Investigation on pyrolysis of coal from Ulan-Ovoo deposit in Mongolia
Автор: Purevsuren B., Bazarova J. G., Ariunaa A., Bazarov B. G., Shagjjav E., Batulzi B., Dorjieva S. G.
Журнал: Вестник Бурятского государственного университета. Химия. Физика @vestnik-bsu-chemistry-physics
Статья в выпуске: 2, 2020 года.
Бесплатный доступ
The technical characteristics, organic elemental composition of coal and inorganic elemental composition of coal ash of Ulaan-Ovoo deposit have been determined. The thermal stability of coal organic mass has been investigated by thermogravimetric analysis: the mechanisms of the thermal decomposition of the coal organic mass have been explained and the thermal stability indices (T5%, T15% and T25%) has been determined. The coal of Ulaan-Ovoo deposit processed by pyrolysis under vaarious heating temperatures and determined the yields of pyrolysis products such as hard residue, condensed liquid (tar and pyrolysis water) and uncondensed gas products. On the basis of proximate, ultimate, thermogravimetric and FTIR analysis results have been confirmed that the Ulaan-Ovoo coal is a middle rank subbituminous D mark. The liquid tar product of pyrolysis of Ulaan-Ovoo coal was investigated by FTIR and GC/MS analysis. Also the pyrolysis hard residue and activated carbon were characterized.
Composition of Ulan-ovoo coal, sub-bituminous coal, thermogravimetric analysis, thermal stability, pyrolysis resin, activated carbon
Короткий адрес: https://sciup.org/148317077
IDR: 148317077 | УДК: 662 | DOI: 10.18101/2306-2363-2020-2-28-45
Текст научной статьи Investigation on pyrolysis of coal from Ulan-Ovoo deposit in Mongolia
Mongolia is among the 10 coal rich countries in the world with the 175 billion tones of geologically estimated coal resources including high quality bituminous coking coals, subbituminous coals and lignite brown coals. More than 70% of this coal resources belongs to the brown coals [1].
Coal undergoes a series of physical and chemical changes during pyrolysis. Various pyrolysis conditions (i.e. coal type, pyrolysis temperature, atmosphere, heating rate, and catalyst factors) significantly affect these physical and chemical changes [2-5]. On the other hand pyrolysis is an efficient form (method) of treatment of organic material at elevated temperatures in the absence of oxygen. It involves the simultaneous change of chemical composition and physical phase during thermochemical decomposition of organic material by heat and is irreversible [6-8]. As a results of pyrolysis, solid (hard residue), condensed liquid (tar and pyrolysis water) and gas (uncondensed) products can be obtained. Solid product is porous material with higher caloric value which can be used as coke, semicoke, smokeless fuel, adsorbent material and so on. Tar is petroleum like product and can be used as complex raw material for production of chemical substances, gasoline, diesel, oils, bitumen and so on. The gas product can be used as gas fuel after cleaning of nitrogen and sulphur containing pollutants in it [6, 7]. Generally before the pyrolysis experiments of coal necessary carry out thermogravimetric analysis to investigate the mechanism of thermal decomposition coal organic mass and to determine the thermal stability characteristics such as thermostability indices (T 5 % and T 25 %) and to evaluate how are easy for pyrolysis [9, 10]. During last decade we are working on pyrolysis of some organic raw materials including different rank coals [11,12], oil shale [13-15], wood waste [16-18], animal bone [19, 20], cedar shell [21], polypropylene waste [22], milk casein [23-25] and characterization of obtained hard residue, tar and gas product after pyrolysis.
The conversion of coal into oil and gas is a major issue in the country, which will affect the national safety and the economically sustainable development. Therefore more detailed investigations by using of modern instrumental analysis such as FTIR, thermogravimetric and GC/MS analysis and different pyrolytic experimental methods is very important for the future development of coal processing industries in Mongolia. For this reason, the coal of Ulaan-Ovoo deposit has been chosen more detailed investigation on thermal processing including pyrolysis and thermolysis, characterization of obtained solid (hard residue) and liquid products.
The coal sample was used in this study from Ulaan-Ovoo deposit in Mongolia. The Ulaan-Ovoo deposit locates very near to the Mongolian and Russian border in Selenge province and has been mined since 1973.
Experimental
The Ulaan-Ovoo coal was crushed into small pieces 3-6 mm in size and the analytical sample was prepared by powdering the coal into small particles size < 0.2 mm in a steel mill. Analytical sample preparation (MNS 27192001), proximate and ultimate analysis of coal were performed according to Mongolian National Standards MNS 656-79 (moisture content), MNS 652-79 (ash yield), MNS 654-79 (volatile matter yield). The elemental composition of coal was determined by micro analytical instrument 5Е С2000, model CNH-analyser.
The FTIR spectra of coal was obtained on a Nicolet 20-PC FTIR spectrometer with CsI optics and DTGS detector. The KBr disc contained 0.5% finely ground coal sample. All the spectra were measured in the frequency range of 4000 to 400 cm-1, and 32 scans were taken for every sample.
Thermogravimetric analysis of coal was carried out in TG/DTA7200, (Hitachi, Japan). Conditions of analysis were as follows: Sample weight — 5-10 mg. Heating temperature range — 20-1150°C, Heating rate — 20°C/min, Carrier gas-argon, Crucible made by Pt-Rh.
Small-scale pyrolysis experiments of coal sample was performed in a laboratory quartz retort (tube) which could process 1 g of coal sample. The retort was placed in a horizontal electric tube furnace. A chrome-alumel thermocouple was immersed in the tube furnace to measure the actual heating temperature. Pyrolysis experiments have been carried out at different temperatures 200-700°C with constant heating rate of 20°C/min. First of all, the quartz retort with coal sample was heated, for example, to 600°C with heating rate of 20°C/min, and kept at 700°C for 80 min. The retort was connected with a thermostable glass tube heated also in a tube furnace at 80°C for collecting tar. This tube was also connected with an air-cooled glass vessel for collecting pyrolysed water. The glass vessel for pyrolysed water was connected with a thin glass tube for non-condensable gases. The yields of pyrolysed products, including solid residue (biochar), tar (condensed liquid product) and pyrolysed water were determined by weighing, and the yield of gases were determined basing on their differences.
For collection of more quantities of pyrolysis hard residue and liquid tar product have used a bigger scale pyrolysis apparatuce. The vertical cylindrical retort made by stainless steel in which 1 kg of coal sample can be pyrolized. The retort was placed in an electric furnace (model SNOL) with a maximum temperature of 950°C. A chrome-alumel thermocouple was immersed in the coal bed to measure the actual heating temperature with temperature control (potentiometer). The retort was connected with an air-cooled iron tube and water-cooled laboratory glass condenser and a collection vessel for the condensate of liquid product (pitch and pyrolysis water). The noncondensable gases after water-cooled condenser were left the system through a thin glass tube. The experiments were carried out to 500°C temperature and the heating rate was 20°C min-1. The yields of products including solid residue (coal char), tar and pyrolysis water were determined by weighing, and the yield of gases by difference.
The pyrolized (pyrolysis hard residue with size — 0,63-1,50 mm) coal sample (1015 g) of Ulaan-Ovoo deposit was placed in a quarts tube and flowed with nitrogen to remove the oxygen and heated (heating rate — 5oC/min) until 800°C and activated with preheated water steam for 180 min.
Method for separation of tar and pyrolysis water
The liquid product of coal pyrolysis consists of tar and pyrolysed water. They form an unmixed two layers and can be separated easily by separating in a glass funnel. The upper layer is tar (viscous liquid) with black-brown colour and an unpleasant smell. The bottom layer is pyrolysed water (non-viscous liquid) with an unpleasant smell and yellowish in colour. The final cleaning of tar from the pyrolysed water was mixed with thermally treated CaCl 2 and through separation (filtering or centrifuging). The yellowish pyrolysed water has a specific gravity of 0.9227 g/см3 and solid residue of 7.2% after evaporation in room temperature.
Sample separation and GC/MS analysis
Acid and base separation: The sample of pyrolysed tar of coal (1 ml) was dissolved in hexane (10 ml) and extracted 3 times with 20 ml portions of aqueous sulphuric acid (10%) to isolate the basic components and 3 times with 20 ml portions of aqueous sodium hydroxide (10%) to isolate the acidic components. The organic phase (neutral fraction) was washed with distilled water, dried with sodium sulphate and hexane was evaporated. The combined sulphuric acid extracts were neutralized with alkali and extracted 3 times with 20 ml portions of dichloromethane and the organic phases (basic fraction) were dried and evaporated to the required concentration for GC/MS analysis. The combined sodium hydroxide extracts were acidified and extracted in a similar fashion to yield the acidic fraction.
Chemical class separation: An aliquot (0.2 ml) of the neutral fraction was applied to column separation. The glass column (10 x 1.0 cm) was packed with activated alumina (4.0 g) and elution was performed by using hexane, toluene and dichloromethane. Hexane (10 ml) was used as an eluent to separate alkanes and alkenes (aliphatic compounds). The second fraction (aromatics) was eluted in 10 ml of toluene. The third fraction (polar compounds) was eluted with 10 ml dichloromethane. Aliquots of three fractions were freed of solvents by evaporation at room temperature (20°C) and dissolved in dichloromethane to a suitable concentration for GC/MS analysis.
GC/MS analysis: TSQ 8000 Trace 1310 Gas Chromatography-Mass spectrometer was used for identifying and quantifying organic compounds contained in the fractions. Capillary column; TR-5 ms 60 m x 0.25 mmx 0.25 μm, Oven program: 50°C — 1 min, 8°C/min to 320°C, hold time at 320°C 30 min; Injection volume 1 μl, Carrier gas and its flow: He 1.5 ml/min. Inlet temperature 250°C. MS transfer line temperature 250°C. Ion source temperature 250°C. Scanned masses 45-550 amu.
Results and discussion
The results of ultimate and proximate analyses of coal samples from Ulaan-Ovoo (UO) deposit are shown in Table 1.
Results of proximate and ultimate analysis of Ulaan-Ovoo coal in Table 1 show that the caloric value and carbon content are higher and the content of sulphur is lower, which is a good for environmental point of view. These results have been confirmed that the coal Ulaan-Ovoo deposit belongs to the class of D mark according to Russian genetic technological classification (GOST 25543-82).
The analytical coal sample of Ulaan-Ovoo deposit tested by FTIR analysis for the characterization of coal organic mass and the results are shown in Fig. 1.
Table 1
Proximate and ultimate analyses of Ulaan-Ovoo coal, (%)
|
Moisture Wa , % |
Ash Ad , % |
Yield of volatile matter Vdaf, % |
Sulphur Sdaf % |
Caloric value, Q s daf, кcal кg-1 |
Carbon Cdaf , , % |
Hydroge Hdnaf , % |
Oxygen and others (N + O)daf, % |
|
5.1 |
4.9 |
44.8 |
0.76 |
7040.0 |
75.2 |
5.0 |
19.8 |
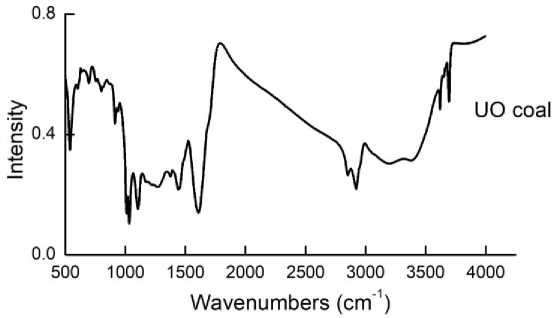
Fig. 1. The FTIR spectra of coal of Ulaan-Ovoo deposit
In the FTIR spectra, Ulaan-Ovoo coal can be recognized as: 1339 and 1497 cm-1 for deformation vibrations of -CH, -CH 2 and –CH 3 groups in saturated aliphatic structures; 1800, 1780 and 1600 cm-1 strongest band for > C=O bonds in acids, ketones, aldehydes and quinines; 2859, 2978 cm-1 for stretching vibrations of -CH, -CH 2 and –CH 3 groups in saturated aliphatic structures; and 3400 cm-1 for stretching associated vibrations of -OH groups in aromatic rings and aliphatic structures [26-28].
For investigation of the mineral matter of the Ulaan-Ovoo coal have obtained pure ash of the coal after completely burning the analytical coal sample at 950oC and tested it by FTIR analysis (Fig. 2) and determined mineral composition by using of roentgen fluorescence analysis (Fig. 3 and Table 2).
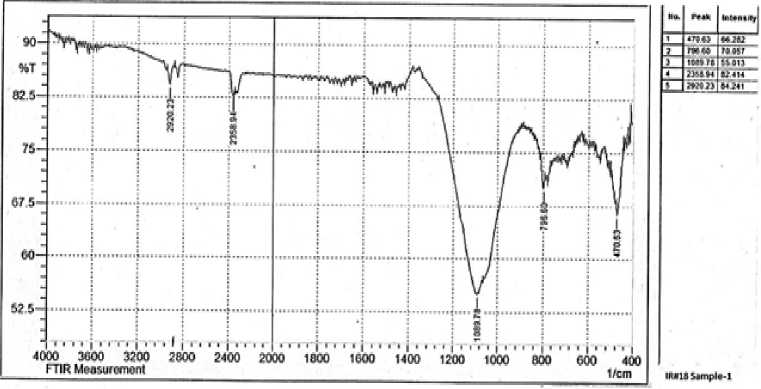
Fig. 2. The FTIR spectra of coal ash of Ulaan-Ovoo deposit.
The FTIR spectra of coal ash of Ulaan-Ovoo deposit in Fig. 2 show that the most intensive and biggest peak at 90-1300 cm-1 belongs for the Si-O- and Ca-O- groups in SiO 2 and CaO which have highest contents in the coal ash of Ulaan-Ovoo deposit. Other peaks with lower intensities at 400-900 см-1 are for -O-Al-; -O-Fe-; -O-Mg-groups in Al 2 O 3 , Fe 2 O 3 and MgO in coal ash.
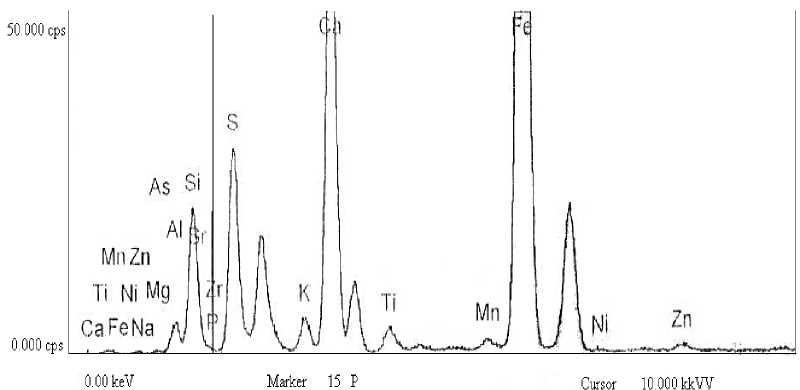
Fig. 3. The spectra of roentgen fluorescence analysis of coal ash of Ulaan-Ovoo deposit
Table 2
|
Elemental composition, % |
Oxide compostion, % |
||
|
Na |
2,220 |
Na 2 O |
2,993 |
|
Mg |
1,047 |
MgO |
1,763 |
|
Al |
2,788 |
Al 2 O 3 |
5,268 |
|
Si |
34,223 |
SiO 2 |
73,210 |
|
P |
1,308 |
P 2 O 5 |
2,996 |
|
S |
1,497 |
SO 3 |
3,739 |
|
K |
0,307 |
K 2 O |
0,370 |
|
Ca |
4,744 |
CaO |
6,638 |
|
Ti |
0,167 |
TiO 2 |
0,279 |
|
Mn |
0,039 |
Mn 2 O 3 |
0,056 |
|
Fe |
1,863 |
Fe 2 O 3 |
2,664 |
|
Zn |
0,012 |
ZnO |
0,014 |
|
Rb |
- |
Rb 2 O |
- |
|
Sr |
0,030 |
SrO |
0,035 |
|
O |
49,754 |
- |
- |
The elemental and chemical composition of Ulaan-Ovoo coal ash, %
The date of mineral composition of Ulaan-Ovoo coal ash in Fig. 3 and Table 2 show that highest content has silicon oxide, relatively low content have aluminum, iron and calcium oxides, and manganese, zinc and strontium oxides have very lowest. The mass ratio between sum of oxides (Fe 2 O 3 + CaO + MgO + Na 2 O + K 2 O/SiO 2 + Al 2 O 3 + TiO 2 = 0.18 < 1) less than 1 which is in indication of acidic character of the ash of Ulaan-Ovoo coal [29].
The thermal decomposition of Ulaan-Ovoo coal in argon atmosphere was analyzed by TG analysis. The results of TG analysis from coal is shown in Fig. 4.
The heating of the Ulaan-Ovoo coal sample at temperatures in the range of 25-1020oC in argon atmosphere, with heating rate 40°C/min shows that the thermal decomposition of coal ends with a 56.4% weight loss and 43.6% hard residue 1020oC (Fig. 4). The TG curve in Fig. 2 consists of different temperature intervals (steps) such as 25-250; 250-550; 550-850; 850-1020oC [13]. In the first step (25-250oC) the weight loss is due to the release of some absorbed gas and moisture from the coal sample. In the second step (250-550oC) intensive thermal decomposition of the organic matter of the coal samples starts forming liquid (tar and pyrolysis water) and gas products. In the third step (550-850oC) the weight loss strongly decreases, which is an indication for ending thermal decomposition and starting carbonization of the coal sample. In the fourth step (850-1020oC) the weight loss lowly increases, which is related with the release of gas, e.g. CO2, H2, CO from the mineral matter of coal sample. From the TG curve in Fig. 2 have been determined the following thermal stability indices of the Ulaan-Ovoo coal as Т5% = 82.9oC; Т15% = 429.3oC; Т25% = 517.9oC. First minimum peak of DTA at 105oC shows a endothermic reaction process related with releasing adsorbed gas and moisture from the coal sample. And a big exothermic reaction peak at 378oC related with intensive thermal destruction of the coal organic mass of the sample. If you look at DTG curve releasing of moisture and adsorbed gas at about 80oC and also thermal destruction reactions of coal organic mass at about 467oC are with maximum rate.
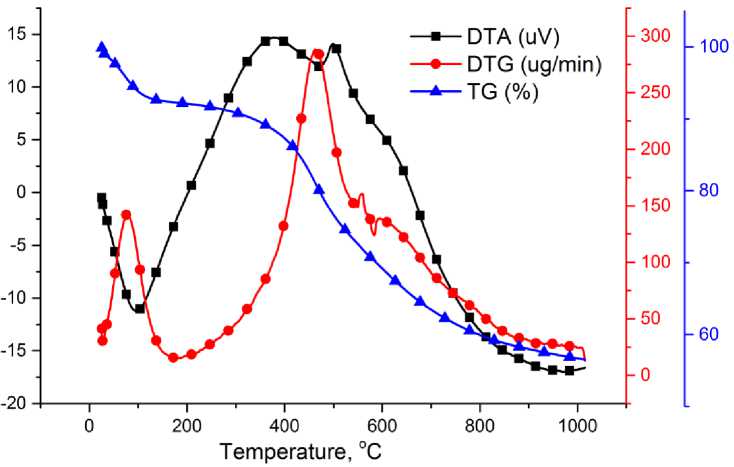
Fig. 4. The TG analysis of Ulaan-Ovoo coal
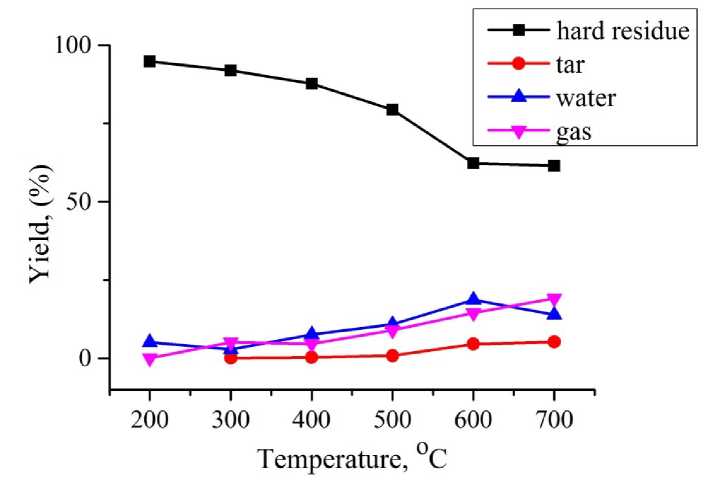
Fig. 5. The results quartz retort pyrolysis experiments of Ulaan-Ovoo coal
The Ulaan-Ovoo coal was pyrolized in standard quartz retort at heating temperatures from 200 to 700oC for 80 min. and determined the yields of hard, liquid and gas products pyrolysis in each temperature ( Fig. 5).
During the pyrolysis of coal the yield of hard residue (main product) decreases until 600oC and the yields of pyrolysis products such as tar and pyrolysis water (liquid products) and gases gradually increase with increasing of heating temperature. The most important pyrolysis product tar began to release at about 300°C, reached the maximum tar yield was 5.23% at about 700°C and it was chose as an optimal heating temperature. At the temperature range of 600-700°C increasing the weight loss stopped and continues constantly and also the yields of tar and pyrolysis water (slightly decreasing) without increasing and the yield of gases slightly decreasing (Fig. 5). These results show that the pyrolysis process of Ulaan-Ovoo coal ended at this temperature range and continues a carbonization process of pyrolysis hard residue in it.
The Ulaan-Ovoo coal was pyrolized in a bigger scale retort for collection of more tar at the chosen optimal heating temperature — 700°C and determined the yield of pyrolysis products with hard residue (58.3%), liquid products (22.2%) and gas (19.5%). The yield of all liquid and gas products (41.7%) show that there is an intensive thermal decomposition of the coal organic mass with higher degree of conversion. As it is known that the organic mass of subbituminous coal is characterized with lower thermal stability than bituminous coal and therefore they are more suitable for gasification and liquefaction.
So collected tar was separated from the pyrolysis water by separating in a glass funnel and used for next analysis. The tar is viscous liquid with black-brown color and bad smelts.
First of all so purified pyrolysis tar of Ulaan-Ovoo coal tested by FTIR analyses and the results are shown in Fig. 6.
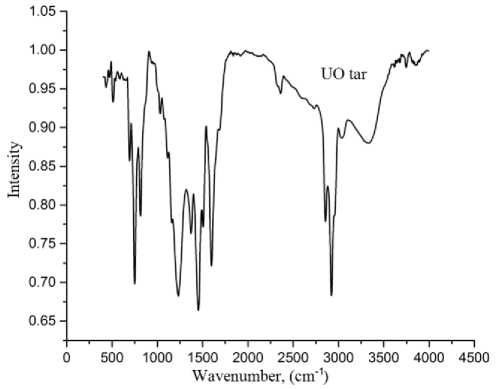
Fig. 6. The FTIR spectra of tar after pyrolysis
In the FTIR spectra of tar product after pyrolysis, several absorption bands were observed for H of aromatic -CH group at 815-877 cm-1, 750 cm-1 and for weak absorption of phenol and alcohol C-O valence at 1267 cm-1, 1114 cm-1 and 1036 cm-1, for — CH 3 aliphatic deformation absorption at 1456 cm-1 and 1379 cm-1, for aromatic C = C variations in valence at 1610 cm-1, 1514-1541 cm-1, for high intensity absorption of aliphatic CH-CH 2 , -CH 3 groups at 2957, 2922, 2851 cm-1 and for absorption of OH, amino groups involved in the hydrogen bond at 3394 cm-1 [26-28]. Therefore tar product of Ulaan-Ovoo coal after pyrolysis consists of mainly aliphatic, aromatic and aromatic-aliphatic compounds.
Some physical characteristics such as specific gravity, flamable temperature and combustion temperature of the tar have been determined (Table 3).
Some physical characteristics of the pyrolysis tar of Ulaan-Ovoo coal
Table 3
|
Sample |
Specific gravity, g/cm 3 |
Flamable temperature, °С |
Combustion temperature, °С |
|
Coal of Ulaan-Ovoo deposit |
1.0625 |
46 |
60 |
The chemical composition of the pyrolysis tar of Ulaan-Ovoo coal have been determined by chemical analysis in group organic compounds ( ) and the results are given in Table 4.
Chemical compostion of the pyrolysis tar of Ulaan-Ovoo coal, %
Table 4
|
Free carbons |
Organic acids |
Organic bases |
Phenolic compounds |
Asphaltenes |
Neutral oils (preasphaltenes) |
|
10.3 |
1.9 |
0.9 |
2.8 |
11.2 |
72.9 |
The determined chemical composition of the pyrolysis tar of Ulaan-Ovoo coal in Table show that the most part of tar is preasphaltenes (Neutral oils) and organic acids, organic bases and phenolic compounds are in lowest contents.
The tar was tested by an atmospheric distillation and obtained several fractions with different boiling temperature ranges. The determined yields of all fraction are given in Table 5.
The date in Table 5 show that the middle and heavy fractions have a higher yields than that of light fraction and residue of distillation. All fractions strongly differ with the color of each fraction and they can be used as gasoline (light fraction), diesel (middle fraction), neutral oils (heavy fraction) and bitumen (residue of distillation) after purification of organic acids, organic bases and phenolic compounds. It is to emphasize that the loss or gas releasing during the distillation was higher — 40.48%.
Table 5
|
Boiling temperature range, °С |
The yield of fractions, % |
The name of fractions |
The color of fractions |
|
71- 180 |
10.29 |
Light |
Yellow |
|
180-250 |
19.29 |
Middle |
Brown |
|
250-320 |
18.53 |
Heavy |
Black-brown |
|
More than 320 |
11.39 |
Residue |
Black |
|
loss |
40.48 |
The yields of fractions of atmospheric distillation of the pyrolysis tar of Ulaan-Ovoo coal, %
The solubility of the pyrolysis tar of the Ulaan-Ovoo coal determined in hexane, toluene, methylenchloride and methanol (1:1 volume ratio) by silicagel chromatograpy and chemical composition of the each separated fractions by GC/MS analysis. The yield of separated fractions by column chromatography method are shown in Table 6.
Table 6
The yield of separated fractions ) and chemical composition of the pyrolysis tar of Ulaan-Ovoo coal by column chromatography
|
Sample |
Hexane soluble fraction (H), % |
Benzene soluble fraction (B), % |
Methylene chloride and methanol (1:1 volume ratio) soluble fraction (M), % |
Total separated fractions, % |
|
Ulaan-Оvoo pyrolysis tar |
0.175 |
3.6 |
8.14 |
11.9 |
The results of silicagel column chromatograpy of the pyrolysis tar show that the content of methylene chloride soluble polar fraction is higher than that of hexane soluble aliphatic and benzene soluble aromatic fraction of the pyrolysis tar of Ulaan-Ovoo coal.
So isolated hexane solouble, benzene soluble and methylene chloride soluble fractions of the pyrolysis tar of Ulaan-Ovoo coal were analyzed by GC/MS analysis and the results are summarized in Table 7.
Have been identified 15 compounds as type of alkanes, cycloalkanes and alkens from total registered 65 GC/MS peaks of hexane soluble aliphatic fraction of the pyrolysis tar of Ulaan-Ovoo coal in Table. Also have been identified 13 compounds as type of benzene, naphthalene, phenanthrene, anthracene, pyrene and their derivatives and heteroatomic aromatic compounds from total registered 28 GC/MS peaks of benzene soluble aromatic fraction of the pyrolysis tar of Ulaan-Ovoo coal. Mostly alcho-holic type of 8 compounds have been identified from total registered 25 GC/MS peaks of methylene chloride soluble polar fraction of the pyrolysis tar of Ulaan-Ovoo coal.
Table 7
The results of the GC/MS analysis of the Ulaan-Ovoo coal pyrolysis tar
|
Groups |
Compounds |
Contents,% |
|
Aliphatic |
Alkane, cycloalkane |
15.13 |
|
Alken |
8.46 |
|
|
Aromatic |
Benzene and its derivatives |
29.84 |
|
Naphthalene and its derivatives |
20.05 |
|
|
Phenanthrene, anthracene and pyrene and their derivatives |
8.61 |
|
|
Heteroatomic aromatic compounds |
11.27 |
|
|
Polar |
Alchohol |
5.67 |
|
others |
- |
As mentioned above the pyrolysis hard residue is the main product of the pyrolysis of Ulaan-Ovoo coal it’s technical characteristics determined and compared with that of initial coal of Ulaan-Ovoo deposit.
The results of determined technical characteristics of initial coal sample and hard residue after pyrolysis are shown in Table 8.
Table 8
The main technical characteristics of solid product of pyrolysis of Ulaan-Ovoo coal
|
The coal of Ulaan-Ovoo deposit |
Moisture, Wa, % |
Ash, Ad, % |
Volatile matter, Vdaf, % |
|
Initial coal sample |
5.1 |
4.9 |
44.8 |
|
Hard residue after pyrolysis |
0.8 |
24.5 |
3.2 |
The dates in Table 8 show that the yield of volatile matter of pyrolysis hard residues decreased more than 14 times than that of initial coal which is an indication of very intensive thermal decomposition of the organic mass of Ulaan-Ovoo coal during the pyrolysis.
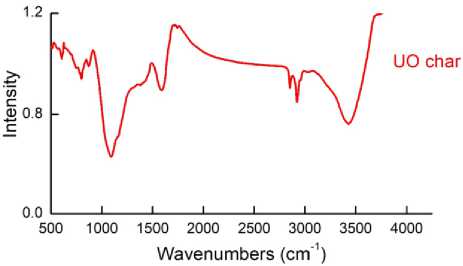
Fig. 7. The FTIR spectra of Ulaan-Ovoo coal pyrolysis hard residue (char)
During the thermal decomposition of coal organic mass and releasing the formed liquid and gas product after pyrolysis the hard residue characterizes a carbonized solid product with high developed porosity structure and it can be used as an environmentally friendly smokeless fuel and an adsorbent material.
The pyrolysis hard residue of Ulaan-Ovoo coal was tested by FTIR and C13 NMR analysis and the results are shown in Fig. 7 (FTIR spectra ) and 8 ( 13C NMR spectra).
In the FTIR spectra of Ulaan-Ovoo coal pyrolysis hard residue (char) a strong and big absorption band was observed for H of aromatic –CH group at 1100 cm-1 and for H of aliphatic –CH 3 , -CH 2 and –CH 1 groups at 2800-2920 cm-1 with much lower intensities than aromatic –CH groups.Also a bigger and unshurp absorption band for H of –OH and –NH groups at 3400-3600 cm-1. Therefore the pyrolysis hard residue of Ulaan-Ovoo coal consists of mainly aromatic, less aliphatic and also aromatic-aliphatic groups in the carbonized macromolecul.
In the 13C NMR spectra of Ulaan-Ovoo coal pyrolysis hard residue aromatic C at 110-150 ppm, aliphatic C of –CH 3 , -CH 2 and –CH 1 groups at 50-70 ppm were observed. The higher intensity of aromatic C is a cleirly indication for more aromatization process of the hard residue during pyrolysis and carbonization.
The pyrolysis hard residue of Ulaa-Ovoo coal was activated at 800oC under flow of water steam for 180 min and determined the main characteristics of obtained activated carbon (Table 9). The optimal heating temperature (800oC ) and time (180 min) of activation vs yield of activated carbon and iod munber, % were determined preliminary.
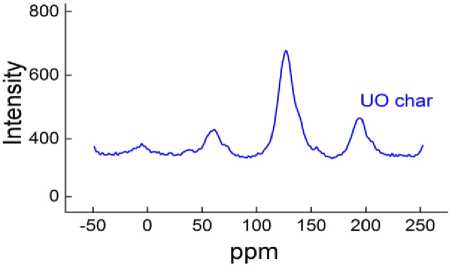
Fig. 8. The 13C NMR spectra of coal pyrolysis hard residue of Ulaan-Ovoo deposit
Table 9
The main characteristics of obtained activated carbon of Ulaan-Ovoo coal
|
Sample of Ulaan-Ovoo coal |
Time of activation, min. |
Yield of activated carbon, % |
Iod number, mg/g |
Iod numbe r, % |
Ash, Ad, % |
Yield of volatile matter, V daf , % |
|
Pyrolysis hard residue |
- |
- |
44,0 |
13,9 |
17,7 |
2,4 |
|
Activated carbon |
180 |
74,7 |
113,0 |
35,6 |
19,1 |
2,7 |
The date in Table 9 show that the most important characteristic is iod number of obtained activated carbon it is increased more than 2,5 times in comparison with that of pyrolysis hard residue which is also has certain porosity structure (Fig. 9-A) before activation.
The SEM photograph of initial Ulaan-Ovoo coal sample (A) and activated carbon (B) of Ulaan-Ovoo coal char are shown in Fig. 9.
The SEM photograph of activated char (pyrolysis hard residue) of Ulaan-Ovoo coal in Fig. 9 clearly shows that has a high developed porosity structure with different sizes (micro, mezzo and macro) pores and the Ulaan-Ovoo coal can be used for preparation of activated carbon (adsorbent material) after carbonization (pyrolysis) and activation (by preheated water steam). As mentioned above the SEM photograph of initial Ulaan-Ovoo coal sample (A) in Fig. 9 shows also some micro pores in it.
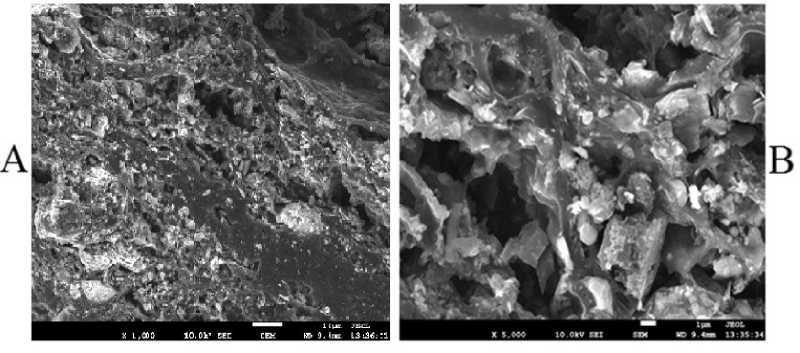
Fig. 9. The SEM photograph of initial Ulaan-Ovoo coal sample (A) and activated carbon (B) of Ulaan-Ovoo coal char
Conclusion
-
1. On the basis of proximate, ultimate, and FTIR analysis have been confirmed that the Ulaan-Ovoo coal is a middle rank D mark subbituminous coal and it is suitable for thermal processing including pyrolysis and thermolysis.
-
2. The thermal decomposition of Ulaan-Ovoo coal investigated by TG, DTA and DTG curves of thermogravimetric analysis and the thermal stability characteristics such as thermal stability indices (T 5 % and T 25 %) determined and evaluated by using of TG curve.
-
3. The pyrolysis of Ulaan-Ovoo coal carried out at different heating temperatures vs the yields of obtained solid, liquid and gas products. The results of pyrolysis experiment of Ulaan-Ovoo coal show that 58.3% of coal organic mass remained as a hard residue at 700°C after pyrolysis and this heating temperature was chosen as optimal temperature in which the yield of all liquid and gas products is higher — 41.7 %. This is an indication of intensive thermal decomposition of the coal organic mass with higher degree of conversion.
-
4. The FTIR spectra of the purified pyrolysis tar of Ulaan-Ovoo coal show that the tar consists of mainly aliphatic, aromatic and aromatic-aliphatic compounds.
-
5. The determined chemical composition of the pyrolysis tar of Ulaan-Ovoo coal by chemical analysis show that the most part of tar is preasphaltenes (neutral oils) and organic acids, organic bases and phenolic compounds are in lowest contents.
-
6. The yields of fractions with different boiling temperature ranges after atmospheric distillation of the pyrolysis tar of Ulaan-Ovoo coal show that the middle and heavy fractions have a higher yields than that of light fraction and residue of distillation.
-
7. The results of silicagel column chromatograpy of the pyrolysis tar show that the content of methylene chloride soluble polar fraction is higher than that of hexane soluble aliphatic and benzene soluble aromatic fraction of the pyrolysis tar of Ulaan-Ovoo coal.
-
8. The chemical composition of each separated fractions after silicagel chroma-tograpy studied by GC/MS analysis and have been identified 15 compounds as type of alkanes, cycloalkanes and alkens from total registered 65 GC/MS peaks of hexane soluble aliphatic fraction of the pyrolysis tar of Ulaan-Ovoo coal in Table. Also have been identified 13 compounds as type of benzene, naphthalene, phenanthrene, anthracene, pyrene and their derivatives and heteroatomic aromatic compounds from total registered 28 GC/MS peaks of benzene soluble aromatic fraction of the pyrolysis tar of Ulaan-Ovoo coal. Mostly alchoholic type of 8 compounds have been identified from total registered 25 GC/MS peaks of methylene chloride soluble polar fraction of the pyrolysis tar of Ulaan-Ovoo coal.
-
9. The determined main technical characteristics of solid product of pyrolysis of Ulaan-Ovoo coal show that the yield of volatile matter of pyrolysis hard residues decreased more than 14 times than that of initial coal which is an indication of very intensive thermal decomposition of the organic mass of Ulaan-Ovoo coal during the pyrolysis.
-
10. The pyrolysis hard residue of Ulaa-Ovoo coal was activated at 800oC under flow of preheated water steam for 180 min and determined main characteristics of obtained activated carbon show that the iod number of obtained activated carbon it is increased more than 2,5 times in comparison with that of pyrolysis hard residue.
-
11. The SEM photograph of activated char (pyrolysis hard residue) of Ulaan-Ovoo coal clearly shows that has a high developed porosity structure with different sizes pores.
Список литературы Investigation on pyrolysis of coal from Ulan-Ovoo deposit in Mongolia
- Purevsuren B. Coal is the main source of energy / In Abstracts of papers, Second Korean and Mongolian Energy Conference. Yonsei University^ Seoul, 2007. — P. 13.
- Nikca S. Flashchain theory for rapid coal devolatilization kinetics. 2. Impact of operating conditions // Energy Fuels. — 1991. -V. 5, № 5. — P. 665-73
- Arenillas A, Rubiera F, Pevida C, Pis J. A comparison of different methods for predict-ing coal devolatilisation kinetics // J. Anal. Appl. Pyrol. — 2001. — V. 58. — P. 685-701.
- Safarova M, Kusy J, Andel L. Pyrolysis of brown coal under different process conditions // Fuel. — 2005. — V. 84, № 17. — P. 2280-2285.
- Wu D., Liu G., Chen S., Sun R. An experimental investigation on heating rate effect in the thermal behavior of perhydrous bituminous coal during pyrolysis // J. Therm. Anal. Calo-rim. — 2015. — V. 119, № 3. — P. 2195-2203.
- Purevsuren B., Davaajav Ya., Erdenechimeg R. Investigation on coals from some Mon-golian deposits. — Toonotprint publisher: Ulaanbaatar, 2010. — Р. 14-22 (in Mongolian).
- Avid B., Purevsuren B., Temuujin J. Bituminous Coals of Mongolia: Occurrence and Characteristics / In: Advances in Energy Research Morena J. Acosta (ed.), 22, Chapter 7, Nova Publishers: New York, 2016. — Р. 159-178.
- Avid B., Sato Y., Maruyama K., Yamada Y., Purevsuren B. Effective utilization of Mongolian coal by upgrading in a solvent // Fuel Process. Tech. — 2004. -V. 85. — P. 933-945.
- Purevsuren B., Davaajav Ya. Investigation on pyrolysis of some organic raw materials, Toonotprint publisher, Ulaanbaatar, (2006) 11-28 (in Mongolian).
- Purevsuren B. Selected works on synthesis of polymers and pyrolysis of organic raw ma-terials and characterization of obtained products. — Toonotprint publisher, Ulaanbaatar, 2003. Р. 81-87.
- Ariunaa A., Li B.Q., Li W., Purevsuren B., Munkhjargal Sh. et al. Coal pyrolysis under synthesis gas, hydrogen and nitrogen // J. Fuel Chem. Tech. — 2007. — V. 35, № 1. — P. 1-4.
- Purevsuren B., Davaajav Ya. Thermal analysis of casein // J. Ther. Anal. & Calorimet. — 2001. — V. 65. — P. 147-152.
- Avid B., Purevsuren B. et al. Pyrolysis and TG analysis of the Shivee Ovoo coal Mongo-lia // J. Ther. Anal. & Calorimet. — 2002. — V. 68. — P. 877-885.
- Avid B., Purevsuren B. Chemical composition of organic matter of the Mongolian Khoot oil shale // Oil shale. — 2001. — V. 18, № 1. — P. 18-23.
- Avid B., Born M., Purevsuren B., Undrakh N., Tuvshinjargal A. Thermal behavior of the Khoot oil shale in the different conditions // Oil shale. — 2003. — V. 20, № 1. — P. 47-55.
- Munkhjargal Sh., Purevsuren B. Products of pyrolysis of wood wastes // Reports of the Institute of Chemistry, MAS, 1998. — Р. 25-28.
- Otgonchuluun D., Purevsuren B., Ariunаа А. Investigation on pyrolysis and characteri-zation of solid and liquid products after pyrolysis of wood waste // Reports of the Mongolian University of Science and Technology. — 2014. — V. 2. — P. 68-171.
- Purevsuren B., Otgonchuluun D., Ariunaa A. Pyrolysis of wood waste and characteriza-tion of obtained products // Reports of the Mongolian Chemical Society. — 2015. — V. 10. — P. 21-30.
- Purevsuren B., Avid B., Gerelmaa T., Davaajav Ya., Morgan T.J. et al. The characteri-zation of tar from pyrolysis of animal bones // J. Fuel Chem. Tech. — 2004. — V. 83. — P. 799-805.
- Batbileg S., Purevsuren B., Davaajav Ya., Narangerel J. Investigation on chemical com-position of tar from cedar shell // Scientific Reports of Institute of Chemistry and Chemical Technology. — 2015. — V. 2. — P. 92-97. (in Mongolian)
- Purevsuren B., Davaajav Ya., Karaca F., Morgan T.J., George A. et al. Pyrolysis of waste polypropylene and characterization of the tar. Europ // J. Mass Spectro. — 2009. — V. 15, № 1. — P. 23-33.
- Purevsuren B., Davaajav Ya. Investigation on pyrolysis of casein // J. Ther. Anal. Calo-rimet. — 2001. — V. 66. — P. 743-748.
- Purevsuren B., Avid B., Tesche B., Davaajav Ya. A biochar from casein and its proper-ties // J. Mater. Sci. — 2003. — V. 38, № 11. — P. 2347-2351.
- Purevsuren B., Avid B., Davaajav Ya., Herod A., Kandiyoti R. et al. Estimation of the molecular mass range of the tar from pyrolysis of casein by gas chromatography-mass spec-trometry, probe mass spectrometry and size-exclusion chromatography with 1-methyl-2-pyrrolidinone as eluent // Europ. J. Mass Spectro. — 2004. — V. 10. — P. 101-108.
- Purevsuren B., Davaajav Ya., Genadiev V., Kotsev H., Glavchev I. Investigation of the liquid tar product from pyrolysis of yak-milk casein and its application in curring of epoxy res-in // Bulgarian Chemical Communications. — 2013. -V. 45, № 2. — P. 157-160.
- Narangerel J. Fundamentals of coal chemistry and technology. — UB, 2011. -388 p.
- Kazitsyn L. A., Kupetskaya N. B. Application of UV, IR and NMR spectroscopy in or-ganic chemistry. — M.: Nayka, 1971. — P. 19-61.
- Monkhoobor M., Batchimeg G. Molecular structure and spectroscopy. — UB, 2009. — P. 30-77.
- Battulga N. The decomposition products, structure and chemical composition of Alagto-goo coal. — Ph. D. Dissertation in Chemistry. — UB, 2008. — 109 p.


