Influence of synthesis conditions on chabazite zeolite crystallization in LiOH solution
Автор: Mamedova G.A., Nasirli G.F., Aliyev T.A.
Журнал: Природные системы и ресурсы @ns-jvolsu
Рубрика: Экология
Статья в выпуске: 1 т.14, 2024 года.
Бесплатный доступ
The chabazite zeolite of potential practical importance has been obtained based on the natural mineral of Nakhchivan Autonomous Republic. Chabazite has a wide range of application and therefore its optimal synthesis conditions have been determined. The chabazite zeolite of potential practical importance has been obtained based on the natural mineral of Nakhchivan. The hydrothermal synthesis has been carried out in Morey type autoclaves made up of 45MNFT stainless steel with a volume of 20 cm3, and with the filling coefficient of F = 0.8. The hydrothermal crystallization experiments have been carried out generating a temperature gradient Т = 0 and without stirring of the reaction mass. The optimal conditions established in this study for the synthesis of chabazite zeolite with a 100 % degree of crystallinity are as follows: temperature of 100°C, alkaline solution of 10-20% LiOH and processing time of 50 hours. It was shown that at temperature below 100°C mordenite+chabazite+quartz is present in the reaction products, and at temperature above 100°C, chabazite+clinoptilolite+albite, chabazite+clinoptilolite+cristobalite, chabazite+clinoptilolite were obtained. At a concentration of LiOH below 10%, mordenite+quartz+chabazite are present in crystallization products, and at a concentration of LiOH above 20%, clinoptilolite+chabazite, hydrosodalite+albite+cristobalite have been obtained. Moreover, when processing time is below 50 hours, mordenite+quartz+chabazite crystallizes, and when processing time is over 50 hours, we obtain clinoptilolite+chabazite+cristobalite, hydrosodalite+albite was obtained. The initial sample and the product obtained were studied by X-ray diffraction (2D PHASER “Bruker” (CuKα, 2θ = 20-80°)), thermogravimetric (NETZSCH STA 449F3 STA449F3A-0757-M) and scanning electron microscopy (Hitachi TM-3000).
Chabazite, crystallization temperature, zeolite, processing time, formation of zeolite
Короткий адрес: https://sciup.org/149146359
IDR: 149146359 | УДК: 541.183.12+549.67+546.28 | DOI: 10.15688/nsr.jvolsu.2024.1.5
Текст научной статьи Influence of synthesis conditions on chabazite zeolite crystallization in LiOH solution
DOI:
The most important representatives of microporous substances, zeolites, have remained one of the most intensively studied classes of chemical compounds for many years because of their great structural diversity and wide practical application as ion-exchange materials and molecular sieves. Zeolites are actively used in industry for the purification, drying, and separation of various components, as catalysts for the most important technological processes, for the extraction of radioactive isotopes from liquid wastes from the nuclear industry, for the removal of impurities that pollute the atmosphere, etc.
Clarification of the optimal conditions for the synthesis of one another zeolite, that is, temperature, alkaline of the medium, and processing time, creates a scientific basis for further research.
Chabazite zeolites are built from double 6membered rings linked by tilted 4-membered rings to give the overall structure [1]. This results in a tri-dimensional pore channel system.
For this purpose, we carried out a series of experiments on the effect of temperature, alkalinity of the medium, and treatment time on the crystallization process of the practically important zeolite chabazite. It should be noted that chabazite is one of the most practically important zeolites; namely, it is used as a catalyst for the conversion of oxygenates to olefins [2], the conversion of methanol to light olefins [3], the selective dehydrogenation of ethane [4], the catalytic reduction of NOx [5], and as an adsorbent of CO2, N2, and radionuclides [6].
An analysis of the scientific literature showed that chabazite can be obtained from various structural types of the starting components in hydrothermal conditions. Previously, it has been reported that the synthesis of chabazite in hydroxide and fluoride media uses either N,N,N-trimethyl-1-adamantammonium
(TMAda), N,N,N-dimethylethylcyclohexyl ammonium (DMECHA) cations, or a mixture thereof [7]. Pure phase SSZ-13 Cha zeolite with wide range of Si/Al ratio has been synthesized in a fluoride-free media by dry gel conversion method where the conversion of SSZ-13 from aluminosilicates precursor gel occurs under water vapor [8], in the presence of a small amount of N,N,N-dimethylethylcyclohexylammonium bromide under solvent-free conditions [9], from USY zeolites and tetraethylammonium [10], using choline chloride, by the hydrothermal conversion of FAU zeolite in benzyltrimethylammonium hydroxide media, transformation using only Na-type FAU zeolite, from low-cost NaY and Al-rich beta precursors, by heating partial de-aluminate kaolin for 2 hours at 130 °C, the step heating procedure, in which the reaction composition is heated to 90 °C for 40 h, followed by a rapid heating to 103°C, which is maintained for 2 hours, and then the completion of the synthesis at 90 °C for one more hour [11].
As you know, synthetic zeolites surpass their natural counterparts in their physicochemical properties. Since synthetic chabazite has better characteristics in comparison with natural ones and has a wide field of application, the aim of this paper is to synthesize and optimize the conditions for obtaining of potential practical importance chabazite zeolite with a 100% degree of crystallinity and phase purity of the natural mineral of Nakhchivan, and the effects of the alkaline solution concentration, crystallization temperature, and processing time on the formation of chabazite zeolite were studied in detail.
It should be noted that the hydrothermal synthesis of chabazite based on natural zeolitecontaining tuff is being carried out for the first time. Synthesis on natural zeolite-containing tuff has a low cost and is environmentally friendly, for ease of handling and large-scale production.
Experimental. Lithium hydroxide (flake, 99% purity, Alfa Aesar GmbH & Co. KG, Germany) has been used without further purification. The natural samples have been obtained from the zeolite horizon in the northwest of the Kyukyuchai river, where zeolite content varies in the range of 75–80%. The samples have been thoroughly washed with distilled water and dried at a temperature of 100 °C for three days.
Hydrothermal synthesis of chabazite has been carried out in Morey autoclaves made of 45MNFT stainless steel with a volume of 18 cm3 and a filling coefficient of F = 0.8. The hydrothermal crystallization experiments have been performed generating a temperature gradient Δ Т = 0 and without stirring the reaction mass. The solid-liquid ratio was set to 1:10. After crystallization was completed, the final material was separated from the initial solution, washed with distilled water to remove excess alkali, and dried at 80 °C. For each experiment, 2 g of natural zeolite was used. The stage of preparation of the initial mixture consists of mixing a heat-treated sample of the Nakhchivan mineral in alkaline solutions at room temperature. After mixing the initial component and the alkaline solution, the initial mixture was transferred to the autoclave, and the crystallization process began at various temperatures. The crystalline structure of the original natural mineral was destroyed and recrystallized into a cubic chabazite structure (with a 100% degree of crystallinity that crystallizes within 50 hours).
In order to identify and optimize the process, the synthesis of chabazite has been carried out by varying the conditions: in the temperature range of 80 to 250 °C; in the alkaline solution of LiOH concentration range of 5 to 30%; and in the reaction time of 10–100 hours.
Characterization techniques
The X-ray diffraction measurements were performed using the X-ray analyzer 2D PHASER “Bruker” (Cu Kα radiation, 2 θ = 5– 50°), using NaCl, SiO2 (quartz), and pure zeolites in internal and external standards, respectively. Samples have been placed on a front-mounted plastic sample holder. The measuring conditions have been as follows: step size of 0.15 s/step, nickel filter as incident beam, slit aperture of 0.3°, and scan range of 2 θ = 0.5° to 10°.
The thermogravimetric analysis of the samples has been carried out on a “Derivatograph-Q 1500-D” of the Hungarian company MOM in the dynamic mode in the temperature range of 20–1000 °С. Shooting mode: heating rate of 20°/min; paper speed of 2.5 mm/min; the sensitivity of differential thermal analysis (DTA), difference thermogravimetry (DTG), and thermogravimetry (TG) is 500 mv; ceramic crucibles; the standard is Al2O3.
Elemental analysis of the starting material and the reaction conversion products has been carried out on a Launch of Trition XL dilution refrigerator “Oxford instrument” multichannel X-ray spectrometer. Measurement mode: Pd-anode, voltage of 25 kW, current strength of 70 MA, exposure time of 100 sec., sensitivity limit of 10-2. For analysis, the samples have been prepared as follows: the analyte was diluted with Li2B4O7 flux (ratio 1:10) at a temperature of 1250 °С. The resulting glass has been crushed under a pressure of 20 t/cm2 with a holding time of 1 min.
Scanning electron microscopy analysis (SEM) of the starting materials and reaction products was performed on a high-resolution microscope (an increase of 30000 times) at Hitachi 3000 TM. Low vacuum mode allowed exploring samples without pre-deposition. The sample was placed on a double-sided adhesive tape glued onto a metal disc and vacuumed to a pressure of 10-4 Pa to obtain micrographs.
Results and discussion
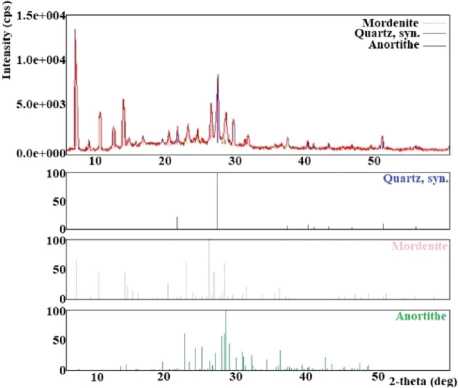
The zeolite tuff of the Nakhchivan deposit in Kyukyuchay was used as a starting material. According to X-ray diffraction and elemental analyses, it was found that 78.5% of the zeolite tuff consists of mordenite (Ca2Na2K2.8Al8.8Si39.2O96·34H2O), 19.5% quartz (SiO2), and 2% anorthite (Ca0.86Na0.14Al1.94Si2.06O8.01). According to elemental analysis, it can be argued that the zeolite tuff of the Nakhchivan Autonomous Republic of
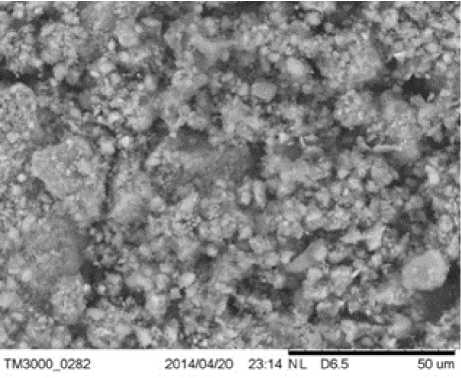
Kyukyuchay deposit was distinguished by phase purity, i.e., the bulk of the sample was concentrated on mordenite. Impurities were present in small quantities. Comparison of X-ray data obtained for the zeolitic tuff of Nakhchivan (Figure 1 (a)) with literature data showed that the studied sample of zeolite consisted mainly of mordenite [12]. The peaks in the diffractogram with interplanar distances d = 3.34 Å, 2.45 Å, 2.28 Å, and 2.12 Å indicate the α-quartz content. Also, a small amount of anorthite (4.30 Å, 3.60 Å, 3.40 Å, and 3.19 Å) was found in the sample composition. SEM image of the Nakhchivan zeolitic tuff is presented in Figure 1 (b), showing that the sample is characterised by an indeterminate surface relief with microcrystals of different sizes on the surface, possibly due to its mineral composition.
Chabazite has been synthesised in alkaline solutions (LiOH) at different temperatures and crystallisation times. The obtained results have shown that chabazite with a high degree of crystallinity has been obtained in alkaline solutions of LiOH ranging from 5 to 30 % at temperatures of 80–250 °C and a processing time of 10–100 hours.
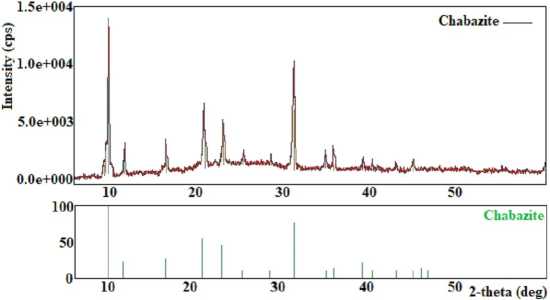
X-ray diffraction patterns of the chabazite and its micrograph are shown in Figure 2 (a) and (b), respectively. Table presents the data from the X-ray diffraction analysis.
According to the X-ray phase analysis, chabazite crystallises in the cubic crystal system with the unit cell parameter a = 9.459 Å.
(a)
Fig. 1. The X-ray diffraction pattern of Nakhchivan zeolitic tuff* (a) and its SEM image (b) Note. *(M – mordenite, Q – quartz, A – anorthite).
(b)
The synthesised chabazite was characterised by a high degree of crystallinity, which is presented in Figure 2 (a) and provides its SEM image in Figure 2 (b). The presented XRD pattern (Figure 2 (a), Table) relates to chabazite obtained under optimum conditions with a 100% degree of crystallinity.
Using the thermogravimetric analysis (Figure 3), the region of dehydration and thermostability of the chabazite have been established. The DTA curve is characterised by one endothermic and one exothermic effect.
The endothermic effect corresponds to the dehydration of the sample with a maximum of 230 °С, at which the weight loss along the TG curve is 24%. The exothermic effect, detected at a temperature with a maximum of 950 °С, according to X-ray diffraction analysis, refers to the destruction of the crystal structure of chabazite and the formation of sanidine, quartz, and anorthite. The diffractogram of the products after 950 °C is shown in Figure 4.
The crystallization of chabazite in solutions of LiOH has been studied at
(a)
Fig. 2. The X-ray diffraction pattern of chabazite with 100% degree of crystallinity (a) and its SEM image (b)

TM3000_2778
2020/02/10 22:42 NL D5.8 30 um
(b)
X-ray diffraction data of the obtained chabazite
|
Chabazite |
|||
|
d exp , Å |
I rel |
hkl |
dca l c, Å |
|
9.44 |
100 |
100 |
9.44 |
|
6.68 |
20 |
101 |
6.68 |
|
5.46 |
25 |
111 |
5.46 |
|
4.24 |
50 |
201 |
4.23 |
|
3.89 |
40 |
211 |
3.86 |
|
3.36 |
10 |
202 |
3.34 |
|
3.14 |
10 |
300 |
3.15 |
|
3.05 |
10 |
301 |
3.00 |
|
2.94 |
70 |
311 |
2.95 |
|
2.62 |
15 |
320 |
2.62 |
|
2.52 |
20 |
312 |
2.52 |
|
2.32 |
10 |
322 |
2.29 |
|
2.23 |
10 |
303 |
2.23 |
|
2.17 |
10 |
402 |
2.11 |
|
2.10 |
15 |
412 |
2.06 |
|
2.07 |
10 |
323 |
2.01 |
|
1.96 |
10 |
422 |
1.93 |
|
1.88 |
10 |
500 |
1.89 |
|
1.87 |
10 |
501 |
1.86 |
Note. dexp – experimentally obtained value of the interplanar distance; Irel – relative intensity of diffraction lines; hkl – Miller indices characterizing the arrangement of atomic planes in the crystal; dcalc – calculated value of the interplanar distance.
temperatures in the range of 80–250 °C. The X-ray diffraction patterns of crystallisation products in the obtained solutions are presented in Figure 5.
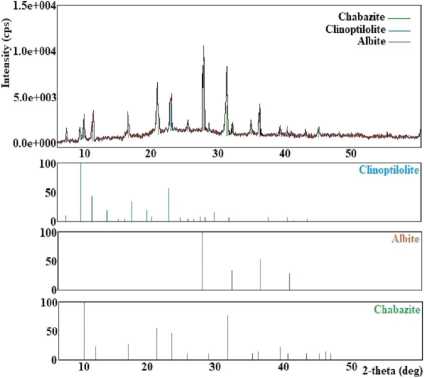
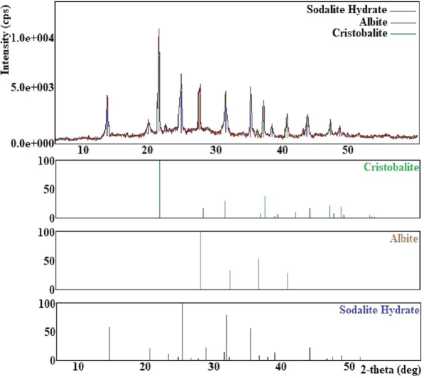
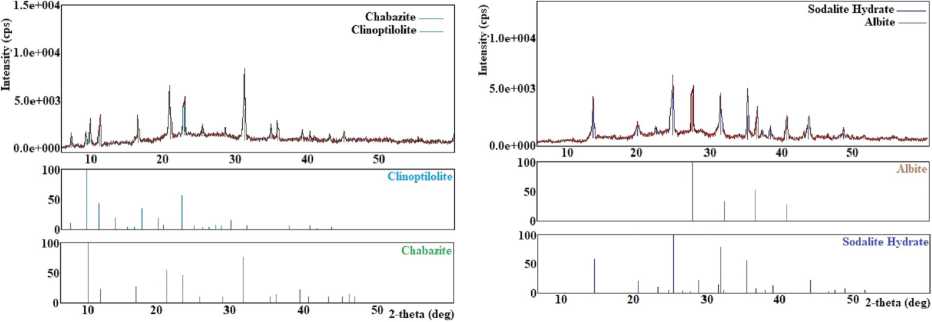
It has been found that at a temperature of 80 °C, crystallization products consist of a mix of chabazite, unreacted mordenite, and quartz (Figure 5(a)). Studies have shown that the optimum crystallisation temperature of chabazite with a high degree of crystallinity is 100 °C. The temperature range of 120–150 °C promotes crystallisation in addition to chabazite, clinoptilolite, and albite (Figure 5 (b)). In the temperature range of 150–200 °C, chabazite, clinoptilolite, and cristobalite (Figure 5 (c)) and at 200–250 °C, chabazite and clinoptilolite (Figure 5 (e)) have been obtained.
The effect of alkaline solution concentration has been studied in solutions of LiOH (in the range of 5–30%).
The obtained results have shown that experiments in the natural mineral LiOH system at a 5% concentration of LiOH crystallise unreacted mordenite, chabazite, and quartz (Figure 5(a)). Pure chabazite with a 100% degree of crystallinity has been obtained in the LiOH concentration range of 10–20%. The study of the influence of the concentration of LiOH solution in the range of 20–25% has shown that clinoptilolite and chabazite are obtained (Figure 5 (e)). A further increase in the concentration of LiOH (in the range of 25–30%) promotes the crystallisation of cristobalite, albite, and hydrosodalite (Figure 5 (d)).
The optimal crystallisation time of chabazite with a 100% degree of crystallinity is 50 hours.
Less than 50 hours of processing time (starting from 10 hours) contribute to the formation of unreacted mordenite, chabazite, and quartz (Figure 5(a)). When processing over 50 hours, that is, in the time interval 60–80 hours, clinoptilolite, chabazite, and cristobalite appeared in the crystallisation products (Figure 5 (c)). In the interval of 80–100 hours, hydrosodalite and albite were obtained (Figure 5 (f)).
Having studied the process of crystallisation of chabazite, the optimal conditions for its synthesis with a high degree of crystallinity were established. The chabazite obtained under optimal conditions differed in phase purity and had a high degree of crystallinity. Chabazite with a 100% degree of crystallinity has been obtained under the following optimal conditions: a temperature of 100 °C, LiOH concentrations of 10–20%, and a processing time of 50 hours.
Conclusions
The natural mineral of Nakhchivan has been used for the synthesis of the potential practical importance of zeolite in chabazite. The effects of temperature, alkaline solution concentrations, and processing time on crystallisation have been investigated. The obtained results have shown that chabazite with a high degree of crystallinity (100%) can be obtained at a temperature of 100 °C, alkaline solution LiOH concentrations of 10–20%, and a processing time of 50 hours.
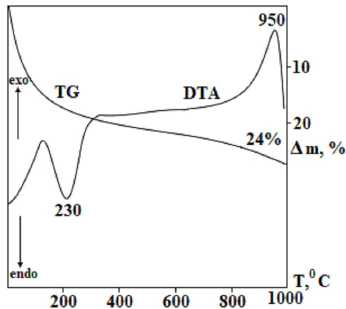
Fig. 3. Thermogram of synthesized chabazite zeolite obtained under optimal conditions
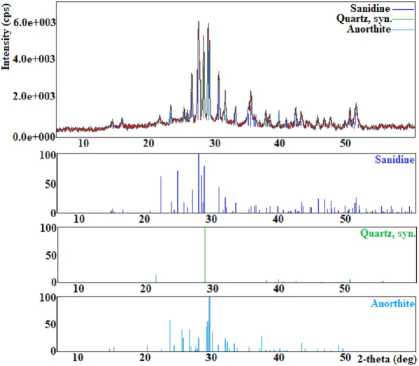
Fig. 4. The X-ray diffraction pattern of the products after 950 °C
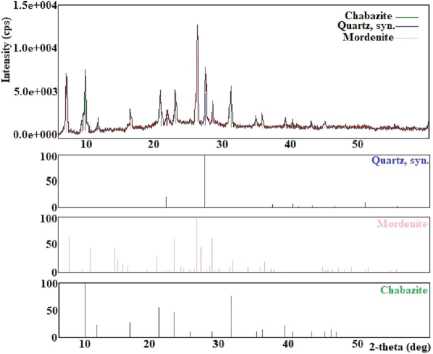
(a)
(b)
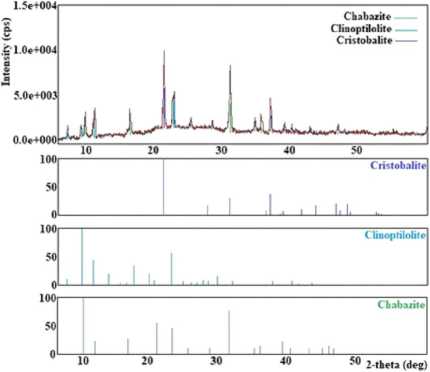
(c)
(d)
(e)
(f)
Fig. 5. X-ray diffraction patterns of crystallization products obtained: (a) at 80 °C, at 5 % LiOH and for 10–50 hours; (b) at 120–150 °C; (c) at 150–200°C and for 60–80 hours; (d) at 25–30% LiOH; (e) at 200–250 °C, at 20–25 % LiOH; (f) for 80-100 hours
Moreover, it was shown that a change in the synthesis conditions (temperature, alkaline solution concentration, processing time) can greatly affect the results of crystallisation. The optimal conditions (temperature of 100 °C, alkaline solution LiOH concentrations of 10– 20%, processing time of 50 hours) for the synthesis of chabazite zeolite with a high degree of crystallinity have been established. Research has shown that, at temperatures below 100 °C, mordenite+chabazite+quartz is present in the reaction products, and at temperatures above 100 °C, chabazite+clinoptilolite+albite, chabazite+clinoptilolite+cristobalite, and chabazite+clinoptilolite were obtained. At a concentration of LiOH below 10%, mordenite+quartz+chabazite are present in crystallisation products, and at a concentration of LiOH above 20%, clinoptilolite+chabazite, hydrosodalite+albite+cristobalite were obtained. And also, when processing time below 50 hours, mordenite+quartz+chabazite crystallized, and when processing over 50 hours – clinoptilolite+chabazite+cristobalite, hydrosodalite+albite was obtained.
Список литературы Influence of synthesis conditions on chabazite zeolite crystallization in LiOH solution
- Yakubovich O.V., Massa W., Gavrilenko P.G., Pekov I.V. Crystal Structure of Chabazite K. Crystallography Reports, 2005, vol. 50, pp. 544-553. DOI: https://doi.org/10.1134/1.1996728
- Machteld M.M., Stephen N.V. Synthesis of Chabazite-Containing Molecular Sieves and Their Use in the Conversion of Oxygenates to Olefins. USA Patent, 2013, no. 8,399,578 B2
- Yashodhan B., Manuel M.-M., Jonathan D.L., et al. Effect of Cage Size on the Selective Conversion of Methanol to Light Olefins. ACS Catalysis, 2012, vol. 2, no. 12, рp. 2490-2495. DOI: https://doi.org/10.1021/cs300558x
- Maeno Z., Wu X., Shunsaku Y., et al. In- Exchanged CHA Zeolites for Selective Dehydrogenation of Ethane: Characterization and Effect of Zeolite Framework Type. Catalysts, 2020, vol. 10, no. 7, pp. 807-817. DOI: https://doi.or g/10.3390/catal10070807
- Anita G., Oliver L.I., Soren B.R., et al. Site- Specific Reactivity of Copper Chabazite Zeolites with Nitric Oxide, Ammonia, and Oxygen. ChemCatChem, 2018, vol. 10, no. 2, pp. 366-370. DOI: https://doi.org/10.1002/cctc.201701357
- Hamza A., Serpil E., Celalettin O., et al. Use of Chabazite, a Naturally Abundant Zeolite, for the Investigation of the Adsorption Kinetics and Mechanism of Methylene Blue Dye. Microporous and Mesoporous Materials, 2016, vol. 235, pp. 78-86. DOI: https://doi.org/10.1016/j.micromeso.2016.08.007
- Long V.D., Son T.L., Raul F.L., Trong D.P. Hydrothermal Synthesis of Alkali-Free Chabazite Zeolites. Journal of Porous Materials, 2020, vol. 27, pp. 1481-1489. DOI: https://doi.org/10.1007/s10934-020-00923-y
- Hasna A.J., Koji M., Kaito O., et al. Synthesis of High Silica SSZ-13 in Fluoride-Free Media by Dry Gel Conversion Method. Microporous and Mesoporous Materials, 2019, vol. 278, pp. 322-326. DOI: https://doi.org/10.1016/j.micromeso.2019.01.006
- Xiong W., Qinming W., Chunyu C., et al. Atom-Economical Synthesis of a High Silica CHA Zeolite Using a Solvent-Free Route. Chemical Communications, 2015, vol. 51, no. 95, рp. 16920-16923. DOI: https://doi.org/10.1039/C5CC05980A
- Nuria M., Manuel M., Avelino C. High Yield Synthesis of High-Silica Chabazite by Combining the Role of Zeolite Precursors and Tetraethylammonium: SCR of NOx. Chemimal Communications, 2015, vol. 51, no. 49, рp. 9965-9968. DOI: https://doi.org/10.1039/C5CC02670A
- Hyunjung L., Prabir K.D. Synthesis of Free- Standing Chabazite-Type Films. Microporous and Mesoporous Materials, 2000, vol. 38, pp. 151-159. DOI: https://doi.org/10.1016/S1387-1811(99)00289-9
- Sánchez-Lуpez P., Antúnez-Garcнa J., Fuentes-Moyado S., et al. Analysis of Theoretical and Experimental X-Ray Diffraction Patterns for Distinct Mordenite Frameworks. Journal of Materials Science, 2019, vol. 54, no. 10, рp. 7745-7757. DOI: https://doi.org/10.1007/s10853-019-03407-w


