International Journal of Eastern Mediterranean Agricultural Research, 2(1), 1-12.
- Dogan, M. (2020). The effects of different sucrose concentrations on the regeneration area of Riccia fluitans l., a medicinal aquatic plant. Journal of Engineering Technology and Applied Science, Vol. 5, (2), 51-58.
- Fuentes, S.R.L., Calheiros, M.B.P., Manetti-Filho, J., and Vieira, L.G.E. (2000). The effects of silver nitrate and different carbohydrate sources on somatic embryogenesis in Coffea canephora. Plant Cell, Tiss. Org. Cult, 60, 5-13.
- Gabryszewska, E.A. (2015). Effect of different sucrose and nitrogen salt levels in the medium and temperature on in vitro propagation of Helleborus niger L. Acta Agrobotanica, 68(2), 161-171.
- George, E.F. (1993). Plant Propagation by Tissue Culture. Part 1. The Technology, 2nd ed. Exegetics Ltd., Edington.
- George, L., Bapat, V., and Rao, P. (1987). In vitro multiplication of Seame through tissue culture. Annals of Botany, Vol. 60 (1), 17-21.
- Gibson, S.I. (2000). Plant sugar-response pathways: Part of a complex regulatory web. Plant Physiol., 124, 1532-1539.
- Gray, D.J., Mcolley, D.W., and Compton, M.E. (1993). High frequency somatic embryogenesis from quiescent seed cotyledons of Cucumis melo cultivars. J. Am. Soc. Hort. Sci., 118, 425-465.
- Honnale, H. and Rao, S. (2013). Direct Somatic Embryogenesis in Sesamum indicum (L.) Cv-E8 from Cotyledon and Hypocotyl Explants. Int J Appl Biol Pharm Technol, 4 (2), 120-127.
- Kamada, H., Kobayashi, K., Kiyosue, T., and Harada, H. (1989). Stress induced somatic embryogenesis in carrot and its application to synthetic seed production. In Vitro Cell Dev. Biol., 25, 1163-1166.
- Karhu, S.T. (1997). Sugar use in relation to shoot induction by sorbitol and cytokinin in apple. J. Am. Soc. Hort. Sci., 122, 476-480.
- Kim Y. H., 2001, Effects of BA, NAA, 2-4, D and AgNO3 treatments on the callus induction and shoot regeneration from hypocotyls and cotyledon of sesame (Sesamum indicum L.). The Korean Soc. Hort. Sci., 42(1): 70-74.
- Lou, H. and Kako, S. (1995). Role of high sugar concentrations in inducing somatic embryogenesis from cucumber cotyledons. Scientia Hortic., 64, 1120.
- Lou, H., Obara-Okeyo, P., Tamaki, M., and Kako, S. (1996). Infuence of sucrose concentration on in vitro morphogenesis in cultured cucumber cotyledon explant. J. Am. Soc. Hort. Sci., 71, 497502.
- Malaghan, S.V., Lokesha, R., Savitha, R., and Ranganatha, A.R.G. (2013). Adventitious shoot regeneration in Sesame (Sesamum indicum L.) (Pedaliaceae) via de-embryonated cotyledonary explants. Research Journal of Biology, Vol. 1, 31 -35.
- Mary, R.J. and Jayabalan, N. (1997). Influence of growth regulators on somatic embryogenesis in sesame.Plant Cell, Tissue Organ Cult., 49, 67-70.
- Murashige, T. and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum, 15, 473-497.
- Naik, P.M., Manohar, S.H., Praveen, N., and Murthy, H.N. (2010). Effects of sucrose and pH levels on in vitro shoot regeneration from leaf explants of Bacopa monnieri and accumulation of bacoside A in regenerated shoots. Plant Cell, Tissue and Organ Culture (PCTOC), 100(2), 235-239.
- Nakagawa, H., Saijyo, T., Yamauchi, N., Shigyo, M., Kako, S., and Ito, A. (2001). Effect of sugars and abscisic acid on somatic embryogenesis from melon (Cucumis melo L.) expanded cotyledon. Scientia Hortic., 90, 85-92.
- Nhut, D.T., Van Le, B., Fukai, S., Tanaka, M., and Van, K.T.T. (2001). Effects of activated charcoal, explant size, explant position and sucrose concentration on plant and shoot regeneration of Lilium longiflorum via young stem culture. Plant Growth Regulation, 33(1), 59-65.
- Pathak, N., Rai, A.K., Kumari, R., Thapa, A., Bhat, K.V. (2014). Value addition in sesame: A perspective on bioactive components for enhancing utility and profitability. Pharmacogn. Rev., 8, 147-155.
- Rasheed, K.A. and Yaseen, S.A. (2013). In vitro shoot multiplication of Asparagus densiflorus as affected by media, sucrose and pH. International Journal of Pure and Applied Sciences and Technology, 17, 28-35.
- Ross, M.K., Thorpe, T.A., and Costerton, J.W. (1973). Ultrastructural Aspects of Shoot Initiation in Tobacco Callus Cultures. Am. J. Bot., 60, 788-795.
- Sayem, M., Maniruzzaman, M., Siddique, S., and Al-Amin, M. (2010). In vitro shoot regeneration through anther culture of Brassica spp. Bangladesh Journal of Agricultural Research, Vol. 35, 331-341.
- Seo, H.Y., Kim, Y.J., Park, T.I., Kim, H.S., and Yun, H.S. (2007). High-Frequency plant regeneration via adventitious shoot formation from de-embryonated cotyledon explants of Sesamum indicum L. In Vitro Cell Dev Biol: Plant, 43, 209-214. https://doi.org/10.1007/s11627-006-9017-2.
- Smeekens, G.S.M. (2000). Sugar-induced signal transduction in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol., 51, 49-81.
- Srivastava, P., Tiwari, K.N., and Srivastava, G. (2017). Effect of different carbon sources on in vitro regeneration of Brahmi Bacopa monnieri (L.) An important memory vitalizer. Journal of Medicinal Plants Research, 5(3), 202-208.
- Taskin, K.M., Ercan, A.G., and Turgut, K. (1999). Agrobacterium tumefaciens-mediated transformation of Sesame (Sesamum indicum L.).Turk J Bot., 23, 291-295.
- Vinterhalter, D.V. and Vinterhalter, B.S. (1999). Hormone-like effects of sucrose in plant in vitro cultures. Phyton., 39(3), 57-60.
- Were, B.A., Gudu, S., Onkware, A., Carlsson, A.S., and Welander, M. (2006). In vitro regeneration of sesame from seedling cotyledon and hypocotyle explants. Plant cell, Tissue and organ culture, Vol. 85, 235-239.
- Yadav, M., Chaudhary, D., Sainger, M., and Jaiwal, P.K. (2010). Agrobacterium tumefaciens-mediated genetic transformation of sesame (Sesamum indicum L.). Plant Cell, Tissue Organ Cult., 103, 377-386.
- Younghee, K. (2001). Effects of nBA, NAA, 2,4-D and AgNo3 treatments on the callus induction and shoot regeneration from hypocotyl and cotyledon of Sesame (Sesamum indicum L. ). Kovean Journal of Horticultural Science and Technology, Vol. 42, 70-74.
- Zimik, M. and Arumugam, N. (2017). Induction of shoot regeneration in cotyledon explants of the oilseed crop Sesamum indicum L. Journal of Genetic Engineering and Biotechnology, 15, 303-30.


