Investigating the ability of diverse native bacillus species to produce hydrolytic enzymes using agar plate assay
Автор: Harba M., Bakri Y., Jawhar M.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 4 т.18, 2022 года.
Бесплатный доступ
Hydrolytic enzymes of Bacillus attracting a great attention due to their various applications in industrial bioprocesses. In this work, out of 525 Bacillus isolates, 40 were screened for their potential capacity to produce hydrolytic enzymes including xylanase, lipase, amylase, pectinase, protease and carboxymethyl cellulose (CMC) using agar plate assay. A pure culture of each isolate was streaked on the surface of agar media containing suitable substrate specific for every enzyme activity, and the diameter of hydrolytic zones were measured. Data showed clear zones around the colonies which were interpreted as evidence of the enzymes activities. The isolates were able to generate at least one of these enzymes among which CMC and protease were the most common enzymes detected in 40 isolates; amylase in 39, xylanase in 36, lipase in 32 and pectinase in 26. Based on 16S rRNA gene sequencing the isolates were classified as B. atrophaeus , B. amyloliquefaciens , B. subtilis , Paenibacillus polymyxa , B. simplex and B. tequilensis . The native B. tequilensis isolates formed the largest zone clearance and had high abilities to produce five hydrolases. The isolates which had the largest enzymatic activity zones and the largest diameter of the clear hydrolysis zones on agar plates were submitted for further research work and enzyme-based industrials.
Xylanase, lipase, amylase, pectinase, protease, carboxymethyl cellulose, bacillus species, agar assay
Короткий адрес: https://sciup.org/143179362
IDR: 143179362
Текст научной статьи Investigating the ability of diverse native bacillus species to produce hydrolytic enzymes using agar plate assay
Microbial hydrolytic enzymes are of great importance in view of their applications in different industrial bioprocesses (Hossain et al., 0 0). Bacillus has been considered as the bacterium of the choice due to its metabolic diversity to produce a high number of these enzymes with useful and/or novel characteristics (Danilova et al., 0 0). Therefore, improvement of new strains for high enzyme production is needed for reducing the cost of the industrial process and might also have some specialized attractive characteristics.
Bacillus species having extracellular xylanolytic, amylolytic, proteolytic, lipolytic, pectinolytic, cellulolytic, chitinolytic activities, which have great commercial value due to their important applications in many industrial bioprocesses ( lrumman et al., 018). These enzymes meet industrial demand since they can excrete extracellularly, facilitating extraction from fermentation media (Raveendran et al., 018; Harba et al., 0 0).
pplication of modern techniques to improve the hydrolytic enzyme production does not invalidate the search for wild organisms producing useful enzymes (Baweja et al., 016). However, identification of naturally occurring bacteria might be the best way to find new strains for producing hydrolytic enzymes for industrial purposes. Furthermore, due to the demand to obtain these enzymes with specific processing characteristics, particularly in developing countries with low technological capabilities, screening isolates using simple in vitro assay will be quite cheap.
Despite the biotechnological prospects of Bacillus sp. in addition to its importance to the host, essential information on its hydrolytic enzyme potential has remained unknown. Furthermore, one of the main problems in screening large number of microbial strains for their enzymatic hydrolases producing ability is the lack of simple and fast reliable screening technique (Tseng et al., 000), therefore, agar plate screening method was tested here. Hence, in the present work, production of six hydrolytic enzymes from a set of Bacillus sp, i.e. B. atrophaeus, B. amyloliquefaciens, B. subtilis, B. simplex, Paenibacillus polymyxa and B. tequilensis was compared under agar media containing specific substrate for each enzyme to determine their potential as sources of industrial enzymes.
MATERIALS AND METHODS
Bacterial isolates
Soil samples (at 4 cm depth) were randomly taken from different geographical regions of Syria. One gram of each sample was mixed with 10 mL of sterile distilled water. Serial dilution was made from 10-3 to 10( mmouneh et al., 011), and 1 mL of each dilution was transferred onto sterilized Nutrient gar (N ) medium and incubated overnight, the colonies of prospective Bacillus sp. were identified according to Wulff et al. ( 00 ).
Identification of the selected isolates
The 16S rRN genes were amplified by PCR using forward and reverse universal primers: BacF (5’ GTGCCT T C TGC GTC-3’) and BcaR (5’-
CTTT CGCCC T TTCC-3’) (Nair et al., 00 ). The PCR reaction mixture contained μl genomic DN , 1x reaction buffer (Tris Cl-MgCl ), 0. mM dNTP, mM MgCl , 1 μM of each primer, and 5U/μl Taq polymerase (Fermentas). PCR steps were as follows: denaturation step at 95 °C for 5 min followed by a second denaturation step at 95 °C for 1 min, annealing for 1 min at 54 °C, an extension at 7 °C for 90 s, and a final extension step of 7 °C for 10 min. total of 30 serial cycles of PCR amplification was performed. PCR products were run on a 1.5% agarose gel, stained with ethidium bromide and then visualized by UV light (30 nm). Before sequencing, PCR products were purified with QI gen gel extraction kit. Sequencing was done using a BI 310 nalyzer (Perklin-elmer, pplied Biosystems, US ), and the sequences were compared using the NCBI database.
Hydrolytic enzymes production
ll the identified isolates were examined for the production of hydrolytic enzymes (xylanase, lipase, amylase, pectinase, protease and CMC) using typical methodologies as illustrated below:
Xylanolytic activity
Xylanolytic activity of the isolates was tested in agar medium supplemented with 05% oat spelts xylan. fter the incubation period, xylanolytic activity of the isolates was detected by clear zones around the colonies using remazol brilliant blue dyed xylan (Ellis and Magnuson, 01 ).
Proteolytic activity
Proteolytic activity was tested by incubating Bacillus isolates on gelatin-agar media (10 g/L gelatin, 5 g/L tryptone, 1 g/L glucose, .5 g/L yeast extract, 0 g/L agar under pH 7) and incubated for 48 h at 30°C. Transparent circles around the colonies were observed after staining with mercuric chloride solution (Fry et al., 1994).
lipolytic activity
For lipolytic activity, the isolates were inoculated on agar media (15 mL/L Tween 80, 5 g/L tryptone, .5 g/L yeast extract, 5 g/L NaCl, 0 g/L agar under pH 7) and incubated for 48 h at 30°C. The appearance of clear zones after staining with methyl red solution indicated the presence of lipolytic activity (Samad et al., 1989).
Pectinolytic activity
For pectinolytic activity, isolates grown on pectic agar medium after it was stained by Cetyl trimethyl ammonium bromide. and examined for the appearance of clear zones to confirm pectinase production (Beg et al., 000).
Cellulolytic activity
For the determination of cellulolytic activity, the isolates were inoculated onto 0.5% CMC-agar plates for 48 h at 30°C. fter incubation, the plates were stained for 10 min with Congo red solution ( g/L) and next destained with NaCl (1 M) for 15 min; clear zones surrounding the colonies indicated cellulase production (Meddeb-Mouelhi et al., 014).
Amylolytic activity
For amylolytic activity, bacteria grown on starch-agar media (10 g/L soluble starch, 5 g/L tryptone, 3 g/L yeast extract, 0 g/L agar under pH 7) solution; transparent zones surrounding the colonies indicated amylase production after staining by remazol dye ( moozegar et al., 003).
Data analysis
Bacterial identification and clear zone observations of hydrolytic enzyme-producing bacillus were analyzed descriptively and presented in figures and tables. Qualitative testing of the hydrolytic enzyme-producing bacillus spp. was done by observing the clear zones around the bacterial colonies and then dividing the diameter of the clear zone with the bacterial colony diameter ( shok et al., 019). The results for the diameter were expressed as relative enzymatic activities.
RESULTS AND DISCUSSION
Based on colony morphology, each distinct morphological character was considered as different bacterial species, and by using 16S rRN gene sequencing method these species were identified as B. atrophaeus, B. amyloliquefaciens, B. subtilis, B. simplex, Paenibacillus polymyxa and B. tequilensis as their sequences confirmed similarities ≤ 98% to their closely related type isolates (Table 1; Fig. 1).
collection of 40 isolates of the Bacillus spp. were screened for their abilities to produce hydrolytic enzymes such as xylanase, lipase, amylase, pectinase, protease and CMC enzymes in agar plate tests. The results of the observations of the enzymatic activity revealed clearance zones around the inoculated sites as a result of hydrolytic enzymes activity for 3 days on agar media containing specific substrate for each enzyme (Fig. ).
Comparative analysis of enzymes activity of Bacillus spp. demonstrated that the tested Bacillus spp. isolates had significantly different levels of enzyme activity through formation of clear zones around the colonies (Fig. 3). It was found that the isolates had the abilities to produce at least one of these enzymes among which CMC and protease were the most common enzymes detected in 40 isolates; amylase in 39, xylanase in 36, lipase in 3 and pectinase in 6 (Table ). However, Bacillus sp. had the highest CMC hydrolysis zone (3.8 cm) followed by protease hydrolysis zone ( .3 cm). In addition, pectinolytic activity that degrades pectin, a plant-based biopolymer, was detected in rather higher hydrolytic zones (1.87 cm) as shown in Fig ure 3. The diameter of the hydrolysis zone showed the concentration and enzymatic activity produced (Palmer, 1995).
The results showed formation of a clear zone around the colonies on agar medium containing xylan, which indicated the ability of colonies to produce xylanase. This is in line with findings of Varghese et al. ( 017). In addition, the proteolytic clear zone produced by Bacillus sp. occurs due to protease activity which catalyzes the hydrolysis of the protein molecules into large fragments and peptidases the hydrolyze polypeptide fragments into amino acids (Razzaq et al., 019).
The data showed that Bacillus sp. had the ability to produce the amylase enzyme since 39 (97.5%) of the isolates produced detectable quantities of this enzyme in the agar media (Fig. ) which gives a direct visual indication of starch hydrolysis that appeared as clear zones around the bacterial colonies (Dhawale et al., 198 ). dditionally, Bacillus sp. could hydrolyze the Carboxymethyl cellulose contained in the media and Table 1: Bacillus species used in the study.
produced a large CMC hydrolysis zone (3.8 cm) which reflect that the polysaccharides have been degraded into saccharides with shorter chains (Zhou et al., 016).
The present study was focused on screening Bacillus sp. for their abilities to produce hydrolytic enzymes including xylanase, lipase, amylase, pectinase, protease and CMC using agar plate assay. Data showed that Bacillus sp. could generate at least one of these enzymes among which CMC and protease were the most common enzymes detected in 40 isolates, and the largest hydrolysis zones were observed for CMC (3.8 cm) and protease ( .3 cm). The isolates which had the largest enzymatic activity zones and the largest diameter of the clear hydrolysis zone will be tested to characterize these enzymes and determine their biochemical properties.
|
Bacillus Species |
Colony morphology |
|
Atrophaeus Amyloliquefaciens Paenibacillus |
Brown-black, opaque, smooth, circular Creamy white with irregular margins Milky white, thin often with amoeboid spreading |
|
Subtilis |
Fuzzy white, opaque, rough, with jagged edges |
|
Simplex Tequilensis |
Cream, gloss, with irregular margins slightly raised Yellowish, opaque, smooth, circular |
Table 2: Enzymatic activity of Bacillus species used in the study.
|
Characteristics |
B. airophaeus |
B. subtilts |
B. polymyxa |
B. amyloliquejaclens |
B. tequilensis |
B. simplex |
Positive isolate numbers |
Percentage of positive isolates |
|
Isolate numbers |
3 |
20 |
2 |
10 |
4 |
1 |
||
|
Enzymatic activities |
0 |
20 |
2 |
10 |
4 |
0 |
||
|
Xvlanase |
- |
- |
- |
- |
36 |
90 |
||
|
Amvlase |
— |
- |
- |
— |
- |
39 |
97.5 |
|
|
Protease |
- |
- |
- |
- |
- |
- |
40 |
100 |
|
CMC |
4- |
4- |
4 |
4- |
4- |
4 |
40 |
100 |
|
Lipase |
- |
- |
- |
- |
- |
32 |
80 |
|
|
Pectinase |
- |
- |
— |
- |
26 |
65 |
Presence of enzyme activity’ and -: Absent of enzyme activity
bp M 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20
Figure 1: garose gel electrophoresis of 16S rRN of some Bacillus sp. isolates used in the study. M represents the 100-bp DN marker (HinfI; MBI Fermentas, York, U ).
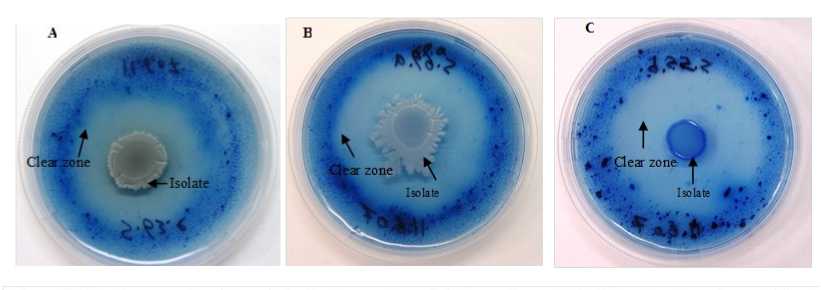
Figure 2: The clear zone of amylase activity by B. atrophaeus (F), B. tequilensis (B) and enbacillus polymyxa (C) on using agar plate assay.
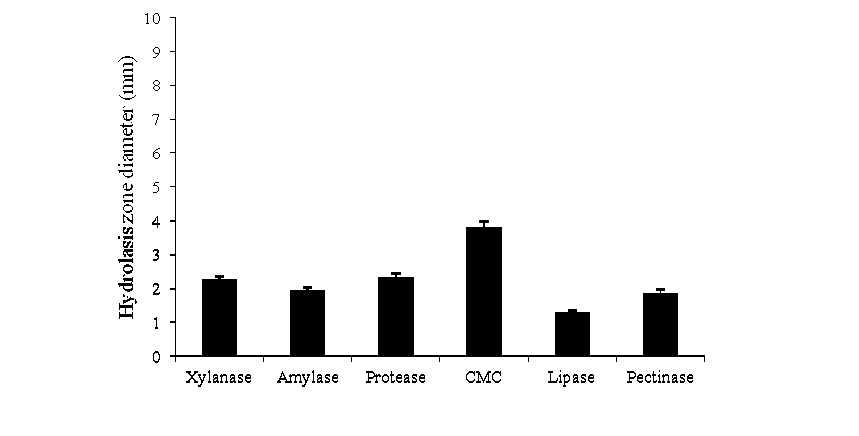
Figure 3: Formation of hydrolysis zones (cm) around the colonies of Bacillus sp . using agar plate method. Error bars display the standard deviation among two biological replicates.
ACKNOWLEDGMENTS
The authors would like to thank the Director General of ECS and the Head of Molecular biology and Biotechnology Department for their much appreciated help throughout the period of this research. Thanks are also extended to Dr. H. mmouneh for his assistance in achieving the experiments.
CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest.
Список литературы Investigating the ability of diverse native bacillus species to produce hydrolytic enzymes using agar plate assay
- Alrumman S, Mostafa, Mostafa YS, Al-Qahtani S., Taha T. 2018. Hydrolytic enzyme production by thermophilic bacteria isolated from Saudi hot springs. Open Life Sci., 2018, 13, 470-480.
- Ammouneh H, Harba M, Idris E, Makee H. Isolation and characterization of native Bacillus Thuringiensis isolates from Syrian soil and testing under insecticidal activities against some insect pests. Turk. J. Agri. Forest. 2011, 35, 421-431.
- Amoozegar MA, Malekzadeh F, Malik KA. Production of amylase by newly isolated moderate halophile, Halobacillus sp. strain MA-2. J. Microbiol. Methods. 2003, 52, 353-359.
- Ashok A, Doriya K, Rao JV, Qureshi A, Tiwari AK, Kumar DS. Microbes producing L-Asparaginase free of glutaminase and urease isolated from extreme locations of antarctic soil and moss. Sci. Rep. 2019, 9,1423.
- Baweja M, Nain L, Kawarabayasi Y, Shukla P. Current technological improvements in enzymes toward their biotechnological applications. Front. Microbiol. 2016, 7, 965.
- Beg QK, Bhushan B, Kapoor M, Hoondal GS. Production and characterization of thermostable xylanase and pectinase from Streptomyces sp. QG-11-3," J. Ind. Microb. Biotech. 2000, 24, 396-402.
- Danilova I, Sharipova M. The Practical potential of Bacilli and their enzymes for industrial production. Front. Microbiol. 2020, 11, 1782.
- Dhawale MR, Wilson JJ, Khachatourians GG, Ingledew WM. Improved method for detection of starch hydrolysis. Appl Environ Microbiol. 1982, 44, 747750.
- Ellis JT, Magnuson TS. Thermostable and alkalistable xylanases produced by the thermophilic bacterium Anoxybacillus flavithermus TWXYL3. ISRN Microbiol. 2012, 517524.
- Fry SM, Huang JS, Milholland RD. Isolation and preliminary characterization of extracellular proteases produced by stains of Xylella fastidiosa from grapevines. Phytopathology. 1994, 84, 357363.
- Harba M, Bakri Y, Jawhar M, Arabi MIE. Identification of a new Bacillus B. amyloliquefaciens isolate with enzymatic and antifungal potential. J. Agroaliment. Processes Technol. 2020, 26, 410-415.
- Hossain TJ, Chowdhury SI, Mozumder HA, Chowdhury MNA, Ali F, Rahman N. Dey S. Hydrolytic exoenzymes produced by bacteria isolated and identified from the gastrointestinal tract of bombay duck. Front. Microbiol. 2020, 11, 2097.
- Meddeb-Mouelhi F, Moisan JK, Beauregard M. A comparison of plate assay methods for detecting extracellular cellulase and xylanase activity. Enzyme Microb. Technol. 2014, 66, 16-19.
- Nair JR, Singh G, Sekar V. Isolation and characterization of a novel Bacillus strain from coffee phyllosphere showing antifungal activity. J. F. Appl. Microbiol. 2002, 93,772-780.
- Palmer T. Understanding Enzymes 4th edition (London:Prentice HallEllis Horwood). 1995.
- Razzaq A, Shamsi S, Ali A, Ali Q, Sajjad M, Malik A, Ashraf M. Microbial proteases applications. Front. Bioeng. Biotechnol. 2019, 7, 110.
- Raveendran S, Parameswaran B., Ummalyma SB, Abraham A, Mathew AK, Madhavan A, Rebello S, Pandey A. Applications of microbial enzymes in food industry. Food Technol. Biotechnol. 2018, 56.
- Samad MYA, Razak CNA, Salleh AB, Zin W, Yunus WM, Ampon K, et al. A plate assay for primary screening of lipase activity. J. Microbiol. Methods. 1989, 9, 51-56.
- Tseng YH, Fang TJ, Tseng SM. Isolation and characterization of a novel phytase from Penicillium simplicissimum. Folia Microbiol. 2000, 45, 121-127.
- Varghese LM, Agrawal S, Sharma D, Mandhan RP, Mahajan R. Cost-effective screening and isolation of xylano-cellulolytic positive microbes from termite gut and termitarium. 3 Biotech. 2017, 7, 108.
- Wulff EG, Mguni CM, Mansfeld-Giese K, Fels J, Lübeck M, Hockenhull J. Biochemical and molecular characterization of Bacillus amyloliquefaciens, B. subtilis and B. pumilus isolates with distinct antagonistic potential against Xanthomonas campestris pv. campestris. Plant Pathol. 2002, 51, 574-584.
- Zhou Y, Wang X, Wei W. et al. A novel efficient ß-glucanase from a paddy soil microbial metagenome with versatile activities. Biotechnol Biofuels. 2016, 9, 36.


