Influence of tert-butyl hydroperoxide and nitrosoglutathione on Escherichia coli cells expressing leghemoglobin
Автор: Kosmachevskaya O.V., Shumaev K.B., Arredondo-peter R., Topunov A.f
Журнал: Журнал стресс-физиологии и биохимии @jspb
Рубрика: Original article
Статья в выпуске: 1 т.3, 2007 года.
Бесплатный доступ
Influence of expressed leghemoglobin on stability of Escherichia coli cells to reactive oxygen and nitrogen species - fert-butyl hydroperoxide and S-nitrosoglutathione was studied. We determined both action of these compounds on cells with different level of leghemoglobin expression and peroxidase activity of the cells. It was shown that priority of pro- or antioxidant properties of leghemoglobin is dependent on its concentration in the cells. In cells with reduced synthesis leghemoglobin mostly functions as prooxidant, in cells with intensive one - as antioxidant
Escherichia coli, leghemoglobin, tert-butyl, hydroperoxide, nitrosoglutathione
Короткий адрес: https://sciup.org/14323459
IDR: 14323459
Текст обзорной статьи Influence of tert-butyl hydroperoxide and nitrosoglutathione on Escherichia coli cells expressing leghemoglobin
It is known that oxidative stress is one of the most widespread stress actions and is result of many negative factors. Important role of generation of reactive oxygen (ROS) and nitrogen (RNS) species in metabolism of different living organisms was shown. ROS because of their prooxidant properties are initiators and intermediates of the majority of reactions taking place under oxidative stress conditions. On the other hand nitric oxide (NO) and other active nitrogen metabolites have both cytotoxic and protective antioxidant properties. So influence of active oxidative stress metabolites (pro- and antioxidants) on living organisms is actively studying. It was also known that hemoglobin-like proteins are compounds actively participating in pro-and antioxidant processes. Heme groups of these proteins can react both with reactive oxygen and nitrogen species.
Sensitivity of microorganisms to toxic activity of oxidants is of specially importance, because one of the main mechanisms of human organism defense against bacterial infection is so-called “respiratory explosion”. It is intensive production of NO, peroxinitrite and ROS (superoxide, hydrogen peroxide, hydroxyl radical and hypochlorite) by macrophages. It was found out that majority of microorganisms defend themselves against ROS with induction of synthesis of antioxidant and reparation enzymes including so-called catalases-peroxidases. It is known that bacterial flavohemoglobin also takes part in detoxification of nitric oxide in different bacteria including E. сoli. It oxidizes NO to nitrate (Gardner et al., 1998). So study of influence of NO and active oxidants on bacterial cell is of doubtless interest.
For study of hemoprotein functioning under oxidative stress conditions we used the model system: Escherichia coli strain expressing leghemoglobin (Lb). Leghemoglobin is plant myoglobin-like protein taking part in regulation of oxygen conditions in nitrogen-fixing legume nodules (Topunov, 1995). At the first time such expression of lupine Lb was made in our joint work with Institute of Bioorganic Chemistry of Polish Academy of Sciences (Sikorski et al ., 1995). Similar work was later made with Lb of other legume plants (Prytulla et al ., 1996; Arredondo-Peter et al ., 1997; Hargrove et al ., 1997; Jones et al ., 1998).
In this work studies on influence of expressed leghemoglobin on stability of E. coli cells to compounds traditionally referred to ROS and RNS – to tert -butyl hydroperoxide ( t -BOOH) and S-nitrosoglutathione (GSNO) were carried out.
MATERIALS AND METHODS
Escherichia coli cells expressing leghemoglobin were used. E. coli TB-1 strain was modified with pEMBL18+:: SyLba plasmid with gene of soybean Lb a by method (Arredondo-Peter et al ., 1997) with modifications. Modified strain was obtained in Autonomy University of State Morelos (Cuernavaca, Mexico) in the laboratory of Dr. R. Arredondo-Peter. The same strain without plasmid was used as control.
The cell suspension (50 µl) stored in glycerol solution at -70o C was sowed into liquid Lb media and grew during 15 h at 37 o C in thermo-shaker (“BioKom”, Russia) with orbital speed 200 rot/min. Obtained culture was used for sowing of 250 ml volume flasks containing 60 ml liquid Lb media to optical density А 600 = 0.02 + 0.03. Culture in experimental flasks was grown at the same conditions. Concentration of cells was measured every 2 h using optical density (OD) at 600 nm in 1 cm optical cuvette. Growth media for strains expressing Lb contained ampicilline in concentration 50 µg/ml.
Red-ox active compounds: tert -butyl hydroperoxide and S-nitrosoglutathione in different concentrations were added to medium at early stage of logarithmic phase, 3 h after sowing (А 600 = 1.2 + 1.5).
For obtaining Lb-containing fraction 7 h and 24 h cell cultures washed of media were sonicated using ultrasonic desintegrator "Soniprep 150” (Japan) at maximal power 23 kHz (4 of 20 s cycles for 7 h cultures and 6 cycles for 24 h cultures) at 0 оC. Sonication solution included 50 mM Tris-HCl buffer pH 7.4, lysozyme (10 mg/ml), and PMSF (28 µM). After it precipitation of cell extract with ammonium sulfate (50-90%) was made.
Peroxidase activity in protein fractions was determined by tinge intensity of o -dianisidine oxidized by hydrogen peroxide under peroxidase action using method (Claiborne, Fridovich, 1979) with modifications. Intensity was spectrophotometrically measured at wavelength 545 nm. Coefficient of micromolar extinction = 0,0128 µM –1cm-1. For determination of peroxidase activity and amount of hemoproteins desalted protein solution in 0.1 M Tris-HCl buffer pH 6.8 was used.
Amounts of hemoproteins were determined using modified method (Riggs, 1981). Difference of peak sizes of pyridine hemochromogene complexes reduced by sodium dithionite minus oxidized by potassium ferricyanide was measured. Differential coefficient of micromolar extinction S ( 556-539 ) = 0,0043 µM –1cm-1, experimentally obtained by us, was used.
Total protein amount in samples was determined using Bradford method (Bradford, 1976).
All spectrophotometric measurements were made on “Beckman Coulter CU-650” spectrophotometer (USA).
Reagents used: LB media, (NH4)2SO4, PMSF, reduced glutathione - “AppliChem” (Germany), lysozyme - “Serva”(Germany), ampicilline, Tris -“ICN” (США), tert -butyl hydroperoxide, potassium ferricyanide - “Sigma-Aldrich” (USA), sodium dithionite, o -dianisidine - “Sigma” (USA), pyridine -“Aldrich” (USA), hydrogen peroxide, HCl, H 2 SO 4 , NaNO 2 , H 3 PO 4 , acetic acid, ethanol, NaOH, KH 2 PO 4 , K 2 HPO 4 - 3H 2 O (Russia). Nitrosoglutathione was synthesized according to method (Shumaev et al ., 2004) with mixing of equimolar amounts of glutathione and NaNO2 directly before experiment..
RESULTS AND DISCUSSION
Experiments on influence on E. coli cells of such inductors of oxidative and nitrosative stress as tert butyl hydroperoxide and S-nitrosoglutathione respectively were carried out. Three types of cells were tested: non-leghemoglobin synthesizing (С-Lb) which contained 0.08 µg heme/mg protein, synthesizing Lb in minor amounts (C ± Lb) - 0.15 цд heme/mg protein, and actively Lb-synthesizing (С +Lb ) – 0.45 µg heme/mg protein.
It was shown that both t -BOOH and GSNO inhibited growth of E. coli cells and this inhibition was concentration dependent. In Table 1 data of influence of these compounds on С+Lb cells are shown.
One can see that tert -butyl hydroperoxide was stronger cytotoxic agent. Since 80 µM concentration it almost totally inhibited growth of E. coli cells.
For further studies we used GSNO in concentration which had small influence on cell growth (200 µM), and t -BOOH in concentration which allowed cells to restore their possibility to growth (40 µM).
In our experiments growth of C-Lb, C+Lb and C ± Lb cultures in logarithmic phase (7 h) was inhibited by tert -butyl hydroperoxide in the same level (inhibition 35-40%). C+Lb and C ± Lb cultures were more sensitive to t -BOOH – their growth level at 24 h was 12% and 40% of control respectively. Growth of С-Lb culture reached level of control one as early as in stationary phase. But on growth curve of C ± Lb culture if t -BOOH itself or together with GSNO was acting minimum in exponential phase was observed (Fig. 1). It is probably connected with appearance of oxoferryl Lb form (heme-Fe,V=O ‘ ) as result of the action of t -BOOH. Oxoferryl forms of hemoproteins are known as strong oxidants. And it is known that leghemoglobin (IV) can be formed as result of Lb reaction with peroxides (Aviram et al. , 1978).
Reactions 1-3 show possible appearance of ferryl Lb form after action of t -BOOH:
heme-FeII + ROOH → heme-FeIII + RO • + OH- (1) heme-FeIII + ROOH → heme-FeIV=O • + ROH (2) heme-FeIV=O • + ROOH → heme-FeIV =O + ROO • + H+ (3).
As it was written above GSNO in concentrations less than 200 µM did not essentially inhibited growth of bacterial cultures studied (Table 1). Even more, GSNO stimulated growth of all types of E. coli cells when t -BOOH induced oxidative stress. Protective effect of GSNO depended on its concentration. It is interesting that restoration of cells growth was obtained even when GSNO was added with t -BOOH in concentrations of latter almost fully suppresses growth of strains studied (Fig. 1).
Data showing difference in intensity of hemoprotein synthesis of culture types are shown on Fig. 2. We measured amount of heme per mg of protein. If compare Figs 2 and 3 it is possible to see that peroxidase activity of E. coli cells was in good correlation with heme amount. Both these parameters also correlated with amount of Lb in cells. So it is possible to say that changes of peroxidase activity can be connected with changes of Lb amount. It is known that Lb can in some cases show so-called “pseudo peroxidase“ activity (Sievers, Ronnberg, 1978).
Changes in peroxidase activity of С +Lb and С ± Lb cultures under the action of t -BOOH and GSNO had different characters. Peroxidase activity of С ± Lb culture was 2 times higher if growth was with GSNO than with t -BOOH. At the opposite side activity of С +Lb culture increased at the presence of t -BOOH in medium. But combination of t-BOOH and GSNO did not resulted in serious increasing of peroxidase activity if compared with control cultures without red-ox active compounds. At the same time t -BOOH and GSNO decreased peroxidase activity of С -Lb culture but such decreasing was less at their joint action.
So at the all types of culture combination of t -BOOH and GSNO resulted in changes in peroxidase activity less than t -BOOH separately. It can be probably explained by generation after interaction of NO with t -BOOH radical products did not participated in free-radical reactions. Several ways of protective action of NO against tert -butyl hydroperoxide-induced oxidative stress which included interaction with hemoproteins were proposed (Gorbunov et al. , 1997; Yalowich et al. , 1999). Direct reaction of NO with ferryl forms of these proteins was also described (Gorbunov et al. , 1995).
Nitric oxide can directly react with ferryl hemoproteins and organic radicals appearing during oxidative stress. Such interaction is going according to reactions:
heme-FeIV=O • + NО • → heme-FeIII-ONO → heme-FeIII + NO 2 - (4)
NО • + RО 2 • → ROОNО (5)
This antioxidant effect of NO can result in decreasing of synthesis of antioxidant enzymes including ones with peroxidase activity. We have also to underline that peroxidase activity of culture intensively synthesizing Lb was always practically by order higher than of С -Lb and С ± Lb cultures.
In some works influence of expressed hemoglobins on bacterial metabolism was studied as well. Changes of E. coli metabolism after expression of Vitreoscilla bacterial hemoglobin (VHb) were reported. For instance, energetic and carbon metabolism of these cells was changed (Dikshit et al. , 1992; Tsai et al. , 1996). It was shown that E. coli cells lacked terminal oxidases restored aerobic metabolism (Dikshit et al. , 1992) and increased their growth under hypoxia conditions (Frey et al. , 2000) in the presence of chimeric VHb containing reductase domen. Data that expressed exogenic Hb can help bacterial cell to be protected against toxic nitrogen compounds (nitrosative stress) (Kaur et al. , 2002, Frey et al. , 2002) were also obtained.
Our data also confirm supposition that Lb in its different oxidative-reductive states can be both pro-and antioxidant. Several possible reactions with reactive oxygen species in which leghemoglobin as oxygen-carrying protein can participate were proposed (Becana, Klucas, 1992):
LbO 2 ↔ Lb3+ +O 2 • - (6)
LbO 2 + O 2 • - + 2H+ → Lb3++ O 2 +H 2 O 2 (7)
Thus it was possible to suppose that leghemoglobin can work as protective protein eliminating reactive oxygen species.
Our data show that priority of pro- and antioxidant properties of leghemoglobin depends on its concentration in the cells. In cells with reduced synthesis of Lb it mainly functions as prooxidant, if synthesis is intensive – as antioxidant. This fact confirm preposition of possible antioxidant role of Lb in its native medium – legume nodule where its concentration is very high (near 3 mM in plant cell cytoplasm). It can be important for supporting necessary oxidative state in symbiotic nitrogenfixing system.
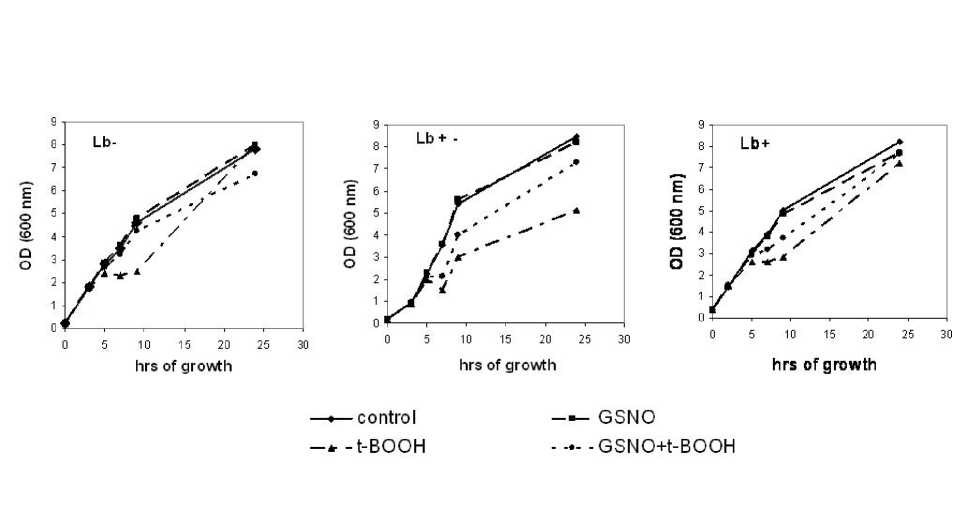
Fig. 1. Growth curves of E. coli cells.
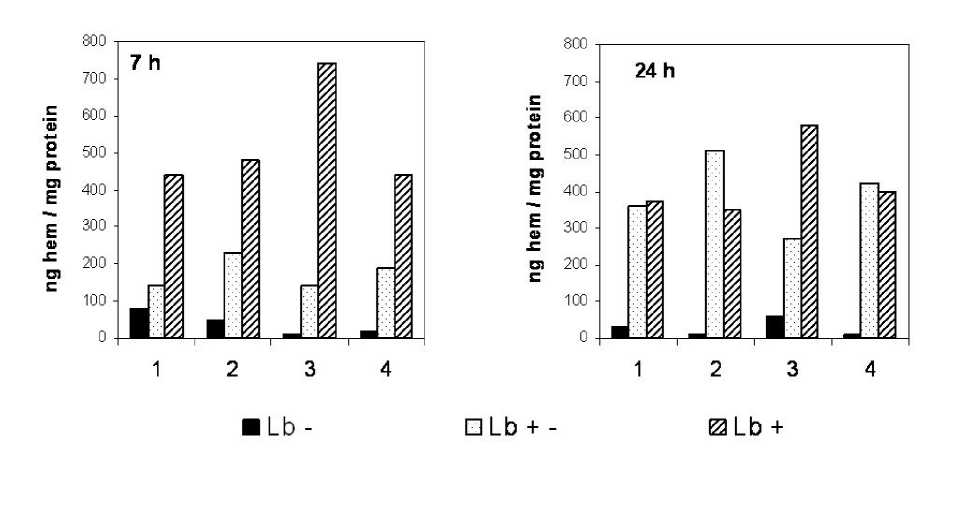
Fig. 2 . Heme content in E. coli cells.
1 – control; 2 – with GSNO (200 µl); 3 – with t -BOOH (40 µl); 4 – with GSNO (200 µl) + (40 µl).
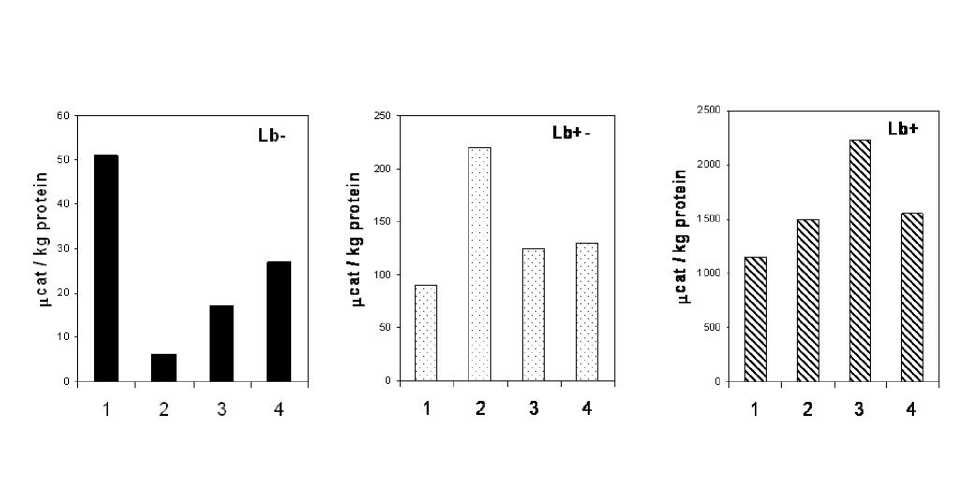
Fig. 3. Total peroxidase activity of soluble proteins of E. coli cells.
1 – control; 2 – with GSNO (200 µl); 3 – with t -BOOH (40 µl); 4 – with GSNO (200 µl) + (40 µl)
Table 1. Influence of GSNO and t -BOOH on growth of E. coli TB-1 cells (Lb+), % of control.
|
C, µM |
t -BOOH |
GSNO |
||||||
|
40 |
80 |
120 |
160 |
150 |
200 |
250 |
500 |
|
|
7 h |
68 |
38 |
26 |
23 |
97 |
94 |
88 |
63 |
|
24 h |
88 |
20 |
12 |
10 |
98 |
96 |
84 |
55 |
ACKNOWLEDGEMENTS
The work was made with support of Russian Foundation for Basic Research (grant 06-04-81054Bel_a).
Список литературы Influence of tert-butyl hydroperoxide and nitrosoglutathione on Escherichia coli cells expressing leghemoglobin
- Arredondo-Peter, R., Moran, J.F., Sarath, G., Peng Luan, and Klucas, R.V. (1997) Molecular cloning of the cowpea leghemoglobin II gene and expression of its cDNA in Escherichia coli. Plant Physiol., 114, 493-500.
- Aviram, I., Wittenberg, B.A., Wittenberg, J.B. (1978) The reaction of ferrous leghemoglobin with hydrogen peroxide to form leghemoglobin (IV). J. Biol. Chem., 253, 5685-5689.
- Becana, M., and Klucas, R.V. (1992) Oxidation an reduction of leghemoglobin in root nodules of leguminous plants. Plant. Physiol., 98, 1217-1221.
- Bradford, M.M. (1976) A rapid and sensitive method for the quantitation of microgram quantities utilizing the principle of protein dye binding. Anal. Biochem., 72, 248-254.
- Claiborne, A., and Fridovich, I. (1979) Purification of the o-dianisidine peroxidase from Escherichia coli B. J. Biol. Chem., 254, 4245-4252.
- Dikshit, R.H., Dikshit, K.L., Liu, Y., and Webster, DA. (1992) The bacterial hemoglobin from Vitreoscilla can support the aerobic growth of Escherichia coli lacking terminal oxidases. Arch. Biochem. Biophys., 293, 241-245
- Frey, A.D., Bailey, J.E., and Kallio, P.T. (2000) Expression of Alcaligenes eutrophus flavohemoprotein and engineered Vitreoscilla hemoglobin-reductase fusion protein for improved hypoxic growth of Escherichia coli. Appl. Environm. Microbiol., 66, 98-104.
- Frey, A.D., Farres, J., Bolinger, C.J.T., and Kallio, P.T. (2002) Bacterial hemoglobins and flavohemoglobins for allevation of nitrosative stress Escherichia coli. Appl. Environm. Microbiol, 68, 4835-4840.
- Gardner, P.R., Gardner, A.M., Martin, L.A., and Salzman, A.L. (1998) Nitric oxide dioxigenase: An enzymic function for flavohemoglobin. Proc. Natl. Acad. Sci. USA, 95, 10378-10383.
- Gorbunov, N.V., Osipov, A.N., Day, B.W., Zayas-Rivera, В., Kagan, V.E., and Elsayed, N.M. (1995) Reduction of ferrylmyoglobin and ferry lhemo glob in by nitric oxide: a protective mechanism against ferryl hemoprotein-induced oxidation. Biochemistry, 34, 6689-6699.
- Gorbunov, N.V., Yalowich, J.C., Gaddam, A., Thampatty, P., Ritov, V.B., Kisin, E.R., Elsayed, N.M., and Kagan, V.E. (1997) Nitric oxide prevents oxidative damage produced by fert-butyl hydroperoxide in erythroleukemia cells via nitrosylation of heme and non-heme iron. J. Biol. Chem., 272, 12328-12341.
- Hargrove, M.S., Barry, J.K., Brucker, E.A., Berry, M.B., Phillips, G.N., Olson, J.S., Arredondo-Peter, R., Dean, J.M., Klucas, R.V., and Sarath, G. (1997) Characterization of recombinant soybean leghemoglobin a and apolar distal histidine mutants. J. Mol. Biol., 266, 1032-1042.
- Jones, D.K., Badii, R., Rosell, F.I., and Lloyd, E. (1998) Bacterial expression and spectroscopic characterization of soybean leghemoglobin a. BiochemJ., 330, 983-988.
- Kaur, R., Pathania, R., Sharma, V., Mande, S.C, and Dikshit, K.L. (2002) Chimeric Vitreoscilla hemoglobin (VHb) carrying a flavoreductase domain relieves nitrosative stress in Escherichia coli: new insight into the functional role of VHb. Appl. Environm. Microbiol., 68, 152-160.
- Prytulla, S., Dyson, H.J., and Wright, P.E. (1996) Gene synthesis, high-level expression and assignment of backbone 15N and 13C resonances of soybean leghemoglobin. FEBS Letters, 339, 283-289.
- Riggs, A. (1981) Preparation of blood hemoglobin of vertebrates. Methods in Enzymology, 76, 5-29.
- Shumaev, K.B., Petrova, N.E., Zabbarova, I.V., Vanin, A.F., Topunov, A.F., Lankin, V.Z., and Ruuge, E.K. (2004) Interaction of oxoferrylmyoglobin and dinitrozyl-iron complexes. Biochemistry (Moscow), 69, 569-574.
- Sievers, G., and Ronnberg, M. (1978) Study of the pseudoperoxidatic activity of soybean leghemoglobin and sperm whale myoglobin. Biochim. Biophys. Acta, 533, 293-301.
- Sikorski, M.M., Topunov, A.F., Strozycki, P., Vorgias, C.E., Wilson, K.S., and Legocki, A.B. (1995) Cloning and expression of plant leghemoglobin cDNA of Lupinus luteus in Escherichia coli and purification of the recombinant protein. Plant Sci., 108, 109-117.
- Topunov, A.F. (1995) Leghemoglobin and its role in regulation of oxygen concentration in nitrogen-fixing legume nodules. Biochemistry (Moscow), 60, 45-49.
- Tsai, P.S., Hatzimanikatis, V., and Bailey, J.E. (1996). Effect of Vitreoscilla hemoglobin dosage on microaerobic Escherichia coli carbon and energy metabolism. Biotechnol. Bioeng., 49, 139-150.
- Yalowich, J.C., Gorbunov, N.V., Kozlov, A.V., Allan, V., and Kagan, V.E. (1999) Mechanisms of nitric oxide protection against fert-butyl hydroperoxide-induced cytotoxicity in iNOS-transduced human erythroleukemia cells. Biochemistry, 38, 10691-10698.


