Interaction of PbO-based oxide melts with crucible materials
Автор: Denisov Viktor M., Belousova Natalia V., Denisova Lyubov T., Kuchumova Oksana V., Zeer Galina M.
Журнал: Журнал Сибирского федерального университета. Серия: Техника и технологии @technologies-sfu
Статья в выпуске: 3 т.4, 2011 года.
Бесплатный доступ
Contact interaction of PbO-GeO2 melts with crucible materials (Au, Ag, Al2O3, BeO) was investigated by sessile drop method. It was found that the contamination of PbO-GeO2 oxides by the components of these crucibles depended on the initial composition of melts.
Melt, oxides of lead and germanium, crucible, gold, silver, contact interaction
Короткий адрес: https://sciup.org/146114585
IDR: 146114585 | УДК: 532.614+546.873
Текст научной статьи Interaction of PbO-based oxide melts with crucible materials
Lead germanates of variable composition are widely used for different technological applications due to their physical and chemical properties [1-4]. Functional properties of these materials depend on conditions of their synthesis and production [5]. Moreover, crucible materials have also a substantial effect, particularly if oxides are in the liquid state. For example, the color of the glasses based on heavy metal oxides depends on the crucible type used for their obtaining [6]. The authors of the last-named work noted that the glasses based on heavy metal oxides were more corrosive in the liquid state by comparison with other glasses. Hence a problem of the choice of crucible material arises, as most of these materials are corroded by liquid glass. As a consequence, the latter is stained resulting in a deterioration of optical quality and an increase of the scattering losses. According to [6], crucibles of Pt and Au are the most corrosion-resistant though there are reasons to assume that crucibles of SnO 2 can show the better resistance [7].
Because of this, the study of contact interaction of PbO-GeO 2 melts with crucible materials (Au, Ag, Al2O3, BeO) is of scientific and practical interest.
Results and discussion
“Melt-substrate” contact interaction was investigated by sessile drop method. All experiments were conducted in air at the separate heating of the sample and the substrate. Photographs of the drops obtained with a Canon EOC 400 Digital were processed using a computer. Analyses of the solidified
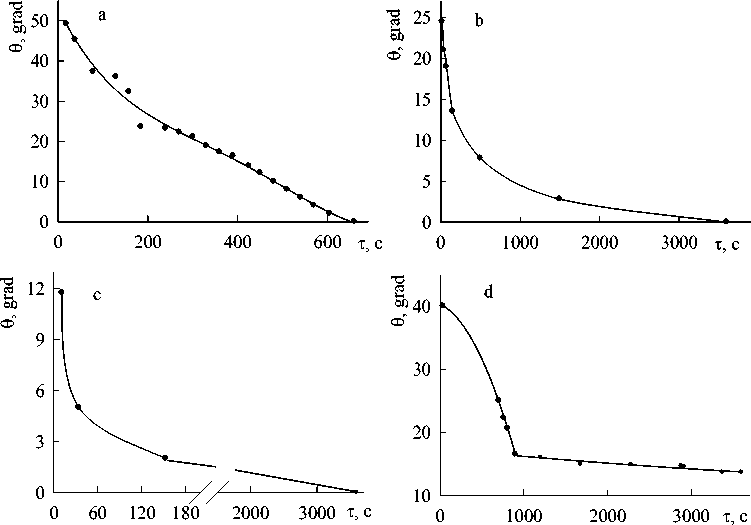
Fig. 1. Contact interaction of PbO – GeO 2 melts with gold. C PbO , at.%: а – 25; b – 30; c – 60; d – 80
drops were carried out using a JEOL JSM 7001F scanning electron microscope and an INCA Energy PentaFETx3 energy dispersive spectrometer.
The study of interaction of solid gold with PbO-GeO 2 melts containing 25; 30; 32,5; 40; 45; 50; 60; 62,5; 66,8; 75; 80; 85; 90 and 100 mol. % of PbO was conducted depending on the time of their contact. The values of transition temperatures of PbO-GeO 2 oxides into the liquid state whereby “meltsubstrate” contact interaction was investigated were taken from [8-10] but sometimes they were refined experimentally with the use of a STA 449 C Jupiter differential scanning calorimeter.
Preliminary results showed that PbO-GeO2 melts react readily with crucible of Al2O3. At a high concentration of PbO in melts, the content of Al 2 O 3 in the solidified samples reaches 3,5 % after the contact of PbO-GeO2 melts with Al2O3 within 30 min. PbO-GeO2 glasses obtained only in crucibles of BeO have a rather good quality. Taking it into account, in all subsequent experiments the given materials were synthesized in these crucibles.
It was found that all PbO-GeO 2 melts didn’t form equilibrium angles on the solid gold and a strong “melt-substrate” adhesion work was observed. As an example, some results on the interaction of PbO-GeO 2 melts with gold are presented in Fig. 1. The similar data were obtained also for other melts.
The color of all PbO-GeO2 drops regardless of the initial composition is noticed to change after experiments and to take on greenish tint. It can be a confirmation of the presence of chemical reactions in the “melt-substrate” system.
As indicated above, crucible materials can influence the color of resulting glasses of heavy metal oxides (PbO-GeO2, PbO-TeO2, PbO-Bi2O3-Ga2O3 and so on [6]). Such effect was observed as Bi2O3-SiO2, Bi 2 O 3 -PbO-Ga 2 O 3 , Bi 2 O 3 -PbO-Ga 2 O 3 -GeO 2 , Bi 2 O 3 -GeO 2 -Li 2 O were obtained in the crucibles of Pt and – 297 –
iflum * Electron image 1


Ge Ka1

Pb Ma1

Au La1


0 Ka1

Ge Ка1
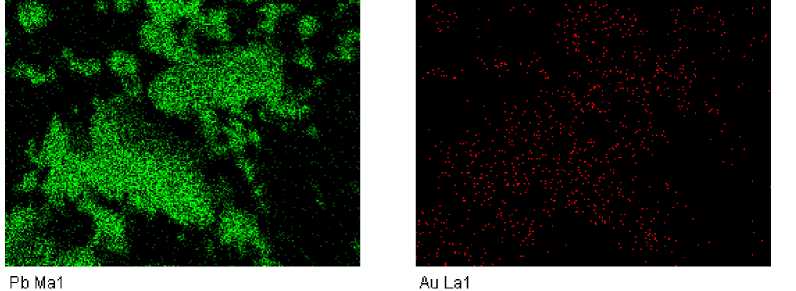
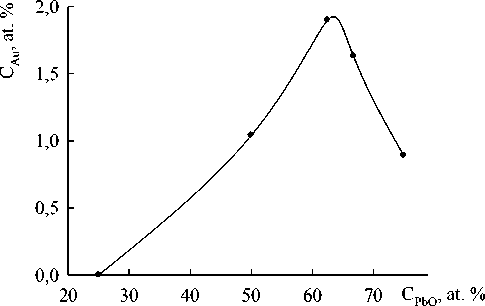
Fig. 4. The content of gold in PbO – GeO 2 alloys after the contact interaction of melts with the solid substrate (τ c)
Au [11]. At the same time Sanz O. et al . [11] emphasize that the color of obtained Bi 2 O 3 -based glasses can be determined by not only the crucible type but also Bi2O3 concentration, melting temperature and oxygen pressure. They distinguish two different coloration mechanisms of the glasses that can take place simultaneously: (i) due to the dissolution of Au and Pt nanoparticles from the crucibles, (ii) due to the segregation of Bi nanoparticles formed by a thermoreduction of Bi 2 O 3 . The amount of incorporated metal increases with the heavy metal oxide content as well as with the temperature in the case of glasses containing Bi 2 O 3 , whereas it decreases with the temperature if PbO is present.
The glasses containing metal nanoparticles of silver and gold were observed to be red in the transmitted light and green-gray in the reflected one [12].
Characteristic X-ray spectra of fragments of some solidified drops after the contact with gold were obtained with the use of energy dispersive microanalysis. They correspond to the energy spectra of gold (Fig. 2 and 3). It can be seen that gold is distributed practically uniformly in the PbO-GeO2 drops. The amount of gold in these drops depends on the melt composition (Fig. 4).
Gold almost doesn’t oxidize and the solubility of oxygen in it is near zero [13]. The authors of [14] noticed the difficulty of the generation of oxygen forms on the massive gold owing to the high energy barrier of oxygen adsorption. This allows assuming a physical dissolution of Au in the PbO – GeO2 melts.
Furthermore, the contact interaction of the PbO – GeO2 melts (45; 55; 80; 85; 90 and 100 mol. % of PbO) with solid silver was investigated. At a high concentration of PbO, very fast spread of the drops over the silver surface is found to take place. The solidified drops of PbO – GeO2 change color from translucent (45-55 mol. % of PbO) to deep-brown (80-100 mol. % of PbO).
Conclusions
The contact interaction of the PbO – GeO2 melts with Au, Ag, Al2O3 and BeO was studied using sessile drop method. Strong adhesion of these melts to the metal substrates was ascertained. The (PbO-GeO2)-Al2O3 interaction is accompanied by the dissolution of solid alumina in the molten glasses.


