Isolation and purification of heterotetrameric catalase from a desiccation tolerant cyanobacterium Lyngbya arboricola
Автор: Kapoor Shivali, Tripathi S.N., Shrivastava Alpana
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 1 т.9, 2013 года.
Бесплатный доступ
The desiccation tolerant cyanobacterium Lyngbya arboricola, isolated from bark surfaces of Mangifera indica, possessed up to four stable isoforms of catalase in addition to other antioxidative enzymes, for several years under a dry state. Purification of the two most persistent isoforms of catalase (Cat) has been undertaken by employing acetone precipitation, ethanol: chloroform treatment, gel filtration and ion exchange chromatography. The two isoforms of catalase remained almost unchanged on varying matric and osmotic hydration levels of mats of the cyanobacterium. The purification procedures resulted in a 1.3 % yield of purified single isoform (0.22 mg mL -1 protein) with 709 Units mg -1 specific activity and a purity index of 0.83. Five millimolar of dithiothreitol (DTT) was observed to be pertinent in maintaining the optimum redox state of the enzyme. The purification procedures additionally facilitated the simultaneous elimination and procurement of phycoerythrins (PE) and mycosporine-like amino acids (MAA). Each purified isoform gave a single band (~45kDa) upon SDS-PAGE and denaturing urea isoelectric focusing (IEF) depicted the presence of 2 subunits each of CatA and CatB. The monoisotopic mass and pI value of CatA and CatB as revealed by LC-MS analysis and internal amino acid sequencing was 78.96, 5.89 and 80.77, 5.92, respectively, showing resemblance with CatA of Erysiphe graminis subs. hordei and CatB of Ajellomyces capsulata. The heterotetrameric monofunctional catalase (~320 kDa), due to its stability in the form of resistance to ethanol: chloroform, its thermoalkaliphilic nature and the presence of innumerable hydrophobic amino acid residues (~40%), thus exhibited its potential for biotechnological applications.
Chromatography, denaturing isoelectric focusing, desiccation, electrophoresis, lyngbya arboricola, stable catalase
Короткий адрес: https://sciup.org/14323711
IDR: 14323711
Текст научной статьи Isolation and purification of heterotetrameric catalase from a desiccation tolerant cyanobacterium Lyngbya arboricola
Abbreviations: Cat, Catalase; DTT, dithiothreitol; IEF, isoelectric focusing; MAA, mycosporine like amino acids ; PE, phycoerythrins; KPB, potassium phosphate buffer; PMSF, phenylmethane-sulphonyl fluoride; ß-ME, beta mercaptoethanol
Metabolic disorders in an oxygen-enriched environment often result in the generation of reactive oxygen species (ROS) such as superoxide, hydroxyl radical, and hydrogen peroxide leading to oxidation of cellular biomolecules causing breakdown of normal cellular, membrane, and reproductive functions (Kranner and Birtic, 2005; Abele, 2002; Marx, 1985). In order to maintain normal growth and function, as well as to neutralize the lethal effects of ROS, organisms have evolved specific antioxidant defenses. Catalase (H2O: H2O oxidoreductase; EC 1.11.1.6), a ubiquitous enzyme that was one of the first enzymes isolated in a state of high purity (Schonbaum and Chance, 1976), efficiently catalyzes the decomposition of hydrogen peroxide into oxygen and water and thus is a significant component of antioxidative defense systems in aerobic organisms. Potential applications of catalases in medicine and industry have led to numerous attempts to engineering this protein (Shaked and Wolfe, 1988). Catalase has also gained attention in recent years because of its link to cancer, ageing in humans and animals (Melov et al., 2000; Preston et al., 2001; Turdi et al., 2007). Also the application of catalases for the elimination of H2O2 in textile bleaching effluents would not only allow the recycling of enormous amount of water used for dyeing but would also have advantage over the use of unfavorable high quantities of salt concentrations such as sodium bisulfite and act as a more environmentally friendly alternative to existing chemical treatments (Thompson et al., 2003; Tzanov et al., 2001; Gujelj et al., 2001). Most of the commercial catalases available in the market are optimally active at 20-50oC and at neutral pH. This necessititates the requirement of new thermoalkalistable catalases which can act at temperatures above 50oC and pH values above 9.0.
Catalases are not restricted to one protein type structurally, functionally, or by sequence. In general, there are three classes of catalase: the typical or monofunctional catalases, the catalase-peroxidases and the Mn-catalases or pseudocatalases (Zamocky and Koller 1999,
Thompson et al ., 2003). Additionally the monofunctional catalases are divided into three phylogenetic clades arising from a minimum of two genes duplication - two distint clades or subgroupings of small subunit enzymes and one clade of large subunit enzymes (Chelikani et al. , 2004; Klotz et al. , 1997). It has been demonstrated that catalases belonging to clade 2 mainly exhibit unusual resistances to physical and chemical denaturation. Catalase HPII was found to show thermal as well as pH stability by showing considerable activity till 60oC which declined after 80oC with a T m value 83oC (Goldberg and Hochman 1989; Switala et al. , 1999). The thermal stability as well as resistance property of catalase to treatment with chloroform and ethanol has been widely exploited for purification of the enzyme (Weiting et al. , 1990).
Cyanobacteria, one of the most primitive oxygenic phototrophs were probably the first organisms to develop elaborate mechanisms for the detoxification of partially reduced oxygen species. Desiccation tolerant terrestrial cyanobacteria, mainly exhibiting subaerial growth, due to oxygen rich atmosphere are supposed to generate considerable ROS and also to possess defense mechanisms against damages expected due to generation of ROS mainly during the phases of drydown (dehydration) followed by desiccation (dry) and then subsequent recovery of metabolism (rehydration) at their natural habitats (Potts, 2001; Tripathi and Maurya, 2001). Cyanobacteria are mostly known to possess bifunctional catalase peroxidase (KatG), Mn-catalases (MnCat), and peroxiredoxins (Zamocky et al., 2008). However, in spite of being the largest group of H2O2 degrading enzymes, typical catalases are very uncommon in cyanobacteria. Until now the only one complete and nonfused gene in which all essential amino acids of typical catalase is conserved is found in Nostoc punctiforme PCC73102. Phylogenetic studies have futher revealed that it belongs to clade 3 of small subunit catalases that contain haem b as the active site and use NADPH as a second active cofactor (Bernroitner et al., 2009). Also incomplete catalase genes have been reported in Cyanothece sp. ATCC51142 and Synechococcus elongatus PCC7942.
The genus Lyngbya has a prominent ecological role in marine ecosystems and is a group rich in bioactive secondary metabolites (Gerwick et al. , 2008; Hoffman, 1994). Recently the whole genome analysis of Lyngba majuscula 3L was done to gain insight into potential microbial interactions and gauge the natural product synthesis of this genus (Jones et al. , 2011). Also there are reports of purification and isolation of a catalase and its gene from other microbes like Aspergillus species (Chandrashekar, 2011) and Bacillus sp. (Wang et al. , 2011) but none have reported catalase from any cyanobacterial species yet. Tripathi and Srivastava in 2001 were the first to report the presence of active catalases, and dismutases in desiccation tolerant cyanobacterium Lyngbya arboricola .
Catalases are known to retain its stability for longer duration, being active in freeze-dried permafrost samples (Gilichinsky et al., 1992) over a period of million years. But the presence of stable and active catalase in cells of this desiccation tolerant terrestrial cyanobacterium Lyngbya arboricola stored in desiccated state for two years is not only reflective of the ability of the cyanobacterium to maintain structural and functional integrity of their macromolecules including proteins under extremes of desiccation, but also signifies for better availability of stable catalase in dry mats of this cyanobacterium.
Due to the low structural stability of most of catalases much work in recent times has been devoted to increase their stability. This makes this cyanobacterium excellent source material for future exploitation of such enzymes for bioengineering and scientific research. This study will also be one of the first reports of presence and purification of catalases in a dessication tolerant cyanonbacteria which may serve as potential sources of thermophilic industrial catalases.
Unfortunately, structural, functional and molecular basis of stability of catalases from the cyanobacterium have not been explored at the desired level. In order to exploit this activity in the future, an understanding of the characteristics of the enzyme is required, necessitating the development of procedures for purification of catalase from the cyanobacterium.
Besides cyanobacteria being useful potential sources for antioxidants and antioxidative enzymes, they also contain significant amounts of unusual phycobiliproteins (PBPs), especially (PEs) (Tripathi et al. , 2007) and UV-absorbing pigments MAAs and scytonemins (Garcia-Pichel et al. , 1993) that are important in biotechnology but pose great problems for the purification of catalases. Thus, this work also describes a process of recovering these water soluble pigments as by-products of the catalase purification process. In addition we have also developed procedure(s) to maintain the pertinent level of redox state of the cell-free extract to minimize denaturation of the enzymes, i.e., catalases.
This method of purification and isolation of antioxidant enzyme catalase with simultaneous recovery of biotechnologically important PEs and UV screening MAAs, may also save the individual efforts and costs to extract these pigments from this cyanobacterium.
MATERIALS AND METHODS
-
(i) Cyanobacterial material: L. arboricola inhabiting bark surfaces of Mangifera indica from the campus of Banaras Hindu University faces 8-9 dry months during the hot summer and cold winter seasons due to a considerably low amount of rainfall (2-12%). However, 3-4 months of a warm and moist rainy season with torrential rainfall (90%) and intermittent breaks result in uncertain wetting and drying and provide relatively better conditions for growth of the cyanobacterium (Tripathi et al. , 1990/91). Collection of the cyanobacterial samples was performed by scraping cyanobacterial mats from bark at the end of the rainy season (during the last week of September); after removing any adherent soil particles, the intact mats were used for further analysis.
Under natural habitat, rehydration (wetting) of dry mats of L. arboricola is on direct availability of water; whereas, dehydration (drying) results due to loss of water from wet mats to the atmosphere. In other words, it can be said that under natural habitats, rehydration and dehydration of the cyanobacterium can be controlled by regulating the osmotic and matric availability of water, respectively. So, osmotic water potential of the cyanobacterial mat was controlled by incubating the mat to the solutes of the medium (water potential solutions) on filter papers; whereas, matric water potential of the mats was controlled by equilibrating the mats in the atmosphere of a solution of defined water potential (isopiestic control, Harris et al. , 1970; Potts and Friedman 1981).
In order to obtain the cyanobacterium under growing conditions, the dry cyanobacterial mats were placed over filter paper pre-soaked with double distilled water at 0 MPa, for 72 h at 25oC and 72 μmol photon m-2s-1 light intensity. To maintain hydration levels, the growing and dry natural mats of L. arboricola were incubated at different osmotic or matric water potentials by equilibrating the mats over sodium chloride solutions and saturated solutions of different salts equivalent to different water potentials (0, -2.8, -21, -39, -167, and -355 MPa) at 25oC as described by Brock (1975) and Tripathi and Maurya (2001). For rehydration of dry mat, the mat was incubated on the filter paper soaked with the required osmotic water potential solutions for the required incubation period; whereas, for dehydration the 72 h grown mat at 0 MPa osmotic water potential was in the atmosphere of the required matric water potential solution for the required time period.
-
(ii) Application of reducing agents: The impact of some reducing agents in optimization of redox state of the enzyme was studied by incubating catalase (0.05 mg protein per lane) with varying concentrations (0-20 mM) of cysteine, DTT and ß-ME, respectively, for 30 min at 4oC and monitoring changes in the enzyme activity by native-PAGE gel. The optimum redox state of the enzyme was evaluated by monitoring the concentration of reductant at which an optimum enzyme activity could be recorded. The levels of sharpness and intensity on native-PAGE gel were related to the state of the enzyme.
-
(iii) Purification of catalase: Purification of the enzyme was achieved by employing acetone precipitation, ethanol-chloroform treatment, gel filtration, ion-exchange chromatography and electroelution processes. These procedures were
carried out at 4oC unless otherwise stated, and the purity of catalase at each step of purification was determined using spectroscopy and PAGE.
-
(a) Preparation of cell-free extract: The cell-free extract used for purification of the enzyme was obtained by crushing cyanobacterial mats (25 g) to powder in liquid nitrogen and homogenizing the powder with 110 mL extraction buffer containing 100 mM potassium phosphate buffer (KPB, pH 7.5), 1 mM Na-EDTA and 1 mM phenylmethane sulfonyl fluoride (PMSF) at 4oC. The homogenate was sonicated at 130 W, 20 kHz for 5 min at 50% amplitude using an ultrasonicator (Sonic & Materials, USA) and centrifuged at 25,000 × g at 4oC for 1 h. The supernatant (cell-free extract) was filtered with 0.45 μm cellulose-acetate membrane filter (Millipore, USA) and was used to obtain catalase enriched fraction of the extract.
-
(b) Sequential acetone precipitation: The catalase enriched fraction was acquired by addition of acetone (20-60%) to the respective supernatants obtained after stepwise addition of acetone to cell free extract. The cell-free extract was mixed with chilled acetone (20%), kept overnight at 4oC for precipitation and the supernatant and precipitate were obtained by centrifuging the mixture at 17,000 × g for 30 min at 4oC. The supernatant was lyophilized, re-dissolved in extraction buffer and was further undertaken for second and third acetone fractionation (40& 60%) successively as before. Catalase enzyme activity was measured in the supernatants and pellets obtained at each step of acetone treatments.
-
(c) Ethanol: chloroform treatment : The enzyme-enriched fractions collected after acetone precipitations were mixed with cold ethanol (95%) and chloroform in a ratio of 10:5:3 (v/v). After vortexing vigorously for 2-5 min, the mixture was
centrifuged at 17,000 × g at 4oC for 30 min. Out of the three layers formed, the top aqueous layer displaying high catalase activity was collected and further purified; the dense solid middle layer containing denatured proteins and some pigments was used for purification of PBPs.
-
(d) Column chromatography: Gel filtration - One milliliter of the enzyme extract (the top aqueous layer after lyophilization) containing 2 mg protein mL-1 was filtered with a 0.22 µm filter and loaded onto a Hi-Prep Sephacryl (16/60) S-300 HR column (Akta Prime Plus, GE Healthcare) that had been washed and pre-equilibrated with ~ 200 mL 100 mM KPB (pH 7.5) and extraction buffer, respectively. The enzyme was eluted at a flow rate of 30 mL h-1. Two-milliliter fractions with maximum enzyme activity were pooled and concentrated using dialysis sacks (12 kDa cut off, Sigma, USA). After dialysis overnight against 10 mM Tris-HCl (pH 8.0) at 4oC, the pooled fraction was applied to a DEAE-Sephadex A-50 column.
Ion exchange chromatography - One milliliter of dialyzed sample (1.5 mg protein mL-1) obtained after gel-filtration was applied to a DEAE-Sephadex A-50 column (20 × 1.5 cm) that was preequilibrated and was thoroughly washed with 10 mM Tris (pH 8.0) at a flow rate of 24 mL h-1 to remove any unbound proteins (mainly, MAA); bound proteins were eluted using a linear gradient of NaCl (0-1.0 M) in 10 mM Tris-HCl (pH 8.0). Ten milliliters (each fraction of 2.5 mL) was collected and desalted overnight at 4oC. Fractions showing high catalase activity at each gradient were pooled, concentrated using 10 kDa cut-off filters (Ultrafree-MC, Millipore, USA) and used for further analysis.
-
(iv ) Determination of purity index : The purity index for catalase (PI catalase ) at each step of purification was expressed as A 405 /A 280 , where A 405
and A 280 stand for absorbance at 405 & 280 nm, respectively. Also, the ratios of A 565 /A 280 and of A 320 /A 280 corresponding to PE and to MAA, respectively, were determined in order to monitor the level of pigment impurity persisting at each step of purification.
Protein concentration was also determined using the Lowry method, and lysozyme (1 mg protein mL-1) was used as the standard (Lowry et al. , 1951).
-
(v) Native PAGE : The cell-free extract and samples obtained at each step of purification were evaluated by native- PAGE for catalase activity using a vertical slab gel apparatus (Miniprotein-II, BioRad, USA) on 8% polyacrylamide gel supported with glycerol following the method of Davis (1964) with modifications as described by Tripathi and Srivastava (2001).
-
(vi ) Electroelution of individual isoforms : Individual isoforms of the enzyme were electroeluted from the native-PAGE of the enzyme by adopting the procedure (with minor modifications) developed by Harrington (1990). The native-PAGE was carried out using the enzyme fraction obtained after 60% acetone precipitation. Packing of the gel slices in the electroeluter was performed by using loading buffer containing 20 mM Tris-HCl (pH 8.0), 100 μM DTT, 1 mM PMSF, 1 mM EDTA and 10 % (v/v) glycerol. The eluates were collected at 8-10 mA per tube for 3-4 h at 4˚C in elution buffer containing 20 mM Tris-HCl (pH 8.0) and 5 mM DTT and were further concentrated with a 10 kDa cut-off filter (Ultrafree-MC, Millipore USA) to the desired protein concentration.
-
(vi i) Enzyme characterization :
-
(a) SDS PAGE : 15% polyacrylamide resolving gel supported with 0.1 % SDS and 5% stacking gel) was
used for further analysis of purity and estimation of molecular weight of the isoforms of catalase obtained in the eluate of DEAE-Sephadex column or after electroelution from native PAGE following the method of Sambrook (Sambrook et al. , 1989). The proteins on gels were visualized by staining with 0.25% (w/v) Coomassie Brilliant Blue R-250 in 45% methanol and 10% acetic acid and destained with 45% (v/v) methanol and 7% (v/v) glacial acetic acid. Molecular weights were analyzed using low molecular weight markers as standards (MW-SDS-70, Sigma, USA).
-
(b) IEF: The isoform(s) of catalase purified by ion-exchange chromatography and by electroelution were further evaluated by denaturing urea-IEF on a 10% polyacrylamide gel with 2% (v/v) Ampholine (pH 3.5-10.0, Sigma, USA) (Robertson et al. , 1987) in 8 M urea using a vertical slab gel apparatus (Miniprotein-II, Bio-Rad, USA). Samples (10-30 µg protein/lane) were incubated with equal volumes of urea-IEF sample solution [8 M-urea, ampholytes (2%), β-ME- 50 μL, bromophenol blue- 50 μg in water] for 5 min at room temperature, applied to bottom of wells and overlaid with a 1% ampholyte, 15 % glycerol solution. Focusing was performed using 50 mM NaOH and 20 mM acetic acid as catholyte and anolyte solutions, respectively, and the gel was run at 250 V for 30 min followed by 450 V (constant current) for another 2 h at 4˚C. After electrophoretic separation, the gel was fixed for 2030 min with constant shaking at room temperature in the IEF fixing solution [Trichloroacetic acid (12% w/v) and Sulfosalicyclic acid (4 % w/v)] in deionized water. Afterward, it was rinsed in destaining solution (the same as that used for SDS-PAGE gels) for 10 min. The protein bands in IEF gels were
visualized by Coomassie Blue staining as described for SDS-PAGE gels.
-
(c) Amino acid sequence analysis : The pI and molecular weights of individual subunits and internal amino acid sequence analyses were outsourced to The Centre for Genomic Application (TCGA), New Delhi, India. The internal amino acid sequences of catalases were analyzed using LC-MS by excising the gel bands after denaturing-IEF, performing tryptic digestion of the protein, and separating and fragmenting them on a reverse phase column. The data were analyzed by matching with Xcalibur/database/uniprot.fasta, and the deduced amino acid compositions of the catalases used as reference (with GenBank gene sequence primary accession numbers in parentheses) were CATA_ERYGR (Q8X1P0) EC.1.11.1.6) and CATB_AJECA (Q9Y7C2) EC 1.11.1.6).
-
(d) Enzyme assay : Catalase activity in enzyme samples (cell-free extract or purified) was determined by monitoring the decrease in absorbance of H 2 O 2 as described by Tripathi and Srivastava (2001) with certain modifications. The reaction was initiated by addition of 4.4 mM H 2 O 2 to a 3 mL reaction mixture containing 2.86 mL of KPB (50 mM, pH 7.5) and 100 µL of enzyme extract (1.4 mg protein mL-1), and catalase activity was measured by recording the amount of H 2 O 2 (extinction coefficient (ε) of H 2 O 2 =43.6 M-1cm-1) consumed. One unit (U) of catalase activity was defined as the amount of enzyme required to degrade 1 μmol of H 2 O 2 min-1 at 25°C. The kinetic parameters were determined with the catalase solution using standard assay with varying substrate concentrations in the range of 3-460 mM. The apparent K m value and V max for the enzyme was estimated by analysis of data by Michaelis-Menten/ Lineweaver-Burk plots.
-
(e) Effect of pH and temperature : The effect of pH on catalase activity was evaluated in the range of pH 3.5-11.0 by employing different buffers, 50 mM sodium acetate buffer (pH 3.6-5.6), 50 mM K-phosphate buffer (pH 5.7-8.0) and 50 mM Glycine-NaOH buffer (pH 8.6-10.6). 100 µL enzyme solutions (1.4mg mL-1) was mixed with 2.86 mL of buffer of desired pH and incubated at 25˚C for 1 h and enzyme activity was measured using the standard assay. Also, the effect of temperature (20-80˚C) on catalase activity was evaluated by incubating the enzyme solution in standard reaction mixtures at each temperature for 1 h. The effect of all the above was expressed as Enzyme Relative activity (%) by taking the pH and temperature at which maximum enzyme activity was recorded as optimum (100%).
The effect of different concentrations of reducing agent DTT (0 -20 mM) on catalase activity was determined by incubating the reaction mixture containing 100 µl of the enzyme solution along with DTT at 25˚C for varying time periods. However, relative enzyme activity (%) was calculated by taking the enzyme activity recorded in the native enzyme without the presence of any additive compound taken as control (100%).
RESULTS
-
(i) Selection of the catalase isoform(s) for purification : An apparent variability in the number of catalase isoforms from four in the present study, to three, reported earlier by Tripathi and Srivastava in 2001, in the mats of L. arboricola harvested from the same natural habitat poses a problem for selection of the isoform(s) to be selected for purification. Native PAGE reflecting CAT activity in the natural mats grown at 0 MPa osmotic water potential for 72 h when incubated matrically
-
(ii) Optimization of redox state of the enzyme : From the native PAGE of catalase isoforms on applying varying reducing agents, we observed that the level of sharpness and intensity of isoforms of catalase decreased from 10 mM onwards on treatment with both cysteine and DTT, with maximum being observed at 5 mM DTT/cysteine treated enzyme [Supplementary Fig. S2 (a) A, B, C)]. On the other hand, upon addition of ß-ME, the sharpness and intensity of the bands were comparatively greatly reduced. At a much lower concentration of 2 mM ß-ME, the bands of native
enzyme were reduced to a thin diffuse band; increasing ß-ME concentration to 20 mM resulted in an almost complete loss of bands [Supplementary Fig. S2 (a) D]. Similar observations were recorded when measuring relative enzyme activity on incubation of the enzyme with varying concentrations of DTT. Maximum enzyme activity (~99 and 90%) was recorded at 5mM DTT on varying time period from 15 to 120 min [Supplementary Fig. S2 (b]. Significantly higher enzyme activity (~65 and 50%) than control was also observed on incubation of enzyme for 15 and 120 min. with 10mM DTT. Henceforth, DTT up to 5 mM was used to maintain the redox state to obtain structurally and functionally stable enzyme during this study.
-
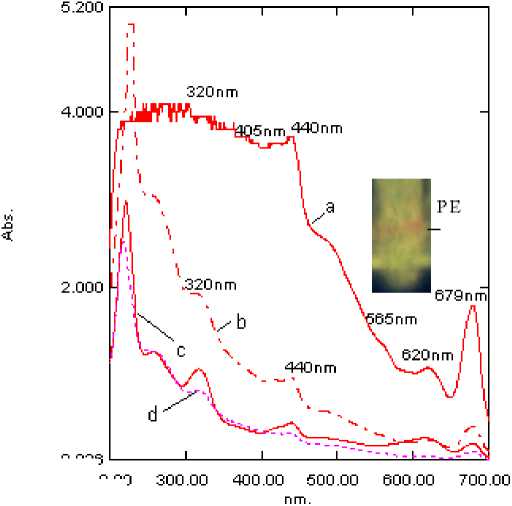
(iii) Purification steps : Absorption spectra of the cell-free extract obtained from the cyanobacterium with different concentrations of proteins and the enzyme extract (upper aqueous layer) collected after ethanol: chloroform treatment is presented in Supplementary Fig. S3. The presence of peaks in UV region (260-360 nm) representing MAA and syctonemins, 440, 679 nm of chlorophylls,and 565, 620 nm of phycobiliproteins besides the heme peak (405 nm) in the crude extract (a) clearly indicated the presence of additional accessory pigments in this cyanobacterium. Removal/recovery of these was achieved through successive purification steps and the success of catalase purification was checked at each step in terms of purity index (A 405 /A 280 ), decreasing ratio of A 565 /A 280 and of A 320 /A 280 in terms of protein content as illustrated in Supplementary Fig. S1. Also catalase activity at each step of purification and during fractionation procedures was checked through gel electrophoresis to ensure purity as given in Fig. 2a; , Fig.3 and Supplementary Fig. S4a, b.
The data of purification of catalase from Lyngbya arboricola are summarized in Table 1. The purified catalase was stored as a concentrated solution at -20oC without any significant loss in enzyme activity. Purification of the two most persistent isoforms [2 and 3; See Fig. 2 (a), E] of catalase with purity index of 0.83 and 0.6, respectively, contributing to 45 % and 40% of the total proteins and yield of 1.3 % was achieved by this procedure. Fig. 3 shows the SDS-PAGE profile of the subsequent purification steps of through which successful procurement of two major isoforms (2 and 3) of catalase was achieved. However electroelution of catalase isoforms from the native PAGE of the partially purified enzyme (after 60% acetone precipitation) supported the isolation of all the four individual isoforms separately (Fig. 2b) if required.
-
(iv) Characterization of catalase :
-
(a) Electrophoresis: The purity of the each of the isoforms isolated by precipitation steps and by electroelution was verified by presence of a single band at ~45kDa in SDS-PAGE (Fig. 4a). Urea-IEF and internal amino acid sequencing of subunits of individual isoforms obtained after ion-exchange chromatography and electroelution revealed the presence of 4 subunits in a single isoform, with the fourth subunit overlapping the third (Fig. 4c). The subunits 1 & 4 and 2 & 3, when analyzed for their internal amino acid sequences, molecular weight and pI revealed greatest homology and sequence matching with CatB (80.77 kD, pI:5.92) of Ajellomyces capsulata and CatA (78.96 kD,pI:5.89) of Erysiphe graminis subs. hordei , respectively (Fig.
5). Thus, an individual isoform of the catalase enzyme is heterotetrameric (MW equivalent to approximately 320 kDa), and each of its dimers is composed of two heteromeric subunits, where each one resembles either CatA or CatB, as depicted by the line diagram in Supplementary Fig. S5.
-
(b) Kinetic Properties : Catalase activity recorded with varying H 2 O 2 concentration indicated a gradual but steady increase in the enzyme activity till 150 mM attaining almost a stationary state at 200 mM. Nevertheless, on further increasing the concentration from 200 mM to 450 mM a gradual lowering was observed with ~ 50 % of enzyme activity being recorded at 450 mM. Linear curve fitting to the Lineweaver-Burk equation yielded a K m value of 10.27 mM and V max of 11.70 mM min-1 (Fig.6).
-
(c) Effect of pH and temperature : The activity of catalase as a function of pH and temperature showed that the catalase from the cyanobacterium being a typical catalase was active over a broad pH range (pH 6.0-10.5); optimal activity (100%) being recorded at pH 8.0. Noticeably, nearly 40% and 80 % of the optimum catalase activity was also recorded at lower (below 5.5) and higher (above 8.0) pH (Fig. 7).
The impact of temperature on enzymatic activity showed that the cyanobacterium catalase exhibited considerable activity in the range 20-70oC with optimum temperature for activity recorded at 50oC. Nearly 80% of the optimum activity was recorded in the range 20oC-55oC which gradually declined to 60% and 40% at 70oC and 80oC respectively (Fig. 7)
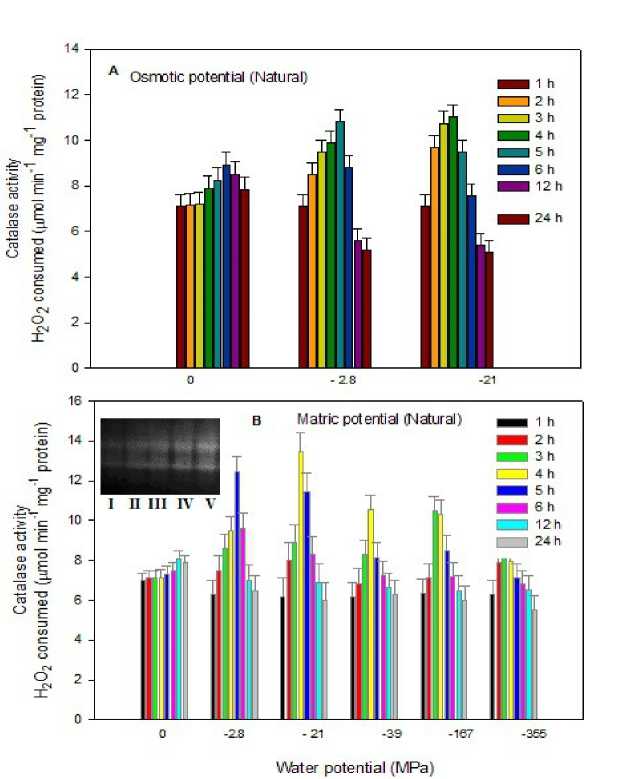
Figure 1: Catalase activity in the dry mats of L. arboricola incubated at different time periods, (A) osmotically and also in its osmotically grown mats on further (B) matric treatment. (Inset B: Lanes I, II, III, IV & V represent native PAGE of catalase isoforms in the osmotically (0 MPa for 72 h) grown mats on matric incubation for 48 h at 0, -2.8, -4.5, -11.2 and -21 MPa, respectively.
(a)
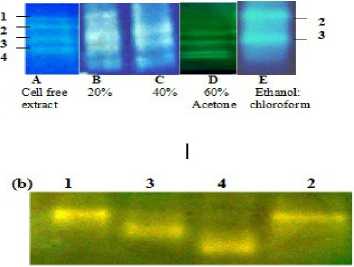
Figure 2: Native-PAGE (8%) of catalase activity in (a) respective enzyme enriched fractions at each step of purification; catalase activity in (A) cell free extract, in supernatant fraction after (B) 20% , (C) 40% acetone fractionation, in pellet fraction after (D) 60% acetone precipitation, (E) uppermost aqueous ethanol fraction (E2, see-Supplementary Fig. S1);.(b) four electroeluted catalase isoforms (corresponding to isoforms 1, 3, 4, 2 respectively procured from the native PAGE of the enzyme fraction obtained after 60% acetone precipitation as described in Materials and Methods
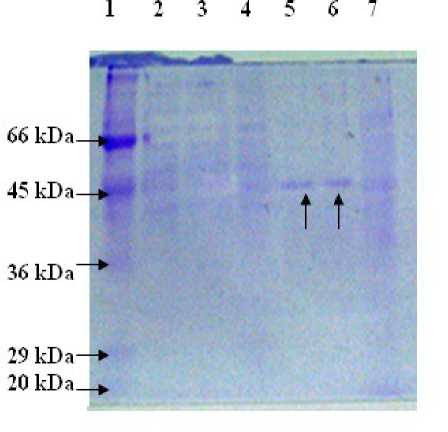
Figure 3: SDS-PAGE of catalase containing fractions after each purification step: (Lane 1) molecular mass standards; (Lane 2) cellular extract; (Lane 3) pellet fraction after 60% acetone precipitation; (Lane4) ethanol fraction after ethanol: chloroform treatment; (Lane 5, 6) purified isoform 2 and 3 of catalase separated by DEAE-ion exchange and (Lane 6) peak fraction after gel filtration.
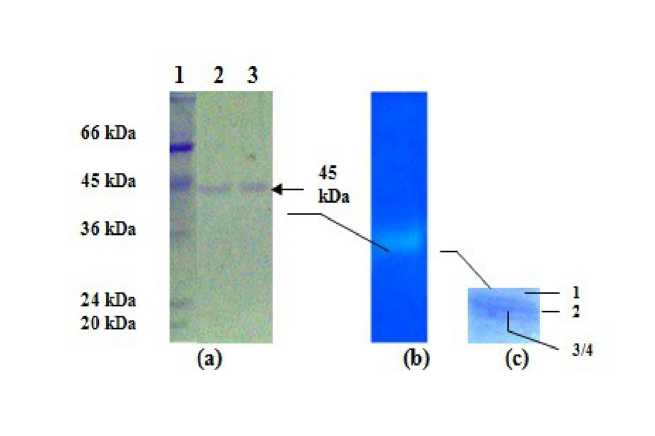
Figure 4: SDS PAGE, native PAGE and denaturing urea isoelectric focusing of single isoform of purified catalase. (a) Denaturing SDS (15%) polyacrylamide gel stained with Coomassie Brilliant Blue. Lane 1 -Molecular mass standard proteins; Lane 2 & 3, purified catalase isoforms (second and third) showing single band at 45 kDa, (b) Native (8%) PAGE stained for catalase activity showing electroeluted single isoform of catalase (c) Urea (8 M) IEF gel with Coomassie Brilliant Blue R-250 staining showing presence of four subunits (3 & 4 are superimposed) in the individual isoform of purified catalase.
A. Sequence of CatA
|
1-10 |
11-20 |
21-30 |
3140 |
11-50 |
51-60 |
61-70 |
71-80 |
81-90 |
91-100 |
л |
||
|
1 |
MRL5LHHLLG |
LSGLWASCP |
YK5GEDQEYH |
ISHIQARGID |
SEFLDQFKVE |
DSNSYLTTDA |
■SGPICDDASL |
KAGERGPTL1 |
EDFIFRQKIQ |
HFDHERVPER |
s |
|
|
101 |
AVHARGAGAY |
GTFTSYADHT |
NITM5FU15 |
itGKETPVFVR |
FSTVAGSRGS |
ADTVRDVHGF |
ATRFY7DEGN |
FDIVGKNIPV |
FFI1DAILFP |
DLVHAVKP3P |
||
|
201 |
CSEIPQAATG |
HDSAHDFFSQ |
QPSTLHTLFW |
AMSGHGIPRS |
YRHMDGFUVH |
imviDisK |
SKL’/KHHKKT |
KQGKA5LVWE |
EAQILAGKHP |
BFHRQDEWDE |
||
|
301 |
INAGHGPEHE |
LGVQIVDEED |
VQAFGFDUD |
PTKFLPEELV |
FVTILGKHKL |
TMPTNYFAE |
TEPMFGPGH |
EVRGTOFSDD |
PLLSGRIYSY |
LDTQINRNGG |
||
|
401 |
PNFE3LF/NR |
PRTKVHHNm |
DGAGQOIFIHT |
MPYSFHSL |
SGGNPKQANQ |
TKGRGFFIAP |
SRKWGSLHR |
GIAS5FADVW |
SQPRHFYIISL |
IPSE($FLVN |
||
|
501 |
AIRFEISQLK |
SDLIKKNILM |
QLNRVSNDLA |
TRVAAVIGYK |
PLDPSPEFYT |
NAITDY17IIF |
GKPLFSWGF |
TVGILASTSS |
STSISQAAQL |
AISFSSRGIR |
||
|
601 |
AVIVGESLLS |
GTDQTYSSAD |
AIAFDAVWI |
MGAETLFGPV |
AKPNILFPSG |
RPSQILHDAY |
RWGKPVGAVS |
KASWLEPLP |
GTKNQGGVYR |
VESVNELATS |
||
|
701 |
IAKGLETFRF |
VDRFPLDS |
||||||||||
|
V |
||||||||||||
B. Sequence of CatB
|
1-10 |
11-20 |
21-30 |
3140 |
41-50 |
51-60 |
61-70 |
71-80 |
81-90 |
91-100 |
|
|
1 |
HRSLKLILAS |
ASWSATCPY |
E5GEMPN5QN |
GPLDRRHDIL |
SDPTKjFLSK |
FYIDDEQSVL |
nCVGGPIED |
QH5LKAGKRG |
PTLLEDFIFR |
еда EITHER |
|
101 |
WERAVHARG |
AGARGVFTSY |
NHHSHIIAAS |
FLiffiAGKOTF |
vfvrfst™ |
SRG5VDSARD |
1HGFATRLYT |
deghfb™ |
NVPVTFIQDA |
ISFPDLIHAV |
|
201 |
KPQPDSEIPQ |
AATAHDTAWD |
FFSQQPSSLH |
ALE¥AMSGHG |
IPRSMRHVDG |
WGVHIFRLVT |
DEGHSTLVKF |
RWKILQGRAG |
LVWEEAQALG |
GKliPDFHRQD |
|
301 |
LWDAIESGRY |
PEWELGFQLV |
NEADQSKFDF |
DLLDPTKIIP |
EELVPFTPIG |
ЮГЛЖТРЮ! |
YFAEIEQIMF |
QPGHWRGID |
FTDDPLLQGR |
LYSYLDTQLN |
|
401 |
RHGGPNFEQL |
PINRPRIPFH |
tMRDGAGQ.4 |
FIPLNTAAYT |
PNSMSKGFPQ |
QMJRTHITRGF |
FTAPGRMVNG |
PLVRELSP5F |
NDVWSQPRLF |
YNSLTVFEKQ |
|
501 |
FLVNMFEM |
SEVRSETVRK |
NVIiaLNKVD |
NDLARRVALA |
IGVEPPSPDP |
TFYHNKnVP |
1GTFGTNLLR |
LtELKIALLT |
RDDGSniAE |
QLRAAFHSA1I |
|
601 |
HKVDIVLVGS |
SIDPQRGVNH |
IYSGADGSIF |
DAVIWGGLL |
ISASIQYPRG |
EtPLRIITDAY |
AYGKPVGAVG |
DGSNEALRDV |
l4aagg:ash |
GLDQPGVYI5 |
|
701 |
NDVSEAYVRS |
VLDGLTAYRF |
LMRFPLDR |
— |
Figure 5: Protein sequencing of subunit 1&4 (B) and subunit 2&3 (A) [Ref. Fig. 4 (c)], showing sequence similarity with CatB and CatA of Ajellomyces capsulata and Erysiphe graminis subs. hordei, respectively.
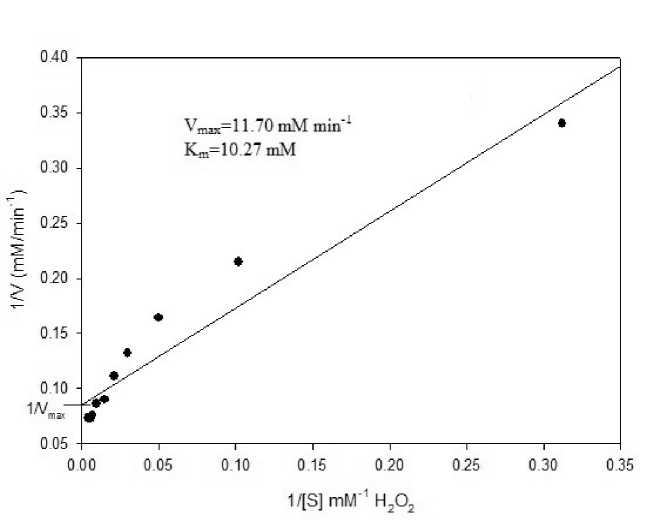
Figure 6: Lineweaver-Burk plot of reaction velocity of catalase purified from L. arboricola for estimation of kinetic parameters of enzyme. The enzyme assays were conducted at various hydrogen peroxide concentrations under standard assay conditions, as described in the Materials and Methods section.
Table 1: Purification characteristics of catalase from L. arboricola .
|
Purification Steps |
Volume (ml) |
Total Protein (mg) |
Enzyme activity (U/ml) |
Specific activity (U/mg) |
Purification fold |
Yield % |
|
Crude Extract |
65 |
159.75 |
4620 |
29.05 |
1 |
100 |
|
1st acetone precipitation |
||||||
|
(20:80) |
51 |
99.20 |
4200 |
42.30 |
1.45 |
90 |
|
2nd acetone precipitation |
||||||
|
(40:60) |
40 |
88.7 |
3900 |
43.90 |
1.51 |
84.4 |
|
3rd acetone precipitation |
||||||
|
(60:40) |
29 |
63.6 |
2850 |
44.81 |
1.54 |
61.6 |
|
Ethanol: chloroform |
||||||
|
(5:3) |
21 |
39.6 |
2070 |
52.2 |
1.79 |
44.8 |
|
Sephacryl S-300HR |
12 |
19.1 |
1900 |
99.4 |
3.42 |
41.12 |
|
DEAE-Sephadex A-50 |
5 |
1.1 |
780 |
709.09 |
24.43 |
1.3 |
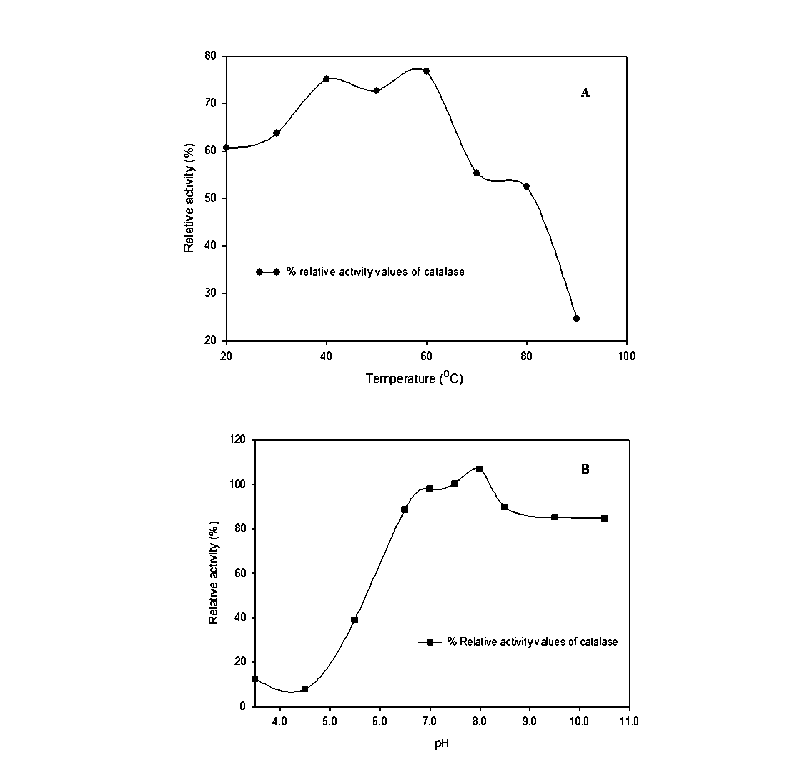
Figure 7: Effect of pH, temperature on purified catalase from L. arboricola . Catalase activity was assayed at different temperature (A) and pH (B) as described in Materials and methods.
DISCUSSION
Catalases from different organisms have a broad range of subunit sizes, a variety of quaternary structures, at least two different prosthetic groups, and even substantially different sequences. A number of catalase isoforms are reported to be present in organisms depending on the growth phases, types of cells and tissues, and also on genetic variability. Loewen and Switala (1987) have shown multiple numbers of catalases upon varying the growth conditions of Bacillus subtilis. Also catalases have been reported to exist in multiple forms in many higher plants, such as loblolly pine, spinach, cotton, wheat, tobacco (Mullen and Gifford, 1993; Garcia et al., 2000). Tissues from different parts of castor seedling exhibited variability in the catalase subunits (Ota et al., 1992). Chandlee and colleagues and Skadsen and Scandalios have shown that maize has three genetically different catalase molecules observed in a tissue specific and age-dependent manner (Chandlee et al., 1983; Skadsen and Scandalios, 1987). Such qualitative and quantitative variations in catalases, mainly monofunctional ones, are commonly attributed to cellular stress responses that are performed by regulating the enzyme, most commonly at the transcriptional level, and occasionally at the translational level, as well as through post-translational modifications and proteolysis. Zamocky et al., 2004 have also investigated the response of soil bacterium Commamonas terrigena N3H to various forms of oxidative stress and have isolated and studied the most abundant isoform. In the present study as evident from the observations made regarding activity (Figs. 1 A, B) and also regarding the number of isoforms (Fig. 1 B inset) of catalase upon varying the level of hydration (osmotic as well as matric), the cyanobacterium also displayed variability in its activity and in the number of isoforms. Such variability in the isoforms appears to be due to variations in the abiotic conditions of the habitats along with the oxygen-rich habitat that the cyanobacterium may encounter at its habitat (Talpasayi and Tripathi 1982). Such observations can also be correlated to the possession of higher levels of ROS enzymes in the cavity of Azolla (Canini et al., 1991) and bubbling cultures of Anabaena cylindrica with higher levels of O2-rich air (Tel-Or et al., 1986) Nevertheless, there are few reports regarding the occurrence of a varying number of isoforms in other cyanobacteria. Most of the studies until date have been related to activity of the enzyme catalase- peroxidase. Mutsuda and colleagues have shown the presence of a single isoform of 150 kDa catalase-peroxidase in Synechococcus PCC 7942 (Mutsuda et al., 1996) and Obinger and coworkers demonstrated the presence of catalase and o-dianisidine peroxidase activity in the cytosolic extracts of the cyanobacterium Anacystis nidulans with similarity to prokaryotic catalase-peroxidase in having single isoform (Obinger et al., 1997). Thus our study will be the first of its kind to report multiple isoforms of catalase in a desiccation tolerant terrestrial cyanobacterium Lyngbya arboricola which also shows variation in its isoforms on exposure to desiccation stress.
Cyanobacteria have evolved different mechanisms to withstand extremes of desiccation. It has already been seen that the cyanobacteria change the level of – SH content, mainly protein – SH, on varying the hydration level, and thus maintain the redox state of their cells during different degrees of dehydration and rehydration. In general, DTT, ß-ME, Na-Asc, cysteine and NaBH4 are used for regulation of thiol reactions (Habeeb, 1972). Compared to other reducing agents, a minimum concentration of DTT (10 mM) was recorded be more effective in maintaining high levels of –SH in dry cells of a desiccation-tolerant cyanobacterium Scytonema geitleri obtained from the rooftop of a building (Paul, 1998). This finding was consistent with earlier findings by Cleland (1964) and Wolf (1993). Nevertheless, in the present study activity of catalase detected on native PAGE as well as spectrophotometrically reflected DTT (5mM) was more effective in bringing the redox state of the enzyme closer to the functionally stable state, and higher concentrations of DTT resulted in functionally unstable enzyme (Supplementary Fig. S2a,b). Also similar reports of effect of thiol stress on Streptomyces coelicolor (Vekaria et al., 2007) showed an induction of catalaseA on exposing the organism to 10 mM DTT. In this study it was shown that specific activity of catalase increased by a factor of >8 upon exposure to 15mM DTT whereas there was no significant change in activity of superoxide dismutase.
Interestingly it was observed that in eukaryotes catalaseA, an enzyme normally observed in oxidative stress, was induced under thiol stress too. Thus, to maintain the redox state of cell-free extract of a cyanobacterium growing in subaerial habitats and to obtain significantly high yield of purified catalase , addition of 5mM DTT is recommended prior following the process of purification of proteins in particular.
Due to a lack of awareness about the type of catalase, and also the abundance of a number of interfering biomolecules in desiccation-tolerant cyanobacteria, selection of the procedure for purification of the enzyme was a critical aspect. As seen from the absorption spectrum (Supplementary Fig. S3), the Soret peak at 405 nm representing the heme group of the enzyme is not so prominent. The phenomenon of shielding of absorption peaks attributed to acetone soluble photosynthetic pigments has been observed in these terrestrial cyanobacteria (Tripathi, 1983). The shielding of the absorption peaks of the photosynthetic pigments was further observed due to the large number of substances with absorption maxima in the UV region that were later recognized as MAAs and scytonemins. It is likely that these UV pigments are responsible for the shielding of absorption peaks of heme in the present case also. In addition, PBPs (mainly PE, as shown in the inset of Supplementary Fig. S3), which have more or less same molecular weight as some isoforms of catalase, are also needed to be removed during enzyme purification.
The cyanobacterial catalase behaves like a typical catalase showing a true Michaelis Menten behaviour with saturation kinetics at 100 mM and above. Though there was inhibition in catalase activity above 250 mM till a H2O2 concentration of 450 mM but (50%) of activity was still recorded showing that there was not complete inhibition/inactivation of catalase at high substrate concentrations. This is in accordance with the behaviour of some typical catalases which are not saturable even up to 200 mM H2O2 (Jang et al., 2004; Yumoto et al., 2000). This may also be due to the thermostability of the cyanobacterium catalase as its reactions to substrate concentrations are similar to the novel thermo-stable catalase from Thermus brockianus which shows no substrate inhibition and inactivation of the enzyme at H2O2 concentrations up to 450 mM (Thompson et al., 2003).
The ability of catalases to remain stable in a desiccated state may be attributed to the presence of hydrophobic amino acids (Supplementary Fig. S6), which contribute nearly 40% of the total amino acid residues in catalase, as determined by internal protein sequences of catalase (results not shown). The stability may also be due to formation of disulfide bonds between two molecules of cysteine in the different subunits. Further catalase activity over a broad pH and temperature range (Fig.7) are indicative of the typical and monofunctional nature as well as the thermostability and pH stability of the enzyme. Similar observations were reported from catalase-peroxidases of therrmoalkaliphilic bacteria Bacillus sp. and catalases from Thermus brockianus (Gudelj et al., 2001; Thompson et al., 2003) which shows promising stabilities at high pH and temperature. Nearly 80% of the optimum catalase activity recorded at pH 9.0-10.5 of the cyanobacterium catalase is higher than the 4-6% of activity observed in Bacillus sp. at pH 9-10 and similar to the substantial catalase activity recorded over a broad pH range of 6-10 in Thermus brockianus catalase. The observations of temperature are also comparable to those recorded in Bacillus sp. (Gudelj et al., 2001) which showed temperature optima at 55oC and nearly 50% of enzyme activity at 70oC as compared to temperature optima at 50oC and 40% of enzyme activity at 70oC for L. arboricola catalase. The optimum activity is lower than that reported for Thermus brockianus which recorded a temperature optima at 90oC (Thompson et al., 2003) but higher than the Mn-catalase from Thermoleophilum album which had activity over a temperature range of 25-60oC with an optimum temperature for activity at 35oC (Allgood and Perry 1986).
On the basis of the above observations, it can be said that the heterotetrameric, monofunctional catalase with dimers of CatA and CatB, purified from L. arboricola with its high number of isoforms is novel among catalases detected so far in cyanobacteria. The detection of enzyme activity and the persistence of isoforms under extreme dehydration, resistance to ethanol: chloroform, presence of many hydrophobic amino acid residues along with the observations of pH and temperature demonstrated the stability of catalase and makes it a valuable source material for industrial applications, particularly by implementing the purification procedures of the enzyme with mass cultures of the cyanobacterium on a large scale. Besides the purification procedure described herein helped in simultaneously purifying 3 biotechnologically important biomolecules which could be a significant and cost effective protocol as compared to other methods. Further study of the structural and functional characteristics of this enzyme and its amino acid sequence analysis would give us a greater insight into the possible mechanisms of its stability and allow exploitation of its survival strategies for its biotechnological potential.
ACKNOWLEDGEMENT
We gratefully acknowledge the Head, Department of Botany, for providing laboratory facilities, and University of Grants Commission, New Delhi for financial support.
REFRENCES
Abele, D. (2002) Toxic oxygen: The radical Life- giver. Nature , 420(6911): 27
Allgood, G.S. and Perry J.J. (1986) Characterization of a Manganese-Containing Catalase from the Obligate Thermophile Thermoleophilum album . J. Bacteriol . 168 , 563-567.
Bernroitner, M., Zamocky, M., Furtmuller, P.G., Peschek, G.A. and Obinger, C. (2009) Occurrence, phylogeny, structure, and function of catalases and peroxidases in cyanobacteria. J. Exp. Bot. 60 , 423-440.
Bohm, G.A., Pfleiderer, W., Boger, W. and Scherer, P. (1995) Structure of a novel oligosaacharide mycosporine amino acid ultraviolet A/B sunscreen pigment from a terrestrial cyanobacterium Nostoc muscorum. J. Biol. Chem . 27 , 8536-8539.
Bonnichsen, R. (1955) Methods in Enzymology , vol. 2, Academic Press, New York, pp. 781-784.
Brock, T.D. (1975) Effect of water potential on Microcoleus (cyanophyceae) from a desert crust. J. Phycol. 11 , 316-320.
Calera, J.A., Sanchez-Weatherby, J., Lopez-Medrano, R. and Leal, F. (2000) Distinctive properties of the catalase B of Aspergillus nidulans . FEBS Lett . 475 , 117-120.
Canini, A., Galiazzo, F., Rotilio, G. and Grilli-Caiola, M. (1991) Superoxide Dismutase in the Symbiont Anabaena azollae Strasb . Plant Physiol . 97 , 34-40.
Chandlee, J.M., Tsaftaris, A.S. and Scandalios, J.G. (1983) Purification and partial characterization of three genetically defined catalases of maize. Plant Sci. Lett . 29 , 117-131.
Chandrashekar, P.A. (2012) Isolation, Purification and Characterization of Catalase from Aspergillus Species. J. Chem. Biol. Phys. Sci . 2 , 318-324.
Chelikani, P., Fita, I. and Loewen, P.C. (2004) Diversity of structures and properties among catalases. Cell. Mol. Life Sci . 61 , 192-208.
Cleland, W.W. (1964) Dithiothreitol, a new protective reagent for SH groups. Biochem . 3 , 480-482.
Garcia-Pichel, F., Wingard, C.E. and Castenholtz, R.W. (1993) Evidence regarding the UV-sunscreen role of a mycosporine-like compound in the cyanobacterium Gloeocapsa sp . Appl. Environ. Microbiol. 59 , 170-176.
Garcia, R., Kaid, N., Vignaud, C. and Nicolas, J. (2000) Purification and some properties of catalase from Wheat germ ( Triticum aestivum L .). J.Agric. Food Chem . 48 , 1050-1057.
Gerwick,W. H., Coates, R. C., Engene, N., Gerwick, L. G., Grindberg,R., Jones, A. and Sorrels, C. (2008) Giant marine cyanobacteria produce exciting potential pharmaceuticals. Microbe 3 , 277–284.
Gilichinsky, D.A.,Voroyoba, E.A., Erokhina, L.G., Fyodorov-Davidov, D.G. and Chaikovskaya, N.R. (1992) Long term preservation of microbial ecosystems in permafrost. Adv. Space Res. 12 , 255-263 .
Goldberg, I. and Hochman , A. (1989) Purification and characterization of a novel type of catalase from the bacterium Klebsiella pneumoniae. Biochim. Biophys. Acta 991 , 330-336.
Gudelj, M., Fruhwirth, G.O., Paar, A., Lottspeich, F., Robra, K. H., Cavaco-Paulo, A. and Gubitz, G. M. (2001) A catalase-peroxidase from a newly isolated thermoalkaliphilic Bacillus sp. with potential for the treatment of textile bleaching effluents. Extremophiles 5, 423-429.
Habeeb, A.F.S.A. (1972) Reaction of protein sulphydryl groups with Ellman’s reagent. in: C.H.W. Hirs and S.N. Timasheff (eds.) Methods in Enzymology , Vol. 25 , Academic Press, New York, pp. 457-464.
Harrington, M.G. (1990) Elution of protein from gels. Methods in Enzymology , vol. 182 , Academic Press, New York, pp. 488-495.
Harris, R.F., Gardner, W.R., Adebayo, A.A. and Somm, L.E. (1970) Agar dish isopiestic equilibration method for controlling the water potential of solid substrates. Appl. Microbiol . 19 , 536-537.
Jang, M.J., Park, P.J., Jung, W.K. and Kim, S.K. (2004) Purification and characterization of a catalase from the liver of bullfrog, Rana catesbeiana shaw . J. Food Biochem. 28 , 435–448.
Jones, A.C., Monroe, E.A., Podell, S., Hess, W.R., Klages, S., Esquenazi, E., Niessen, S., Hoover, H., Rothmann, M., Lasken, R.S., Yates III, J.R., Reinhardt, R.,Kube, M., Burkart, M.D., Allen, E.E., Dorrestein, P.C., Gerwick, W.H. and Gerwick, L. (2011) Genomic insights into the physiology and ecology of the marine filamentous cyanobacterium Lyngbya majuscula. PNAS, 108(21) , 8815–8820
Klotz, M. G., Klassen, G. R. and Loewen P. C. (1997) Phylogenetic relationships among prokaryotic and eukaryotic catalases. Mol. Biol. Evol . 14 , 951–958.
Kranner, I. and Birtic, S. (2005) A modulating role for antioxidants in desiccation tolerance.
Integr. Comp. Biol. 45 , 734-470.
Loewen, P.C. and Switala, J. (1987) Multiple catalases in Bacillus subtilis. J. Bacteriol. 169 , 3601-3607.
Loewen, P.C., Klotz, M.G. and Hassett, D.J. (2000) Catalase—an "old" enzyme that continues to surprise us. ASM News 66 , 76-82.
Lowry, O.H., Rosebrough, N.J., Farr, A.L. and Randall, R.J. (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193 , 265-275.
Marx, J.L. (1985) Oxygen free radicals linked to many diseases. Science, 235 , 529-531.
Melov, S., Ravenscroft, J., Malik, S., Gill, M.S., Walker, D.W., Clayton, P.E., Wallace, D.C., Malfroy, B., Doctrow, S.R. and Lithgow, G.J. (2000) Extension of life-span with superoxide dismutase/catalase mimetics. Science, 289 , 1567-1569.
Michan, S., Lledias, F., Baldwin, J.D., Natvig, D.O. and Hansberg, W. (2002) Regulation and oxidation of two large monofunctional catalases, Free Radic. Biol. Med . 33 , 521-532.
Mullen, R.T. and Gifford, D.J. (1993) Purification and characterization of catalase from Loblolly Pine ( Pinus taeda L .) Megagametophytes. Plant Physiol. 103 , 477-483.
Mutsuda, M., Ishikawa, T., Takeda, T. and Shigeoka, S. (1996) The catalase-peroxidase of Synechococcus PCC 7942: purification, nucleotide sequence analysis and expression in Escherichia coli . Biochem. J . 316 , 251-257.
Nadler, V., Goldberg, I. and Hochman, A. (1986) Comparative study of bacterial catalase. Biochim. Biophys. Acta, 882 , 234-241.
Nicholls, P., Loewen, P. and Fita, I. (2001)
Enzymology and structure of catalases. In: Sykes AG, Mauk G, [Eds.] Advances in inorganic chemistry. Heme-Fe proteins . New York, Academic, pp. 52–106.
Obinger, C., Regelsberger, G., Strasser, G., Burner, U. and Peschek, G.A. (1997) Purification and characterization of a homodimeric catalase-peroxidase from the cyanobacterium Anacystis nidulans . Biochem. Biophys. Res. Commun . 235 , 545-552.
Ota, Y., Ario, T., Hayashi, K., Nakagawa, T., Hattori, T., Maeshima, M. and Asahi, T. (1992) Tissuespecific Isoforms of catalase subunits in castor bean seedlings. Plant Cell Physiol . 33 , 225-232.
Paul, S. (1998) Biochemical Studies on Survival of a Terrestrial Cyanobacterium Scytonema geitleri under water stress. Ph.D. Thesis. Banaras Hindu University, India.
Potts, M. (2001) Desiccation tolerance: a simple process? Trends in Microbiol . 9 , 553-559.
Potts, M. and Friedmann, E.I. (1981) Effects of water stress on crytoendolithic cyanobacteria from hot desert rocks. Arch. Microbiol . 130, 267-271.
Robertson, E.F., Dannelly, H.K., Malloyand, P.J. and Reeves, H.C. (1987) Rapid isoelectric focusing in a vertical polyacrylamide minigel system. Anal. Biochem . 167 , 290-294.
Sambrook, J., Fritsch, E.F. and Maniatis, T. (1989) Molecular cloning: A Laboratory Manual , Cold Spring Harbor, Laboratory Press, Cold Spring
Harbour, New York.
Schonbaum, G.R., and Chance, B. (1976) Catalase, in: P.D. Boyer (Eds.) The Enzymes , vol. XIII pt. C, 3rd ed., Academic Press, London. pp. 363–408.
Shaked, Z.and Wolfe, S. (1988) Stabilization of pyranose-2-oxidase and catalase by chemical modification. Methods Enzymol. 137 , 599-615.
Skadsen, R.W. and Scandalios, J.G. (1987) Translational control of photo-induced expression of the Cat-2 catalase gene during leaf development in maize. Proc. Natl. Acad. Sci. USA , 80 , 4455-4459.
Talpasayi, E.R.S. and Tripathi, S.N. (1982) Photofixation of carbon in subaerial blue-green algae, in: Proceedings of International Symposium on Biological Nitrogen Fixation. IARI, New Delhi, pp. 138-149.
Tel-Or, E., Huflejt, M.E. and Packer, L. (1986) Hydroperoxide metabolism in cyanobacteria. Archives of Biochem. Biophys . 246 , 396-402.
Thompson, V.S., Schaller, K.D. and Apel, W.A. (2003) Purification and characterization of a novel thermo-alkali-stable catalase from Thermus brockianus . Biotechnol. Prog . 19 , 1292-1299.
Tripathi, S.N. (1983) Effect of temperature on chlorophyll stability of some subaerial blue green algae. Z. Algae Microbiol . 23 , 443-446.
Tripathi, S.N. and Maurya, J.N. (2001) Photosynthetic activities of a Roof-top desiccation tolerant cyanobacterium Scytonema geitleri at varying hydration levels. Algae 16 , 445-455.
Tripathi, S.N. and Srivastava, P. (2001) Presence of stable oxygen scavenging enzymes superoxide dismutase, ascorbate peroxidase and catalase in a desiccation-tolerant cyanobacterium
Lyngbya arboricola under dry state. Curr. Sci . 81 , 197-200.
Tripathi, S.N., Kapoor, S. and Shrivastava, A. (2007) Extraction and purification of an unusual phycoerythrin in a terrestrial desiccation tolerant cyanobacterium Lyngbya arboricola . J. Appl. Phycol. 19 , 441-447.
Tripathi, S.N., Tiwari, B.S. and Talpasayi, E.R.S. (1990/91) Growth of cyanobacteria (blue green algae) on urban buildings. Energy Buildings . 15-16 , 499-505.
Tsuchihashi, M. (1923) Zur Kenntnis des Blutkatalase. Biochem. Z . 140 , 63–112.
Turdi, S., Li, Q., Lopez, F.L. and Ren, J. (2007) Catalase alleviates cardiomyocyte dysfunction in diabetes: role of Akt, Forkhead transcriptional factor and silent information regulator 2 . Life Sci. 81 , 895–905
Tzanov, T., Costa, S., Gubitz, G. M. And Cavaco-Paulo, A. (2001) Dyeing with catalase treated bleaching baths. Color Tech . 117 , 1-5.
Vekaria, H., Sadagopan, K., Adamec, J., Jarori, G.K. and Prabha, C.R. (2007) Thiol stress induces catalaseA in Streptomyces coelicolor . (In: Formatex: Communicating Current Research and Educational Topics and Trends in Applied Microbiology , Méndez-Vilas, A., Ed. 246-254.
Wang, W.,Wang, F., Ji, X., Liu, S.,Yuan, C. and Sun, M. (2011) Cloning and characterization of a psychrophilic catalase gene from an antarctic bacterium. African J. Microbiol. Res. 5 , 31953199.
Weiting, N.I., Trelease, R. N.and Eising. R. (1990) Two temporally synthesized charge subunits interact to form the five isoforms of cottonseed ( Gossypium hirsutum ) catalase. Biochem. J . 269 , 233-238.
Wolf, W.J. (1993) Sulphydryl content of glycinin: effect of reducing agents. J. Agric. Food Chem . 41 , 168-176.
Yumoto,I., Ichihashi, D., Iwata, H. and Istokkovics, A. (2000) Purification and characterization of a catalase from the facultatively psychrophilic bacterium Vibrion rumoiensis S-1T exhibiting high catalase activity. J.Bacteriol . 182 , 19031909.
Zamocky, M. and Koller, F. (1999) Understanding the structure and function of catalases: clues from molecular evolution and in vitro mutagenesis. Prog. Biophys. Mol. Biol . 72 , 1966.
Zámocký, M., Godobíková, J., Ganperík, J., Koller, F., and Poleka , B. (2004 ) Expression, purification, and sequence analysis of catalase-1 from the soil bacterium Comamonas terrigena N3H. Protein Expression and Purification 36 , 115– 123.
Zamocky, M., Jakopitsch, C., Furtmuller, P.G., Dunand, C. and Obinger, C. (2008) The peroxidase-cyclooxygenase superfamily: reconstructed evolution of critical enzymes of the innate immune system. Proteins 71 , 589605.
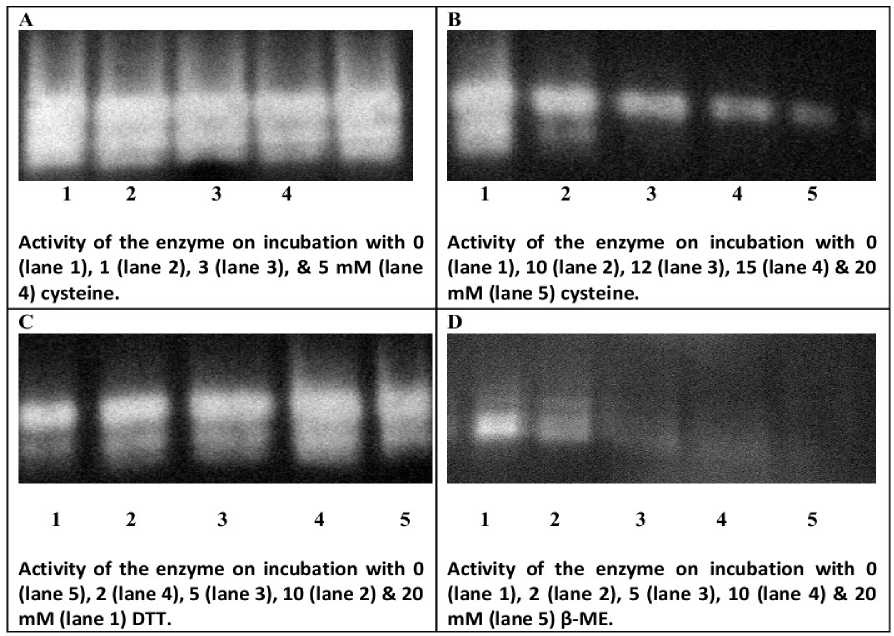
Supplementary Fig.1. Native-PAGE of catalase (0.05 mg enzyme per lane) activity on incubation for 30 min at 4oC with (A) 0-5 mM cysteine & (B) 10-20 mM cysteine, (C) 0- 20 mM dithiothreitol (DTT) and (D) 0- 20 mM Mercaptoethanol (β-ME).
0.036
200.00
Supplementary Fig. 2. UV-visible overlay absorption spectra of cell free enzyme extract (0.5 mg protein ml-1) of L. arboricola having at each step of purification. (a) crude extract, (b) 60% acetone precipitation (c) after ethanol: chloroform treatment, (d) after ion exchange chromatography . (Inset: native-PAGE of catalase isoforms along with PE in the cell-free extract of the cyanobacterium).
Fraction Number
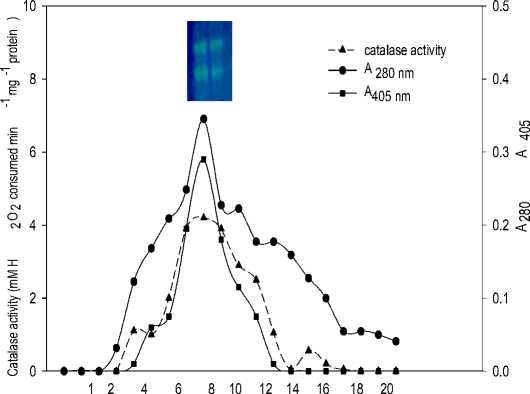
Supplementary Fig.3a. Gel-filtration chromatography of ethanol fraction obtained after ethanol: chloroform treatment during purification of catalase. The enzyme was eluted with 100 mM KPB (pH 7.0) at 30 ml h-1 flow rate and 2 ml fraction volume. (Inset: NativePAGE of catalase isoforms from pooled fractions 7-12 with maximum enzyme activity).
Fraction Number
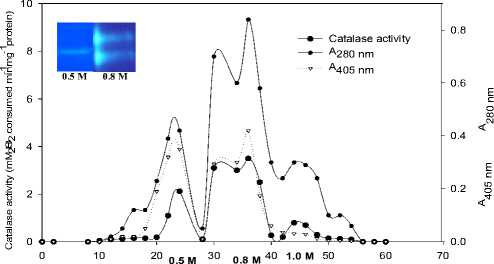
Supplementary Fig.3b. Ion-exchange chromatography of enzyme enriched fractions procured after gel- filtration. The enzyme was eluted by a linear gradient of NaCl (0-1.0 M) in 10 mM Tris-HCl (pH 8.0) at a flow rate of 24 ml h-1 collecting 10 ml at each gradient. (Inset: Native-PAGE of catalase isoforms in the fractions pooled using 0.5 M and 0.8 M NaCl).
Isoform Subunits
IEF
CatB

3/4
------► < > CatA
Sequencing CatA
Список литературы Isolation and purification of heterotetrameric catalase from a desiccation tolerant cyanobacterium Lyngbya arboricola
- Abele, D. (2002) Toxic oxygen: The radical Life-giver. Nature, 420(6911): 27
- Allgood, G.S. and Perry J.J. (1986) Characterization of a Manganese-Containing Catalase from the Obligate Thermophile Thermoleophilum album. J. Bacteriol. 168, 563-567.
- Bernroitner, M., Zamocky, M., Furtmuller, P.G., Peschek, G.A. and Obinger, C. (2009) Occurrence, phylogeny, structure, and function of catalases and peroxidases in cyanobacteria. J. Exp. Bot. 60, 423-440.
- Bohm, G.A., Pfleiderer, W., Boger, W. and Scherer, P. (1995) Structure of a novel oligosaacharide mycosporine amino acid ultraviolet A/B sunscreen pigment from a terrestrial cyanobacterium Nostoc muscorum. J. Biol. Chem. 27, 8536-8539.
- Bonnichsen, R. (1955) Methods in Enzymology, vol. 2, Academic Press, New York, pp. 781-784.
- Brock, T.D. (1975) Effect of water potential on Microcoleus (cyanophyceae) from a desert crust. J. Phycol. 11, 316-320.
- Calera, J.A., Sanchez-Weatherby, J., Lopez-Medrano, R. and Leal, F. (2000) Distinctive properties of the catalase B of Aspergillus nidulans. FEBS Lett. 475, 117-120.
- Canini, A., Galiazzo, F., Rotilio, G. and Grilli-Caiola, M. (1991) Superoxide Dismutase in the Symbiont Anabaena azollae Strasb. Plant Physiol. 97, 34-40.
- Chandlee, J.M., Tsaftaris, A.S. and Scandalios, J.G. (1983) Purification and partial characterization of three genetically defined catalases of maize. Plant Sci. Lett. 29, 117-131.
- Chandrashekar, P.A. (2012) Isolation, Purification and Characterization of Catalase from Aspergillus Species. J. Chem. Biol. Phys. Sci. 2, 318-324.
- Chelikani, P., Fita, I. and Loewen, P.C. (2004) Diversity of structures and properties among catalases. Cell. Mol. Life Sci. 61, 192-208.
- Cleland, W.W. (1964) Dithiothreitol, a new protective reagent for SH groups. Biochem. 3, 480-482.
- Davis, B.J. (1964) Disk electrophoresis. II. Method and application to human serum proteins. Ann. N.Y. Acad.Sci. 121, 404-427.
- Garcia-Pichel, F., Wingard, C.E. and Castenholtz, R.W. (1993) Evidence regarding the UV-sunscreen role of a mycosporine-like compound in the cyanobacterium Gloeocapsa sp. Appl. Environ. Microbiol. 59, 170-176.
- Garcia, R., Kaid, N., Vignaud, C. and Nicolas, J. (2000) Purification and some properties of catalase from Wheat germ (Triticum aestivum L.). J.Agric. Food Chem. 48, 1050-1057.
- Gerwick, W. H., Coates, R. C., Engene, N., Gerwick, L. G., Grindberg,R., Jones, A. and Sorrels, C. (2008) Giant marine cyanobacteria produce exciting potential pharmaceuticals. Microbe 3, 277-284.
- Gilichinsky, D.A.,Voroyoba, E.A., Erokhina, L.G., Fyodorov-Davidov, D.G. and Chaikovskaya, N.R. (1992) Long term preservation of microbial ecosystems in permafrost. Adv. Space Res. 12, 255-263.
- Goldberg, I. and Hochman, A. (1989) Purification and characterization of a novel type of catalase from the bacterium Klebsiella pneumoniae. Biochim. Biophys. Acta 991, 330-336.
- Gudelj, M., Fruhwirth, G.O., Paar, A., Lottspeich, F., Robra, K. H., Cavaco-Paulo, A. and Gubitz, G. M. (2001) A catalase-peroxidase from a newly isolated thermoalkaliphilic Bacillus sp. with potential for the treatment of textile bleaching effluents. Extremophiles 5, 423-429.
- Habeeb, A.F.S.A. (1972) Reaction of protein sulphydryl groups with Ellman’s reagent. in: C.H.W. Hirs and S.N. Timasheff (eds.) Methods in Enzymology, Vol. 25, Academic Press, New York, pp. 457-464.
- Harrington, M.G. (1990) Elution of protein from gels. Methods in Enzymology, vol. 182, Academic Press, New York, pp. 488-495.
- Harris, R.F., Gardner, W.R., Adebayo, A.A. and Somm, L.E. (1970) Agar dish isopiestic equilibration method for controlling the water potential of solid substrates. Appl. Microbiol. 19, 536-537.
- Hoffmann, L. (1994) Marine Cyanophyceae of Papua New Guinea.VI. The genus Lyngbya. S.L. Belg. J. Bot. 127, 79-86.
- Jang, M.J., Park, P.J., Jung, W.K. and Kim, S.K. (2004) Purification and characterization of a catalase from the liver of bullfrog, Rana catesbeiana shaw. J. Food Biochem. 28, 435-448.
- Jones, A.C., Monroe, E.A., Podell, S., Hess, W.R., Klages, S., Esquenazi, E., Niessen, S., Hoover, H., Rothmann, M., Lasken, R.S., Yates III, J.R., Reinhardt, R.,Kube, M., Burkart, M.D., Allen, E.E., Dorrestein, P.C., Gerwick, W.H. and Gerwick, L. (2011) Genomic insights into the physiology and ecology of the marine filamentous cyanobacterium Lyngbya majuscula. PNAS, 108(21), 8815-8820
- Klotz, M. G., Klassen, G. R. and Loewen P. C. (1997) Phylogenetic relationships among prokaryotic and eukaryotic catalases. Mol. Biol. Evol. 14, 951-958.
- Kranner, I. and Birtic, S. (2005) A modulating role for antioxidants in desiccation tolerance. Integr. Comp. Biol. 45, 734-470.
- Loewen, P.C. and Switala, J. (1987) Multiple catalases in Bacillus subtilis. J. Bacteriol. 169, 3601-3607.
- Loewen, P.C., Klotz, M.G. and Hassett, D.J. (2000) Catalase-an "old" enzyme that continues to surprise us. ASM News 66, 76-82.
- Lowry, O.H., Rosebrough, N.J., Farr, A.L. and Randall, R.J. (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265-275.
- Marx, J.L. (1985) Oxygen free radicals linked to many diseases. Science, 235, 529-531.
- Melov, S., Ravenscroft, J., Malik, S., Gill, M.S., Walker, D.W., Clayton, P.E., Wallace, D.C., Malfroy, B., Doctrow, S.R. and Lithgow, G.J. (2000) Extension of life-span with superoxide dismutase/catalase mimetics. Science, 289, 1567-1569.
- Michan, S., Lledias, F., Baldwin, J.D., Natvig, D.O. and Hansberg, W. (2002) Regulation and oxidation of two large monofunctional catalases, Free Radic. Biol. Med. 33, 521-532.
- Mullen, R.T. and Gifford, D.J. (1993) Purification and characterization of catalase from Loblolly Pine (Pinus taeda L.) Megagametophytes. Plant Physiol. 103, 477-483.
- Mutsuda, M., Ishikawa, T., Takeda, T. and Shigeoka, S. (1996) The catalase-peroxidase of Synechococcus PCC 7942: purification, nucleotide sequence analysis and expression in Escherichia coli. Biochem. J. 316, 251-257.
- Nadler, V., Goldberg, I. and Hochman, A. (1986) Comparative study of bacterial catalase. Biochim. Biophys. Acta, 882, 234-241.
- Nicholls, P., Loewen, P. and Fita, I. (2001) Enzymology and structure of catalases. In: Sykes AG, Mauk G, [Eds.] Advances in inorganic chemistry. Heme-Fe proteins. New York, Academic, pp. 52-106.
- Obinger, C., Regelsberger, G., Strasser, G., Burner, U. and Peschek, G.A. (1997) Purification and characterization of a homodimeric catalase-peroxidase from the cyanobacterium Anacystis nidulans. Biochem. Biophys. Res. Commun. 235, 545-552.
- Ota, Y., Ario, T., Hayashi, K., Nakagawa, T., Hattori, T., Maeshima, M. and Asahi, T. (1992) Tissue-specific Isoforms of catalase subunits in castor bean seedlings. Plant Cell Physiol. 33, 225-232.
- Paul, S. (1998) Biochemical Studies on Survival of a Terrestrial Cyanobacterium Scytonema geitleri under water stress. Ph.D. Thesis. Banaras Hindu University, India.
- Potts, M. (2001) Desiccation tolerance: a simple process? Trends in Microbiol. 9, 553-559.
- Potts, M. and Friedmann, E.I. (1981) Effects of water stress on crytoendolithic cyanobacteria from hot desert rocks. Arch. Microbiol. 130, 267-271.
- Preston, T.J., Muller, W.J. and Singh, G. (2001) Scavenging of extracellular H2O2 by catalase inhibits the proliferation of HER-2/Neu-transformed rat-1 fibroblasts through the induction of a stress response. J. Biol.Chem. 276, 9558-9564.
- Robertson, E.F., Dannelly, H.K., Malloyand, P.J. and Reeves, H.C. (1987) Rapid isoelectric focusing in a vertical polyacrylamide minigel system. Anal. Biochem. 167, 290-294.
- Sambrook, J., Fritsch, E.F. and Maniatis, T. (1989) Molecular cloning: A Laboratory Manual, Cold Spring Harbor, Laboratory Press, Cold Spring Harbour, New York.
- Schonbaum, G.R., and Chance, B. (1976) Catalase, in: P.D. Boyer (Eds.) The Enzymes, vol. XIII pt. C, 3rd ed., Academic Press, London. pp. 363-408.
- Shaked, Z.and Wolfe, S. (1988) Stabilization of pyranose-2-oxidase and catalase by chemical modification. Methods Enzymol. 137, 599-615.
- Skadsen, R.W. and Scandalios, J.G. (1987) Translational control of photo-induced expression of the Cat-2 catalase gene during leaf development in maize. Proc. Natl. Acad. Sci. USA, 80, 4455-4459.
- Talpasayi, E.R.S. and Tripathi, S.N. (1982) Photofixation of carbon in subaerial blue-green algae, in: Proceedings of International Symposium on Biological Nitrogen Fixation. IARI, New Delhi, pp. 138-149.
- Tel-Or, E., Huflejt, M.E. and Packer, L. (1986) Hydroperoxide metabolism in cyanobacteria. Archives of Biochem. Biophys. 246, 396-402.
- Thompson, V.S., Schaller, K.D. and Apel, W.A. (2003) Purification and characterization of a novel thermo-alkali-stable catalase from Thermus brockianus. Biotechnol. Prog. 19, 1292-1299.
- Tripathi, S.N. (1983) Effect of temperature on chlorophyll stability of some subaerial blue green algae. Z. Algae Microbiol. 23, 443-446.
- Tripathi, S.N. and Maurya, J.N. (2001) Photosynthetic activities of a Roof-top desiccation tolerant cyanobacterium Scytonema geitleri at varying hydration levels. Algae 16, 445-455.
- Tripathi, S.N. and Srivastava, P. (2001) Presence of stable oxygen scavenging enzymes superoxide dismutase, ascorbate peroxidase and catalase in a desiccation-tolerant cyanobacterium Lyngbya arboricola under dry state. Curr. Sci. 81, 197-200.
- Tripathi, S.N., Kapoor, S. and Shrivastava, A. (2007) Extraction and purification of an unusual phycoerythrin in a terrestrial desiccation tolerant cyanobacterium Lyngbya arboricola. J. Appl. Phycol. 19, 441-447.
- Tripathi, S.N., Tiwari, B.S. and Talpasayi, E.R.S. (1990/91) Growth of cyanobacteria (blue green algae) on urban buildings. Energy Buildings. 15-16, 499-505.
- Tsuchihashi, M. (1923) Zur Kenntnis des Blutkatalase. Biochem. Z. 140, 63-112.
- Turdi, S., Li, Q., Lopez, F.L. and Ren, J. (2007) Catalase alleviates cardiomyocyte dysfunction in diabetes: role of Akt, Forkhead transcriptional factor and silent information regulator 2. Life Sci. 81, 895-905
- Tzanov, T., Costa, S., Gubitz, G. M. And Cavaco-Paulo, A. (2001) Dyeing with catalase treated bleaching baths. Color Tech. 117, 1-5.
- Vekaria, H., Sadagopan, K., Adamec, J., Jarori, G.K. and Prabha, C.R. (2007) Thiol stress induces catalaseA in Streptomyces coelicolor. (In: Formatex: Communicating Current Research and Educational Topics and Trends in Applied Microbiology, Méndez-Vilas, A., Ed. 246-254.
- Wang, W.,Wang, F., Ji, X., Liu, S.,Yuan, C. and Sun, M. (2011) Cloning and characterization of a psychrophilic catalase gene from an antarctic bacterium. African J. Microbiol. Res. 5, 3195-3199.
- Weiting, N.I., Trelease, R. N.and Eising. R. (1990) Two temporally synthesized charge subunits interact to form the five isoforms of cottonseed (Gossypium hirsutum) catalase. Biochem. J. 269, 233-238.
- Wolf, W.J. (1993) Sulphydryl content of glycinin: effect of reducing agents. J. Agric. Food Chem. 41, 168-176.
- Yumoto,I., Ichihashi, D., Iwata, H. and Istokkovics, A. (2000) Purification and characterization of a catalase from the facultatively psychrophilic bacterium Vibrion rumoiensis S-1T exhibiting high catalase activity. J.Bacteriol. 182, 1903-1909.
- Zamocky, M. and Koller, F. (1999) Understanding the structure and function of catalases: clues from molecular evolution and in vitro mutagenesis. Prog. Biophys. Mol. Biol. 72, 19-66.
- Zámocký, M., Godobíková, J., Ganperík, J., Koller, F., and Poleka, B. (2004) Expression, purification, and sequence analysis of catalase-1 from the soil bacterium Comamonas terrigena N3H. Protein Expression and Purification 36, 115-123.
- Zamocky, M., Jakopitsch, C., Furtmuller, P.G., Dunand, C. and Obinger, C. (2008) The peroxidase-cyclooxygenase superfamily: reconstructed evolution of critical enzymes of the innate immune system. Proteins 71, 589-605.


