Interactive effects of salinity and low potassium on growth, physiology response of Houttuynia cordata Thunb. W01-100
Автор: Zou Yu Ting, Dai Sha, Li Jing Ye, Liu Zheng Qiong, Wu Wei
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 4 т.8, 2012 года.
Бесплатный доступ
Houttuynia cordata Thunb. is a plant enrichment in potassium in plant was reported. Salinity and low potassium availability are important environmental factors restricting plant growth and productivity throughout the world. The interactive effects of salinity and potassium on growth, water content, chlorophyll content, lipid peroxidation content, ion accumulations and K+/Na+ ratio, and organic accumulations as well as oxidative enzymes were investigated in Houttuynia cordata Thunb.. Plants of three-leaf-stage were selected for uniformity, then treated with four levels of Na+ (50, 100, 200 mmol/L) and K+ (0, 0.6, 1.2, 2.4 mmol/L) for 20 days. Plant biomass production, ratio of root and shoot, root numbers, water content and MDA content significantly declined in the combined effect of salinity and K+ deprivation, and increased with salinity. However, salinity in conjunction with K+ deprivation led to an increase on leaf chlorophyll content, which even increased with increasing salinity levels. As expected, K+ content in plant was positive correlated with supplementary K+ concentrations, while Na+ was well correlated with salinity, especially enhanced by the interactive effects of salinity and K+ deprivation. Soluble sugar and proline contents remarkable increased by the highest salinity. SOD activity also substantial increased by the highest salinity, and increased with supplementary K+ concentrations. However, elevated CAT and POD activities were not accompanied with an increase in SOD activity.
Salinity, potassium, ion contents, organic accumulation, antioxidant enzymes
Короткий адрес: https://sciup.org/14323680
IDR: 14323680
Текст научной статьи Interactive effects of salinity and low potassium on growth, physiology response of Houttuynia cordata Thunb. W01-100
Symbols and Abbreviations: H. cordata, Houttuynia cordata Thunb.; GAP, Good Agricultural Practices; Na, sodium; K, potassium; MDA, malondialdehyde; SOD, superoxide dismudise; POD, peroxidase; CAT, catalase; H 2 O 2 , hydrogen peroxide; ROS, reactive oxygen species; ·O 2- , superoxide radicals.
Houttuynia cordata Thunb.(H. cordata) W01-100 is a pungent, heart-like leafed perennial herb and constitutes a single species of the genus Houttuynia. H. cordata has been identified as one of the most potential medical and edible wild plant resources (Wu et al., 2005a). It is mainly used to reduce fever to ease malnutrition, clear toxins in body, and also used to treat lung carbuncles, and has anti-bacterial and immunomodulatory properties (Chen et al., 2008) and recently, it has demonstrated considerable efficacy in anti-SARS (Lau et al., 2008). Addition of its young plant is popularly used as wild vegetable (Kim et al., 2001; Nuengchamnong et al., 2009). Due to these advantages of H. cordata, wild resources can not meet the great requirement. It was reported that H. cordata W01-100 was rich in potassium in plant, which had relatively higher absorbed K+ capability (Zou et al., 2011). Intensive research also reported that the optimal potassium concentration for H. cordata W01-100 culturing in MS medium was 1.28 mmol/L K+, which was much lower than other plants’ growth requirement (Xu et al., 2011).
Salinity in agricultural land is a major problem worldwide, placing a severe constraint on crop growth and productivity in many regions, and increased salinization of arable land is expected to have devastating global effects (Sobhanian et al., 2011). Therefore, salinity is one of the most significant abiotic factors limiting crop productivity (Gama et al., 2007; Munnas 1993). Growth inhibition and poor plant performance under saline conditions are attributed to osmotic stress imposed by salinity and to specific ion (Na+ in most cases) toxicity. The effects of mild cases of salt stress are primarily limited to plant growth, development, and crop productivity, but in extreme cases, salt stress can lead to plant death (Aoki et al., 2005).
It was reported that detrimental effects of death of these factors are crucially dependent on a plant’s ability to maintain K+ homeostasis and control K+ transport across cellular membranes (Shabala and Pottosin, 2010). It has also been suggested that the primary reason for salinity induced programmed cell death is the resultant depletion in cytosolic K+ (Hafsi et al., 2010). Study reported that potassium greatly lower ROS production by reducing activity of NAD (P) H oxidases (Cakmak, 2005). Zheng et al. (2008) demonstrated that suitable increment of K alleviated symptoms of the individual salt stress by improving growth of shoots and roots, reducing
MDA content, enhancing activities of anti-oxidative enzymes. These emphasize the pivotal role of intracellular K homeostasis in plant salt tolerance (Shabala and Cuin 2007; Cuin et al. 2008).
Therefore, the present experiment was conducted to study the influence of salinity and potassium deprivation on H. cordata W01-100, which was as a kind of plant enrichment in potassium. Changes in plant biomass, root number, ion and organic solutes and MDA accumulations, as well as anti-oxidative enzymes activities were analyzed.
MATERIALS AND METHODS
Plant materials and growth conditions
H. cordata (accession number, W01-100) was obtained from Good Agricultural Practices (GAP) base of 999 Pharmaceutical Group in China, which is planted as a new line for disease resistance and high-quality and yield (Wu et al., 2004). Its chromosome number is 90 (Wu et al., 2003). The plant first was cultured in Murashige and Skoog’s mediums, when it was at three-leaf-stage, sterile plants were selected. Plants were selected uniform to subject to salt stress treatment for 20 days. The study were both set four levels for sodium (Na+) (0, 50, 100, 200mmol/L) and potassium (K+) (0, 0.6, 1.2, 2.4mmol/L), listing in Table 1. Plant growth in a controlled sterile chamber under 12h of light and 12h of darkness with an average day/night temperature of 24/20 X , and a relative humidity of 50/60% day/night. During the entire cultured period, the photosynthetic photon flux density in the chamber was 30μmolm-2s-1. Light was provided by cool-white fluorescent lamps (Philips, China). The whole plants were harvested for determine.
Growth parameters and water contents
After 20d of treatment, the plants were harvested. Water content and growth parameters including fresh weight of aboveground and underground, dry weight of whole plant, root number were measured. The ratio of root and shoot is fresh weight ratio of underground and aboveground.
Chlorophyll contents
Chlorophyll contents were measured by a portable chlorophyll meter (SPAD-502, Konica Minolta, Tokyo, Japan) of the second fully expanded leaves from the top of individual plants. SPAD value was an indicator of chlorophyll content. The average value of three points per leaf at upper, middle and lower positions was used.
Na+, K +contents
The fresh plant were dried quickly at 105 °C for 30min, and then oven-dried at 70° C for 24h. The dried material was smashed to pass a 0.25mm sieve. The sample powder (0.1g) soaked in 10mL 1mmol/L hydrochloric vibrated for 30min, and filtered, and then take out 5mL settled to volumetric flask of 50mL with 1mmol/L hydrochloric acid. The extraction solution was used to determine Na+ and K+ concentrations by flame photometer. Foliar K+ content was calculated in terms of Ug-1 dry weight (DW).
Proline, soluble sugars determinations
To determine free proline level, 0.5g of leaf samples from each group were homogenized in 3% (w/v) sulphosalycylic acid and then homogenate filtered through filter paper (Xiong, 2008). Mixture was heated at 100°C for 40min in water bath after addition of acid ninhydrin and glacial acetic acid. After cooling the mixture, added 6.0mL of toluene. The chromophore-containing toluene was separated and absorption at 520nm was read, using toluene as a blank. Proline concentration use L- proline for the standard curve.
Soluble sugar content was determined by the method of Xiong (2008). A total of 0.3g of fresh tissue (leaf) was mixed with a 5.0mL aliquot of methanol (80%) in covered glass tubes and boiled at 70° C for 30min. After cooling the mixture, a 1mL aliquot of the extract was mixed with 1.0mL of phenol and 5.0mL of concentrated sulfuric acid. After agitation and cooling of the reagent mixture, A640 was read using methanol as a blank. Soluble sugar concentration was calculated using glucose solution as a standard curve. All the analyses (for proline and sugars) were carried out on both young and old leaves.
Lipid peroxidation
Malondialdehyde (MDA) was assayed as an end product of lipid peroxidation by the 2-thiobarbituric acid (TBA) reaction according to Steemart and Bewley (1980). Leaf samples (0.3g) were homogenized in 3mL of trichloroacetic acid (TCA). An equal volume of 0.5% thiobarbituric acid (TBA) was added and samples incubated at 95 °C for 30 min. The reaction stopped by putting the reaction tubes in the ice bath. The samples then centrifuged at 10000×g for 30min. The supernatant removed, absorption read at 532nm, and the amount of nonspecific absorption at 600nm read and subtracted from this value. The amount of MDA present calculated from the extinction coefficient of 155mM-1cm-1.
Enzyme assays
Leaves (0.2g) were homogenized in a mortar and pestle with 10mL 50mmol/L sodium phosphate buffer (pH 7.8 for SOD, pH 6.0 for POD and 7.0 for CAT) containing 1% insoluble polyvinylpyrrolidone (w/v). The homogenate was centrifuged at 15,000g for 10 min and the supernantant fraction was used as crude extract for enzyme activity. All operations were carried out at 4°C
Superoxidase dismutase (SOD) activity was determined by monitoring its ability to inhibit photochemical reduction of nitroblue tetrazolium (NBT) at 560 n. The reaction mixture (1.5mL) contained 50mM phosphate buffer (pH 7.8), 10μM ethylene diamine tetraacetic acid (EDTA) -Na 2 , 13mM L-methionine, 75μM NBT, 2μM riboflavin and 0.1mL enzyme extract. Riboflavin was added last and tubes were shaken and illuminated with a two 20-W fluorescent tubes. The reaction was allowed to proceed for 15min after which the lights were switched off and the tubes were covered with a black cloth. Absorbance of the reaction mixture was read at 569nm. A unit of activity was determined as the reduction of 50% NBT. Enzyme activity was calculated in terms of U/g FW.
Peroxidase (POD) activity was determined based on guaiacol oxidation. The reaction mixture contained of 1.0mL 50mmol/L sodium phosphate buffer (pH 6.0), 0.4mL 5mmol/L guaiacol, 0.2mL 10mmol/L H 2 O 2 , 0.02 mL enzyme extract and 0.2mL distilled water. Increase in the absorbance due to oxidation of guaiacol was measured at 470nm. One unit of activity was determined by the variety of 0.01/min. Enzyme activity was expressed as U/g FW.
Catalase (CAT) activity was measured according to Aebi (1984). About 2mL reaction mixture containing 0.9mL 100mmol/L sodium phosphate buffer (pH 7.0), 0.3 mmol/L H2O2, 0.1 mL enzyme extract and distilled water to make up the volume to 2mL. Reaction started by adding H2O2 and decrease in absorbance recorded at 240 nm for 5mins, reading one time every minute. CAT activity was computed by calculating the amount of hydrogen peroxide (H2O2) decomposed. One unit of activity was determined by the variety of 0.01/min. Enzyme activity was calculated in terms of U/g FW.
Statistical analysis
Values were presented as means ± standard errors (SE) from three independent treatments. These data were subjected to analysis of variance and Duncan’s multiple range test ( P < 0.05) using SPSS version 17.0.
RESULTS
Growth parameters
Both salinity and K+ deprivation resulted in a significant reduction in plant biomass of H. cordata. Yellow, wilted, shrinkage leaves were observed in salinity and K+ starvation treatments. A considerable decrease in whole plant biomass production was registered when the two constraints were applied simultaneously (Table 1). Plant biomass increased with the increasing K+ when treated with the same salt levels (Table 1). The ratio of root and shoot decreased caused by increasing salinity, while increasing with supplied K+ concentrations excepted in the highest salinity (Table 1). Table 1 showed root numbers were significantly decreased in the combine effect of salinity and K+ deprivation, and decreased with increasing salinity. Root numbers were increased by supplied K+ concentrations under the same salinity.
Water and chlorophyll contents
Table 1 showed that the lowest water content was observed in leaves of plants subjected to the combined effects of highest salt stress and K+ deprivation. Water content increased significantly with increasing K+ regardless of salinity levels. Opposite to the variation of water content, chlorophyll content and salinity concentration showed a positive correlation. High salt stress resulted in water lose in plant and coarse small shrinkage of blade was observed in high salt concentrations treatments. The highest salinity showed the highest chlorophyll content no matter what K+ concentrations was.
K+ and Na+contents
Na+ content in plants increased substantially with increasing salinity, and reached the maximum value at the highest salinity and K+-deficiency treatment (Table 2). But Na+ content significantly decreased by supplementary K+. K+ content was negative with salinity levels, but had a positive correlation with supplementary K+. And K+/Na+ ratio was also positive associated with K+ concentrations in low and mid salinity. However, the K+/Na+ ratio showed no significantly difference in high salinity with different K+ concentrations.
Proline and soluble sugar content
Proline content in fresh leaves of H. cordata W01-100 significantly increased by the combined effect of salinity and K+ starvation, and reached the peak at the highest salinity (Fig. 1a). Plants treated with the same salinity, proline content increased with the supplied potassium concentrations; and the same trend was observed under the same supplementary K+. The soluble sugar content was greatly increased in the highest salinity level (200 mmol/L), and being highest also in the highest salinity in conjunction with K+ deprivation (Fig. 1b). Then under the same salinity, soluble sugar content decreased with increasing K+, but was positive associated with salinity.
Lipid peroxidation
Fig. 2a showed that MDA content was significantly affected by the combined effect of salinity and K+ deprivation, and reached the maximum value at the highest salinity. The supplied K+ decreased MDA content in leaf. Plant being treated in the same K+ concentration, MDA content increased with increasing salinity.
Table 1 K+ and Na+ contents of H. cordata grown in mediums containing different concentrations of K+ and Na+
|
K+ content of plant |
Na+content of plant |
K+ /Na+ ratio |
|
|
K0-50 |
1.32±0.00 D |
2.25±0.05 G |
0.58±0.01 BC |
|
K0.6-50 |
1.69±0.00 C |
2.41±0.02 FG |
0.70±0.01 BC |
|
K1.2-50 |
1.97±0.04 A |
2.22±0.05 G |
0.89±0.00 BC |
|
K2.4-50 |
1.93±0.00 A |
1.91±0.03 H |
1.01±0.02 A |
|
K0-100 |
1.03±0.04 E |
3.73±0.06 C |
0.28±0.01 D |
|
K0.6-100 |
1.40±0.04 F |
3.56±0.06 CD |
0.39±0.01 DE |
|
K1.2-100 |
1.77±0.08 BC |
2.88±0.09 E |
0.61±0.03 CD |
|
K2.4-100 |
1.89±0.04 AB |
2.54±0.02 F |
0.74±0.01 BC |
|
K0-200 |
0.95±0.07 E |
4.77±0.03 A |
0.20±0.02 D |
|
K0.6-200 |
1.36±0.04 D |
4.28±0.09 B |
0.32±0.01 DE |
|
K1.2-200 |
1.40±0.04 D |
3.48±0.07 D |
0.40±0.01 DE |
|
K2.4-200 |
1.36±0.04 D |
2.98±0.03 E |
0.46±0.02 DE |
Note: K 0.6-0 , K 0.6-50 , K 0.6-100 , K 0.6-200 represent K and Na concentration were 0.6 and 0, 50, 100, 200 mmom/L, 0 and 100 mmom/L, respectively. The rest can be done in the same manner. Each value is mean±SE (n=3). Values in the same column followed by the diffierent letters are significantly different at 1% ( P <0.01) according to LSD multiple-range test.
Table 2 The interactive effects of salt and K+ concentrations on growth parameters, water and chlorophyll contents of H. cordata
|
No. |
Dry weights |
Ratio of root/shoot |
Root numbers |
Water contents |
Chlorophyll contents |
|
K0-50 |
4.34±0.16 C |
0.21±0.01 AB |
8.60±0.20 AB |
14.01±0.01 D |
27.55±2.04 DE |
|
K0.6-50 |
4.02±0.01 C |
0.21±0 BC AB |
7.40±0.72 BC |
11.10±0.02 F |
27.29±1.20 DE |
|
K1.2-50 |
6.23±0.03 A |
0.17±0.02 ABC |
7.67±0.18 BC |
22.16±0.01 A |
29.89±0.76 ABCDE |
|
K2.4-50 |
5.19±0.01 B |
0.15±0.01 ABC |
9.73±0.07 A |
12.05±0.05 B |
30.05±0.72 ABCDE |
|
K0-100 |
2.98±0.04 D |
0.14±0.02 CDE |
5.33±0.87 E |
16.93±0 B |
30.8±0.65 ABCD |
|
K0.6-100 |
5.24±0.06 B |
0.14±0.01 CDE |
5.87±0.35 DE |
14.28±0.07 C |
30.44±1.17 ABCDE |
|
K1.2-100 |
5.43±0.22 B |
0.13±0.01 CDE |
6.33±0.29 D |
9.63±0.01 H |
28.85±0.48 CDE |
|
K2.4-100 |
4.21±0.05 C |
0.15±0.01 CDE |
8.33±0.97 BC |
13.12±0.01 E |
29.37±1.07 BCDE |
|
K0-200 |
2.30±0.04 E |
0.03±0.02 E |
0.87±0.68 H |
2.95±0.06 K |
31.5±1.64 ABCD |
|
K0.6-200 |
1.16±0.03 F |
0.02±0.01 E |
0.40±0.23 H |
7.87±0.02 I |
34.29±2.20 A |
|
K 1.2-200 |
2.97±0.09 D |
0.12±0.02 DE |
1.90±0.25 FGI |
6.85±0.05 J |
32.65±0.87 ABC |
|
K2.4-200 |
3.17±0.06 D |
0.04±0.01 E |
3.40±0.20 F |
10.01±0.02 G |
33.44±0.90 AB |
Note: K 0.6-0 , K 0.6-50 , K 0.6-100 , K 0.6-200 represent K and Na concentration were 0.6 and 0, 50, 100, 200 mmom/L, 0 and 100 mmom/L, respectively. The rest can be done in the same manner. Each value is mean±SE (n=3). Values in the same column followed by the diffierent letters are significantly different at 1% ( P <0.01) according to LSD multiple-range test.
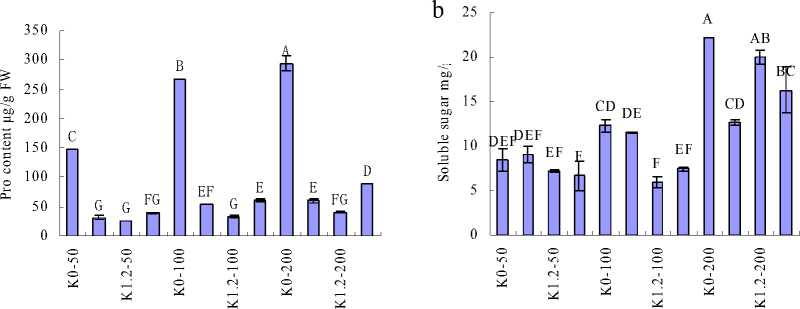
Figure 1 Proline and soluble sugar contents of H. cordata grown in the mediums containing different concentrations of K+ and Na+. (a) Proline content; (b) soluble sugar content. Note: Values are means±SE (n=3), vertical line on top of the bars means SE. Bars carrying different letters are significantly different at P < 0.05.
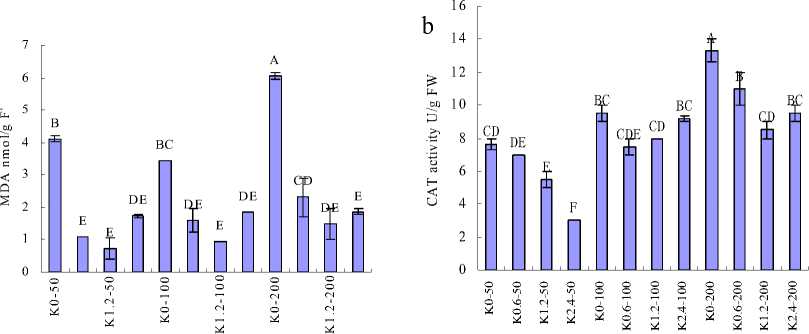
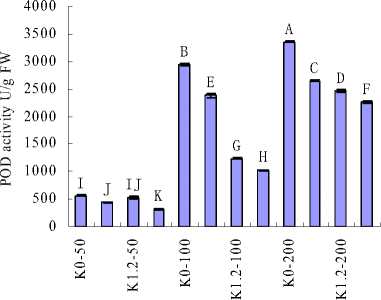
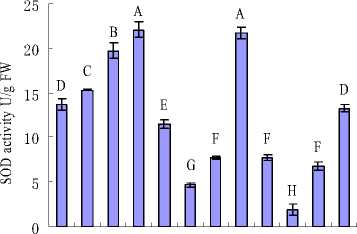
Figure 2 MDA content and Antioxidative enzyme activities of H. cordata grown in mediums containing different concentrations of K+ and Na+. (a) MDA; (b) CAT; (c) POD and (d) SOD. Note: Values are means±SE (n=3), vertical line on top of the bars means SE. Bars carrying different letters are significantly different at P < 0.05.
Enzymes activities
DISCUSSION
Heavy salinity stress generally leads to growth arrest and even plant death (Parida and Das, 2005). In the present study, salinity also led to a reduction in biomass production, but increased with increasing supplementary K+. Growth was more affected by the interactive effects of salinity and K+ deprivation than either factor alone. This could be partially attributed to a substantial reduction in root growth, water content and mineral nutrient absorption in plant, as a result of high osmotic pressure in plant cells. Study reported that salt stress had no significant effect in leaf chlorophyll content (Li et al., 2010). Cha-um et al. (2010) reported that salinity significantly decreased leaf chlorophyll content, and supplementary potassium marked enhanced chlorophyll content. However, in this research, leaf chlorophyll content had a substantial enhance with increasing salinity, reaching the maximum value at the highest salinity. It was suggested that high salinity resulting in much water losing, led to chloroplast shrink, thus leaf chlorophyll content increased (Salin, 1987; Valentina, 2000).
The lipid peroxidation of membranes has been considered a good marker for oxidative damage and results from a degradation of polyunsaturated fatty acids, which severely affects their functionality causing irreversible damage (Halliwell and Gutteridge, 1989). Our data showed a considerable increased of leaf MDA content by increasing salinity, and being highest in plants subjected to the combined effects of salinity and K+ deprivation, which was in well accordance with Hernandez et al. (2010). This reflected the severe oxidative injury of membrane. Supplementary K+ decreased lipid peroxidation content in high salinity treatments. Hafsi et al. (2011) also reported that supplied K+ nutrient obviously declined MDA content of Hordeum . The results confirmed the significance of K+ nutrition in alleviating the detrimental effects of salinity stress on biological membranes. Therefore, the observed increase in the level of lipid peroxidation reflects impairment of membrane integrity.
Plants growth inhibition and poor plant performance under saline conditions are attributed to osmotic stress imposed by salinity and to specific ion (Na+ in most cases) toxicity. To deal with osmotic stress and maintain sufficient turgor pressure required extension growth in roots and shoots, the primary physiological response of plants is to undergo osmotic adjustment through two processes: accumulation of ions in the vacuole and synthesis of organic solutes in the cytosol (Lee et al., 2008). Halophytes in saline conditions usually accumulate inorganic ions in vacuoles to decrease cell water potential because the energy consumption from absorbing inorganic ions is far less than from synthesizing organic compounds (Munns, 2002). In the present study, low salinity had relative higher K+ content in plant, compared to the highest salinity, and enhanced its content with increasing supplied K+. This also reflected in the enhanced plants biomass productions and root numbers in low salinity and higher supplied K+ treatment. The increasion and reduction of Na+ and K+ contens in plants, indicating that a competitive inhibition between the absorption of Na+ and K+, similar problem also reported in Parida and Das (2005) and Li (2010). Not only Na+ and K+ contents, but also K+/Na+ ratio can be used as phyto-physiological for salt stress (Keutgen and Pawelzik, 2008). In this study, increasing K+ concentration enhance K+/Na+ ratio, but showed no significant difference in high salinity. It was may existed a positive feedback regulation that plants cultured in high salinity stressed stimulated root growth to absorb mineral nutrients, but higher osmotic inhibited nutrient transferred to aboveground. Therefore, plants treated in high salinity K+/Na+ ratio showed no difference.
Meanwhile, H. cordata W01-100 can also synthesize compatible organic solutes to prevent dehydration (Lee et al., 2008). Furthermore, some of these organic solutes also can protect biomacromolecules in cytoplasm (Parida and Das, 2005). Soluble sugars and proline accumulation in plant play an important role in turgor maintenance, which consider as an adaptive trait concerned with stress tolerance (Najafi, 2010; Rhodes and Hanson, 1993). In the present study, it evident that soluble sugar and proline contents in fresh leaf of H. cordata W01-100 increased not only with increasing salinity but also with increasing supplementary K+. This suggested that the induction of the synthesis of soluble sugar and proline was associated both salinity and supplementary K+. The data indicated that accumulation of soluble sugar and proline might not only being as osmolyte and protectant, but also have other roles related to K+ physiological metabolic activities. The increase in proline content under stress condition may be due to breakdown of proline rich protein or de novo synthesis of proline (Tewari and Singh, 1991). The correlation between K+ and soluble sugar and proline accumulation should be investigated further.
Recently, a third component, an ROS-induced damage to key macromolecules and proteins, has been added to the list of detrimental effects of salinity (Zhu, 2003; Tester and Savenport, 2003). To minimize the effects of oxidative stress, plant cells have developed a complex antioxidative system, including low-molecular mass antioxidants as well as antioxidative enzymes, such as SOD, POD, CAT (Noctor and Foyer, 1998). SOD represents the first line in the removal of ROS in cells, it converts superoxide (O2-) to hydrogen peroxide (H2O2) and molecular oxygen (O2) (Mittler, 2002). In this work, a decrease in total SOD activity as a consequence of salinity was observed. Preview study had demonstrated that leaf SOD activity increased with salinity levels (Hernandez et al., 2010; Koca et al., 2007), which was against with this study. However, it was implied that an increase of O2- under low salinity compared with high salinity. In another way, elevated SOD activity was not accompanied with an increase in the activities of major H2O2 scavenging enzymes CAT and POD. CAT and POD activities increased as a result of increasing salinity, but decreased with supplementary K+ concentrations when under the same salinity. It was indicated that the increase of O2- might not only accumulation from salinity stress, but also from other sources. It was reported that oxygen-dependence photosynthesis may be “leaking” energy to molecular oxygen, forming ROS such as O2- (Wise and Naylor, 1987; Ort and Baker, 2002). Therefore, it implied that accumulation of O2- might be partially suppressed by the low photosynthesis rate in these treatments. However, it can not exclude the possibility that there might be other antioxidant compounds in H. cordata which could be more effective under salinity stress.
ACKNOWLEDGMENTS
Список литературы Interactive effects of salinity and low potassium on growth, physiology response of Houttuynia cordata Thunb. W01-100
- Aebi, H., (1984). Catalase in vitro. Method of enzymology. 105: 121-126.
- Aoki, A., Kanegami, A., Mihara, M., Kojima,T.T., Shiraiwa, M. and Takahara H., (2005). Molecular cloning and characterization of a novel soybean gene encoding a leucine-zipper-like protein induced to salt stress. Gene., 356: 135-145.
- Cakmak, I. (2005). The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J. Plant Nutr. Soil Sci. 168: 521-530.
- Cha-um, S., Siringam, K., Juntawong, N. and Kirdmanee, C. (2010). Water relations, pigment stabilization, photosynthetic abilities and growth improvement in salt stressed rice plants treated with exogenous potassium nitrate application. Int. J. Plant Prod. 4 (3): 187-198.
- Chen, L., Wu, W., Huang, C.Y., Yang, Y.X. and Zheng Y.L. (2008). Composition and variablity of the essential oil of Houttuynia of China. Chem. Natur. Comp. 44: 778-783.
- Cuin, T.A., Miller, A.J., Laurie, S.A. and Leigh, R.A. (2003). Potassium activities in cell compartments of slat-grown barley leaves. J. Exp. Bot. 54: 657-661.
- Gama, P.B., Nagana, S.I.S., Tanaka, K. and Nakazawa R. (2007). Physiological response of common bean (Phaseeolus Vulg. L.) seedlings to salinity stress. Afri. J. Bio. 2: 79-88.
- Hafsi, C., Romero-Puertas·L, M.C., del Río·L, A., Sandalio, M. and Abdelly C. (2010). Differential antioxidative response in barley leaves subjected to the interactive effects of salinity and potassium deprivation. Plant Soil, 334: 449-460.
- Halliwell, B. and Gutteridge, J.M.C. (1989). Free radicals in biology and medicine. 2nd end, Clarendon, Oxford. pp: 145-176.
- Hernandez, M., Fernandez-Garcia, N., Diaz-Vivancos, P. and Olmos E. (2010). A different role for hydrogen peroxide and the antioxidative system under short and long salt stress in Brassica oleracea roots. J. Exp. Bot., 61 (2): 521-535.
- Keutgen, A.J. and Pawelzik E. (2008). Impacts of NaCl stress on plant growth and mineral nutrient assimilation in two cultivars of strawberry. Environ. Exp. Bot. 65: 170-176.
- Kim, S.K., Ryu, S., No, J., Choi, S. and Kim Y. (2001). Cytotoxicalk aloids from Houttuynia cordate. Arch. Pharm. Res. 24: 518-521.
- Koca, H., Bor, M., özdemir, F. and Türkan I. (2007). The effect of salt on lipid peroxidation, antioxidative enzymes and proline content of sesame cultivars. Environ. Exp. Bot. 60: 344-351.
- Lau, K.M., Lee, K.M., Koon, C.M., Cheung, C.S.F., Lau, C.P., Lee, M.Y.H. and Cheng C.H.K. (2008). Immunomodulatory and anti-SARS activities of Houttuynia cordata. J. Ethnopharmacol., 118(1): 79-85.
- Lee, G., Carrow, R.N., Duncan, R.R., Eiteman, M.A. and Rieger M.W. (2008). Synthesis of organic osmolytes and salt tolerance mechanisms in Paspalum vaginatum. Environ. Exp. Bot. 63: 19-27.
- Li, R., Shi, F. and Fukuda, K. (2010). Interactive effects of various salt and alkali stresses on growth, organic solutes, and cation accumulation in a halophyte spartibina alterniflora (Poaceae). Environ. Exp. Bot. 68: 66-74.
- Mittler, R. (2002). Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7: 405-410.
- Munnas, R. (1993). Physiological processes limiting plant growth insaline soils: some dogmas and hypotheses. Plant Cell Environ. 16: 15-24.
- Munns, R. (2002). Comparative physiology of salt and water stress. Plant Cell Environ., 25: 239-250.
- Najiafi, F., Khavari-Nejad, R.A. and Siah M. (2010). The effects of salt stress on certain physiological parameters in summer savory (Satureja hortensis L.) plants. Journal of Stress Physiology & Biochemistry 6 (1): 15-21.
- Noctor, G. and Foyer, C. (1998). Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol., 49: 249-279.
- Nuengchamnong, N., Krittasilp, K. and Ingkaninan K. (2009). Rapid screening and identification of antioxidants in aqueous extracts of Houttuynia cordata using LC-ESI-MS coupled with DPPH assay. Food Chem. 117: 750-756.
- Ort, D.R. and Baker, N.R. (2002). A photoprotective role for O2 as an alternative electron sink in photosynthesis? Curr. Opin. Plant Biol. 5: 193-198.
- Parida, A.K. and Das A.B. (2005). Salt tolerance and salinity effects on plants. Ecotox. Environ. Safe. 60: 324-349.
- Rhodes, D. and Hanson, A.D. (1993). Quaternary ammonium and tertiary sulfonium compounds in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 44: 375-384
- Tester, M. and Davenport, R. (2003). Na+ tolerance and Na+ transport in higher plants. Annu. Bot., 91: 503-527.
- Tewari, T.N. and Singh, B.B. (1991). Stress studies in lentil (Lens esculenta Moench). Ⅱ. Sodicity-induced changes in chlorophyll, nitrate, nitrite reductase, nucleic acids, proline, yield and yield components in lentil. Plant Soil. 135: 225-250.
- Salin, M.L. (1987). Toxic oxygen species and protective systems of the chloroplast. Physiol. Plant. 72: 681-689.
- Shabala, S., and Pottosin, L.L. (2010). Potassium and potassium-permeable channels in plant salt tolerance. Ion channels and plant stress responses, signaling and communication in palnts. pp: 87-110.
- Shabala, S.N. and Cuin, T.A. (2007). Potassium transport and plant salt tolerance. Physiol. Plant. 133: 651-669.
- Sobhanian, H., Aghaei, K. and Komatsu, S. (2011). Changes in the plant proteome resulting from salt stress: toward the creation of salt-tolerant crops. J. Proteomics: 1-15.
- Stewart, R.R.C., and Ewley J.D. (1980). Lipid peroxidation associated aging of soybean axes. Plant Physiol. 65: 245-248.
- Valentina, M. (2000). Activities of SOD and the ascorbateglutathione cycle enzymes in subcellular compartmnts in leaves and roots of the cultivated tomato and its wild salt tolerant relative Lycopersicon pennellii. Plant Physiol. 110 (1): 42-51.
- Wise, R.R. and Naylor A.W. (1987). Chilling-enhanced photooxidation: evidence for the role of singlet oxygen and superoxide in the breakdown of pigments and endogenous antioxidants. Plant Physiol. 83: 278-282.
- Wu, W., Zheng,Y.L., Chen, L., Wei,Y.M.and Yan Z.H. (2005a). Genetic diversity among the germplasm resources of the genus Houttuynia Thunb. in China based on RAMP markers. Gene. Resour. Crop Evol. 52: 473-482.
- Wu, W., Zheng, Y.L., Yang, R.W., Chen, L. and Wei, Y.M. (2003). Variation of chromosome number and cytomixis of Houttuynia cordata Thunb. from China. Acta. Phytotaxon Sinica. 41: 245-257.
- Wu, W., Zheng, Y.L., Yang, R.W., Chen, L. and Wei, Y.M. (2003). Variation of chromosome number and cytomixis of Houttuynia cordata Thunb. from China. Acta. Phytotaxon. Sin. 41: 245-257.
- Xiong, Q.E. (2008). The course instruction of plant physiology lab laboratory. Sichuan science and technology press. Pp: 88-123.
- Xu, Y.W., Zou, Y.T., HUsaini, A.M., Zeng,J.W., Guan, L.L, Liu, Q. and Wu, W. (2011). Optimization of potassium for proper growth and physioloical response of Houttuynia cordata Thunb.. Environ. Exp. Bot. 71: 292-297.
- Zheng, Y., Jia, A., Ning, T., Xu, J., Li, Z. and Jiang, G. (2008). Potassium nitrate application alleviates sodium chloride stress in winter wheat cultivars differing in salt tolerance. J. Plant Physiol., 165: 1455-1465.
- Zhu, J.K. (2003). Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 6: 441-445.
- Zou, Y.T., Wu, W., Dai, S., Zeng, J.W. and Li, J.Y. (2011). Screening Houttuynia Thunb. for genotypes of the capability of enrichment in potassium. J. Soil Water Conserv. 25 (6): 260-264.


