Использование генов углеводного обмена для улучшения качества клубней картофеля (Solanum tuberosum L.)
Автор: Слугина М.А., Кочиева Е.З.
Журнал: Сельскохозяйственная биология @agrobiology
Рубрика: Обзоры, проблемы
Статья в выпуске: 3 т.53, 2018 года.
Бесплатный доступ
Картофель ( Solanum tuberosum L.) относится к важнейшим сельскохозяйственным культурам во всем мире. Ценные пищевые и технические качества клубней картофеля в основном определяются накоплением в них крахмала. Крахмал состоит из линейных и разветвленных полимеров (соответственно амилоза и амилопектин). Три основные задачи современной селекции картофеля, направленной на улучшение продовольственных качеств клубней, включают увеличение их крахмалистости, получение клубней с повышенным содержанием амилозы или амилопектина, а также ингибирование процесса холодового осахаривания. Современные молекулярные и биотехнологические методы (маркер-опосредованная селекция, получение трансгенных растений, геномное редактирование и т.п.) позволяют изменять желаемые признаки растений. Однако вне зависимости от используемого подхода основополагающий этап, определяющий успешный результат работы, - это правильный выбор гена-мишени, что, в свою очередь, требует детального понимания метаболических путей синтеза и распада целевого продукта в растительных тканях...
Картофель, крахмал клубней, амилоза, амилопектин, холодовое осахаривание, углеводный метаболизм, ферменты углеводного метаболизма, аллельные варианты генов
Короткий адрес: https://sciup.org/142216548
IDR: 142216548 | УДК: 635.21:631.522/.524:577.21 | DOI: 10.15389/agrobiology.2018.3.450rus
Текст обзорной статьи Использование генов углеводного обмена для улучшения качества клубней картофеля (Solanum tuberosum L.)
М.А. СЛУГИНА, Е.З. КОЧИЕВА
Картофель ( Solanum tuberosum L.) относится к важнейшим сельскохозяйственным культурам во всем мире. Ценные пищевые и технические качества клубней картофеля в основном определяются накоплением в них крахмала. Крахмал состоит из линейных и разветвленных полимеров (соответственно амилоза и амилопектин). Три основные задачи современной селекции картофеля, направленной на улучшение продовольственных качеств клубней, включают увеличение их крахмалистости, получение клубней с повышенным содержанием амилозы или амилопектина, а также ингибирование процесса холодового осахаривания. Современные молекулярные и биотехнологические методы (маркер-опосредованная селекция, получение трансгенных растений, геномное редактирование и т.п.) позволяют изменять желаемые признаки растений. Однако вне зависимости от используемого подхода основополагающий этап, определяющий успешный результат работы, — это правильный выбор гена-мишени, что, в свою очередь, требует детального понимания метаболических путей синтеза и распада целевого продукта в растительных тканях. Процесс биосинтеза крахмала, начиная с образования моносахаридных субстратов до формирования крахмального зерна, включает множество реакций и требует координированной работы большого числа ферментов. Кроме того, стоит отметить, что в углеводный метаболизм вовлечены не только ферменты, модифицирующие моно-, ди- и полисахариды, но и регуляторные белки, оказывающие опосредованное влияние на эти процессы, работу которых также необходимо принимать во внимание. С учетом опубликованного ранее обзора (В.К. Хлесткин соавт., 2017), где основное внимание уделено генам, определяющим конкретные физико-химические и технологические свойства крахмала, в настоящем сообщении акцент сделан на современном понимании процессов биосинтеза и распада крахмала и описании известных на сегодняшний день ферментов углеводного обмена с целью выявления ключевых генов, ассоциированных с содержанием крахмала в клубнях. Из большого числа белков, участвующих в метаболизме углеводов в клубнях картофеля, выделены ключевые ферменты, которые как напрямую, так и опосредованно могут осуществлять наиболее важные этапы синтеза и распада крахмала в клубнях. Среди них сахаро-зосинтазы, крахмалфосфорилазы, крахмалсинтазы, гранулосвязанная крахмалсинтаза, крахмалветвящий фермент, α - и β -амилазы, кислая вакуолярная инвертаза, а также ингибиторы инвертаз и амилаз. Определен круг кодирующих эти ферменты генов-кандидатов, аллельные варианты которых могут быть связаны с хозяйственно ценными признаками картофеля. Дальнейшая работа требует анализа аллельных вариантов этих генов-кандидатов у широкого круга сортов, линий и образцов дикорастущих видов картофеля и выявления ассоциаций с желаемыми агрономическими признаками, что позволит использовать их в качестве генов-мишеней для разработки молекулярных маркеров и сайтов редактирования при селекции новых сортов картофеля с заданными признаками.
Kлючевые слова: картофель, крахмал клубней, амилоза, амилопектин, холодовое осахаривание, углеводный метаболизм, ферменты углеводного метаболизма, аллельные варианты генов.
Картофель ( Solanum tuberosum ) — важнейшая мировая продовольственная, кормовая и техническая культура. Картофель возделывается на всей территории Российской Федерации, в разных климатических зонах, расположенных на огромном пространстве от южных границ до полярного круга, являясь одним из основных продуктов питания.
Основу питательной ценности клубней картофеля составляет крахмал. По структуре пищевой крахмал принято делить на гликемический и резистентный, что определяется количественным соотношением двух полимеров — амилозы и амилопектина. Амилоза представляет собой прямую цепь молекул глюкозы, которая переваривается дольше. Амилопектин имеет ряд ответвлений мелких цепочек глюкозы и переваривается быстрее. Таким образом, энергетические и диетические характеристики картофеля
∗ Работа выполнена в рамках КПНИ «Развитие селекции и семеноводства картофеля».
зависят от качественного состава крахмальных зерен. Картофель как техническая культура ценен содержанием крахмала, который используется в производстве клея, глюкозы, биоэтанола, биопластиков и других продуктов и материалов (1-3). В связи с этим одно из важных направлений селекции картофеля — увеличение удельного веса крахмала клубней и создание сортов с повышенным содержанием амилозы или амилопектина. Также не стоит забывать, что экономическая эффективность при возделывании картофеля зависит не только от объемов производства и крахмалистости клубней, но и от длительности их хранения, где слабым звеном опять же остается крахмал. В нормальных условиях клубни содержат в среднем 12-18 % крахмала и 0,5-1,5 % сахаров. Температуры хранения ниже +3 ° С вызывают защитную реакцию клубней на переохлаждение, которая сопровождается интенсивным разложением крахмала и накоплением редуцирующих сахаров (глюкозы и фруктозы). Это так называемый процесс холодового осахаривания (cold-induced sweetening), который ухудшает товарные характеристики клубней картофеля.
Следовательно, на сегодняшний день актуальны три основные задачи: увеличение доли крахмала в клубнях картофеля (крахмалистость), моделирование качественного состава крахмала клубней (соотношение амилозы и амилопектина), а также предотвращение процесса холодового осахаривания и уменьшения количества редуцирующих сахаров. Для их решения необходимо прежде всего определить пути углеводного метаболизма в клубнях, выделить ключевые ферменты, регулирующие эти пути, а также выявить аллели кодирующих их генов, ассоциированные с хозяйственно ценными признаками клубней. Это даст возможность проводить направленную селекцию, основанную на моделировании углеводного метаболизма клубней, чтобы получать картофель с желаемыми свойствами.
Углеводный состав клубней картофеля — это сложный комплексный признак, который контролируется совокупностью генетических и внешних факторов (4). Еще несколько десятилетий назад на физиологическом уровне была определена последовательность реакций биосинтеза и распада крахмала в растительной клетке, которая казалась достаточно хорошо изученной (5). Однако современный анализ геномных и транскрип-томных данных показал, что схемы углеводного метаболизма растительной клетки значительно сложнее: существуют альтернативные метаболические пути, и одна и та же реакция может катализироваться различными ферментами. Было выявлено большое количество белков, регулирующих активность основных ферментов углеводного метаболизма, а также, например, белков-транспортеров, которые определяют пространственную локализацию ключевых реакций.
Таким образом, понимание механизмов углеводного обмена позволит проводить направленную селекцию, выбирать полезные аллели ключевых генов и получать новые сорта с заданными свойствами. Поэтому в настоящий момент поиск генов, влияющих на содержание сахаров и крахмала в клубнях картофеля, вызывает большой интерес у многих исследователей (2-4, 6).
Метаболизм углеводов в клубнях картофеля. Картофельный крахмал состоит из двух полимеров: разветвленного амилопектина и линейной амилозы, структурной единицей которых служит а -глюкоза. Синтез крахмала происходит в пластидах (главным образом, в хлоропластах и амилопластах), где оба полимера формируют нерастворимые гранулы. Крахмал может различаться по структуре зерен, степени полимеризации молекул и физико-химическим свойствам (1, 6, 7).
Метаболизм крахмала происходит как в листьях (в хлоропластах), так и в клубнях (в амилопластах). Большинство реакций протекает преимущественно одинаково, но существуют и некоторые органоспецифические различия. Например, в листьях в течение суток происходит последовательная смена процессов синтеза и распада крахмала. Клубни картофеля, в свою очередь, синтезируют крахмал на протяжении всего своего развития, накапливая его в качестве энергоемкого субстрата. В листьях АТФ, необходимый для синтеза крахмала, образуется в процессе фотосинтеза, а в амилопласты клубней АТФ импортируется из фотосинтезирующих органов. Субстратом для синтеза крахмала в хлоропластах листьев служит АДФ-глюкоза, образованная в результате цикла Кальвина-Бенсона, в то время как в развивающихся клубнях картофеля таким субстратом становится сахароза, поступающая из фотосинтетически активных листьев (3, 8). Биохимические различия путей биосинтеза крахмала в листьях и клубнях картофеля предполагают существование отличительных особенностей и в генетических основах обсуждаемых метаболических процессов в этих органах.
Несмотря на кажущуюся простоту биохимических реакций, в углеводном метаболизме остается множество неразрешенных вопросов. Усложняет понимание наличие большого количества ферментов, возможность осуществления реакций альтернативными путями, до конца не определенная последовательность промежуточных реакций и их субклеточная локализация. Так, неясно, в каких органеллах происходят промежуточные этапы метаболизма крахмала, какие белки осуществляют и контролируют внутриклеточный транспорт сахаров из цитозоля в амилопласты. Поэтому вместо единой схемы метаболизма крахмала существуют несколько альтернативных гипотез (3, 8, 9). Также важно понимать, что для разных периодов жизнедеятельности растений характерны различные метаболические пути. В частности, биосинтез крахмала в листьях, при закладке столонов, в процессе развития столонов, в созревших клубнях и в собранных клубнях при хранении существенно различается.
В рамках настоящего обзора будут рассмотрены вопросы биосинтеза крахмала в растущих клубнях картофеля, в которых идет интенсивное накопление крахмала.
В листьях в процессе фотосинтеза образуется сахароза, которая доставляется в клетки через симпласт или апопласт. В случае апопластного пути сахароза напрямую поступает в клубень по межклеточному пространству, где гидролизуется до глюкозы и фруктозы апопластными инвертазами. Образовавшиеся моносахара с помощью гексозотранспортеров проникают в клетки клубней. Одновременно сахароза проникает в клетки клубней по симпластному пути, используя белки-сахарозотранспортеры. Попадая в цитозоль клеток клубней, сахароза гидролизуется сахарозосинтазой до УДФ-глюкозы и фруктозы.
Таким образом, в результате в цитоплазме клеток клубня накапливается УДФ-глюкоза. Остается спорным вопрос о дальнейших превращениях УДФ-глюкозы и локализации биохимических реакций. По некоторым данным, УДФ-глюкоза преобразуется в глюкозо-1-фосфат, который затем конвертируется в АДФ-глюкозу, в свою очередь, поступающую в амилопласт и там участвующую в реакциях биосинтеза полисахаридов (8). Альтернативная модель предполагает, что в цитозоле клеток клубня УДФ-глюкоза сначала превращается в глюкозо-1-фосфат, а затем в глюкозо-6-фосфат и в такой форме транспортируется с помощью триозо-6-фосфат-ного транслокатора в амилопласт. Внутри амилопластов глюкозо-6-фосфат превращается в АДФ-глюкозу, из которой под воздействием крахмалсин- тезирующих ферментов (крахмалсинтазы, крахмал-ветвящие ферменты и др.) образуется крахмал (8, 10). Оба альтернативных пути предполагают, что непосредственным субстратом для синтеза амилозы и амилопектина в амилопластах служит АДФ-глюкоза (9). Остаток АДФ-глюкозы присоединяется к наращиваемой цепи крахмалсинтазами (SS, starch synthase, EC 2.4.1.21). По мере того, как полисахаридная цепь растет, крахмал-ветвящие ферменты (BE, branching enzyme, EC 2.4.1.18) вносят разветвления, и таким образом синтезируется амилопектин (9). Синтез линейной молекулы — амилозы, в свою очередь, осуществляется ферментом гранулосвязанной крахмалсинтазой (GBSS, granule-bound starch synthase, EC 2.4.1.242).
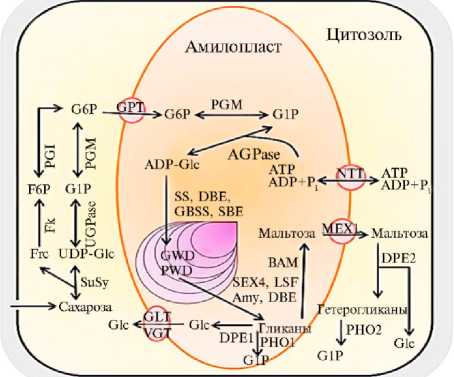
Возможный путь метаболизма крахмала в клубнях картофеля (3). Ферменты: SuSy — сахарозосинтаза, Fk — фруктокиназа, UGPase — УДФ-глюкозо-пирофосфорилаза, PGI — фосфоглюкоизомераза, PGM — фосфоглюкомутаза, AGPase — АДФ-глюкозо-пирофосфорилаза, SS — крах-малсинтазы, LSF — SEX4-подобный фермент, Amy — амилазы, DBE — дебранчинг-фермент; DPE — диспро-порционирующий фермент; PHO — крахмалфосфорила-за, BAM — β -амилазы. Белки-транслокаторы: GPT — транспортер глюкозофосфата, MEX — мальтозотранспортер, NTT — нуклеотидный транслокатор; GLT — глюкозотранспортер; VGT — вакуолярный глюкозотранспортер. Вещества: Frc — фруктоза, Glc — глюкоза, UDF-Glc — УДФ-глюкоза, F6P — фруктозо-6-фосфат, G1P — глю-козо-1-фосфат, G6P — глюкозо-6-фосфат, ADP-Glc — АДФ-глюкоза, ATP — АТФ, P i — неорганический фосфор.
На финальной стадии формируются крахмальные гранулы, имеющие полукристаллическую структуру. Несмотря на то, что точные механизмы этого до сих пор не ясны, считается, что завершающий этап образования крахмальной гранулы зависит исключительно от амилопектина (9). При этом процесс формирования крахмальных зерен специфичен для разных видов растений и разных органов: если в хлоропласте листа имеет место большое коли- чество мелких гранул, то в амилопласте клубня их всего несколько и они очень крупные (9).
Таким образом, биосинтез крахмала, начиная с образования моносахаридных субстратов и до формирования крахмального зерна, включает множество реакций и требует координированной работы большого числа ферментов. Кроме то- го, как уже отмечалось, в углеводный метаболизм вовлечены не только фер- менты, модифицирующих моно-, ди- и полисахариды, но и опосредованно влияющие на эти реакции регуляторные белки, работу которых тоже нужно принимать во внимание.
Первичный этап синтеза крахмала клубней напрямую зависит от работы сахаролитических ферментов, так как именно они способствуют аккумуляции гексоз, которые в дальнейшем включаются в синтез крахмала. Однако конечное накопление крахмала определяется не только скоростью его синтеза, но и интенсивностью распада, так как эти процессы осуществляются одновременно и непрерывно. Описано достаточное количество ферментов, разрушающих крахмал, которые специфичны в отношении гликозидной связи и воздействуют на различные субстраты (амило- за, амилопектин, декстран). Эти ферменты имеют разное генетическое происхождение и принадлежат к различным семействам (7, 11-14).
Ферменты, разрушающие крахмал, можно разделить на две категории — гидролитические ( α - и β -амилазы) и фосфоролитические ( α -гликан-фосфорилазы). Их сравнительная активность может изменяться в зависимости от стадии развития или условий среды. Какая из групп ферментов имеет большее значение — спорный вопрос. По некоторым данным, основной вклад в разрушение крахмала вносит гидролитический путь, хотя фосфоролитический менее энергозатратен (15). Однако само по себе фосфорилирование крахмала не может быть достаточным фактором (16). Возможно, этот процесс делает поверхность крахмального зерна более гидрофильной и, таким образом, более доступной для гидролитических ферментов, дополнительно создавая селективные белок-углеводные и белок-бел-ковые взаимодействия (14, 16-18).
Крахмальные зерна, разрушаясь, превращаются в разветвленные либо линейные формы полигликанов. Затем разветвленные формы преобразуются в линейные гликаны в результате работы снимающих разветвление ферментов, например изоамилаз (isoamylase, EC 3.2.1.68) или декстриназ (dextrin 6- α -glucanohydrolase; EC 3.2.1.142), которые специфичны к α -1,6-гликозидной связи. На завершающем этапе линейные гликаны могут быть разрушены β -амилазами ( β -amylase, EC 3.2.1.2) или крахмалсинтазой (SS, starch synthase, EC 2.4.1.21) до нейтральных сахаров (12).
В итоге последовательной деструкции крахмала в амилопластах образуются метаболиты (триозофосфаты, мальтоза, глюкоза), которые затем с помощью специфичных транспортеров направляются в цитозоль (19). Там они вовлекаются в путь метаболизма гликанов, подвергаясь воздействию цитозольных фосфорилаз — трансглюкозидаз (DPE2, disproportionating enzyme, EC 2.4.1.25), в итоге превращаясь в фосфаты гексоз, которые, в свою очередь, необходимы для биосинтеза сахарозы.
Ключевые гены углеводного метаболизма клубней картофеля. Многие гены метаболизма крахмала организованы в генные семейства (9). Разные представители одного семейства могут играть неодинаковые роли в фотосинтезирующих и запасающей органах (3). Активность ферментов метаболизма крахмала регулируется как на транскрипционном уровне (например, циркадными ритмами или присутствием сахаров) (3), так и посттрансляционно, в том числе за счет белок-белковых взаимодействий и фосфорилирования белков (8).
В 2017 году было проведено масштабное исследование с целью идентификации всех генов, связанных с метаболизмом крахмала, в геноме картофеля (3). В результате идентифицировано 77 геномных локусов, кодирующих ферменты метаболизма крахмала. Для сравнения, в геноме ре-зуховидки Таля ( Arabidopsis thaliana ) известно 46 генов метаболизма крахмала, из которых 44 имеют гомологов в геноме картофеля (3). Кроме того, в геноме картофеля найдены новые изоформы многих ферментов (3).
В таблице приведены известные на сегодняшний день гены картофеля, кодирующие ферменты метаболизма крахмала (3, 20-22).
Было показано, что из 77 описанных геномных локусов, ассоциированных с метаболизмом крахмала в растении картофеля, некоторые гены экспрессируются исключительно в листьях, другие — во всех крахмалсинтезирующих органах, а третьи, самые интересные (в рамках настоящего обзора), — в клубнях. По всей видимости, именно среди последних могут быть гены, ассоциированные с хозяйственно ценными признаками (3). Биоинформатический анализ транскрипционных данных (3) выявил 454
несколько генов, экспрессия которых специфична для клубней картофеля. Наибольший уровень клубнеспецифичной экспрессии наблюдался у генов транслокатора глюкозо-6-фосфата GPT2.1 , сахарозосинтазы SuSy4 , фосфогликанфосфатазы SEX4 , крахмалсинтазы SS5 и крахмал-ветвящего фермента SBE3 .
Гены, кодирующие ферменты углеводного метаболизма у картофеля ( Solanum tuberosum L.) (цит. по 3 с дополнениями)
|
Белок/семейство белков |
1 Гены 1 |
Специфичность экспрессии |
|
ADP-glucose pyrophosphorylase large subunit (АДФ-глюкозо-пирофосфорилаза, большая субъединица) ADP-glucose pyrophosphorylase small subunit (АДФ-глюкозо-пирофосфорилаза, малая субъединица) |
AGPL1, AGPL2, AGPL3 AGPS1.1, AGPS1.2, AGPS2 |
AGPL1 в листьях |
|
Alpha-amylase ( α -амилазы) |
AMY1.1, AMY1.2, AMY23, AMY3, AMY3-like |
AMY1.1 в листьях |
|
Alpha-glucan phosphorylase ( α -гликан- |
PHO1a, PHO1b, PHO2a, |
PHO1b в листьях, PHO1a в |
|
фосфорилазы) ATP-ADP antiporter (ATФ-AДФ антипортер) |
PHO2b NTT1, NTT2 |
клубнях |
|
Beta-amylase ( β -амилазы) |
BAM1, BAM2, BAM3.1, BAM3.2, BAM4, BAM6.1, BAM6.2, BAM6.3, BAM7, BAM9 |
BAM3.1 в листьях |
|
Branching enzyme (крахмал-ветвящий фермент) Disproportionating enzyme (4- α -гликанотранс-феразы) Glucan water dikinase ( α -гликан-Н2О-дикиназа) Glucose transporter (глюкозотранспортер) |
SBE1.1, SBE1.2, SBE2, SBE3 DPE1, DPE2 GWD GLT1 |
SBE3 в клубнях, экспрессия усиливается по мере роста клубней |
|
Glucose-6-phosphate translocator (трансло- |
GPT1.1, GPT1.2, GPT2.1, |
GPT2.1 в клубнях, экспрессия уси- |
|
катор глюкозо-6-фосфата) |
GPT2.2 |
ливается по мере роста клубней |
|
Granule bound starch synthase (гранулосвязанная крахмалсинтаза) Inorganic pyrophosphatase (неорганические фосфатазы) Isoamylase (изоамилазы) |
GBSS1 PPase, PPase-like ISA1.1, ISA 1.2, ISA2, ISA3 |
В клубнях экспрессия выше, чем в листьях |
|
Limit dextrinase (лимит-декстриназа) Maltose excess (мальтозотранспортер) |
LDE MEX1 |
Органоспецифичность не показана, но экспрессия LDE усиливается по мере роста клубней |
|
Phosphoglucan phosphatase (фосфогликан- LSF1, LSF2, SEX4, SEX4-like фосфатазы) Phosphoglucan water dikinase (фосфогликан- PWD Н2О-дикиназа) Phosphoglucoisomerase (фосфоглюкоизоме- PGI, PGI-like1, PGI-like2 разы) Phosphoglucomutase (фосфоглюкомутазы) PGM1, PGM2.1, PGM2.2, pPGM |
SEX4 в клубнях, экспрессия падает по мере роста клубней |
|
|
Starch Synthase (крахмалсинтазы) |
SS1, SS2, SS3, SS4, SS5, SS6 |
SS5 в клубнях |
|
Sucrose Synthase (сахарозосинтазы) SuSy1, SuSy2, SuSy3, SuSy4, SuSy6, SuSy7 Triose-phosphate/phosphate translocator (трио- TPT, TPT-like зофосфатный/фосфатный транслокатор) UDP-glucose pyrophosphorylase (УДФ- UGPase1, UGPase2 глюкозо-пирофосфорилаза Vacuolar Glucose Transporter (вакуолярный VGT3-like глюкозотранспортер) Vacuolar invertase (вакуорярная инвертаза) Pain-1 Invertase inhibitor (ингибиторы инвертаз) INH1, INH2 Amylase inhibitor (ингибитор амилаз) SbAI П р и м е ч а н и е. Пропуски означают отсутствие данных. |
SuSy4 в клубнях, экспрессия усиливается по мере роста клубней |
|
Как известно, в процессе клубнеобразования происходит наиболее интенсивный синтез крахмала (23). Поэтому не только гены с высокой экспрессией в клубнях заслуживают особого внимания, но также и те гены, экспрессия которых растет по мере закладки и развития клубней (3), так как именно они могут быть регуляторами процесса клубнеобразова-ния. К таким генам относятся гены сахарозосинтазы SuSy4, крахмалветвящего фермента SBE3, транслокатора глюкозо-6-фосфата GPT2.1 и декстриназы LDE (3). При этом интересно, что уровень транскрипции гена фосфогликанфосфатазы SEX4, характеризующегося высокой экспрессией именно в клубнях, находится в обратной зависимости от интенсивности роста клубня и синтеза крахмала (3).
Исходя из современных представлений об углеводном метаболизме клубней, можно предложить несколько генов-кандидатов для решения сформулированных выше основных задач современной селекции картофеля (увеличение крахмалистости, повышение содержания амилозы или амилопектина, ингибирование холодового осахаривания). Рассмотрим их подробнее.
Гены, определяющие содержание крахмала. Среди генов, чья экспрессия коррелирует с ростом клубней, наибольший интерес представляет ген сахарозосинтазы SuSy4 . Именно для SuSy4 получено наибольшее количество данных, свидетельствующих о его ключевом влиянии на содержание крахмала в клубнях картофеля (3, 24-27).
Белки семейства сахарозосинтаз (sucrose synthase, EC 2.4.1.13) катализируют реакцию обратимого гидролиза сахарозы в присутствии УДФ до УДФ-глюкозы и фруктозы и встречаются у всех высших растений (28). В растительной клетке SuSy4 присутствует в растворимой форме в цитозоле (24). Сахарозосинтаза — основной фермент, расщепляющий сахарозу в эндосперме злаковых и клубнях картофеля, она обеспечивает субстрат для синтеза крахмала в запасающих органах. Суперэкспрессия гена сахарозо-синтазы SuSy4 в растениях картофеля приводит к увеличению содержания крахмала в клубнях и повышению урожайности (30).
Еще один важный ген, для которого показана ассоциация с повышенным содержанием крахмала в клубнях, — α -гликанфосфорилаза. α -Гликанфосфорилазы (starch phosphorylase, EC 2.4.1.1), представители семейства гликозилтрансфераз 35 (GT35), играют значимую роль в метаболизме углеводов у растений, животных и прокариот (31, 32). В растениях аналоги α -гликанфосфорилаз известны также как крахмалфосфорилазы. Этот фермент осуществляет фосфоролитическую деградацию крахмала и катализирует реакцию обратимого переноса остатка гликозила на конце α -1,4-D-гликановой цепи в присутствии фосфата c образованием глюкозо-1-фосфата. У всех растений имеются две различные формы крахмалфос-форилаз — пластидная и цитозольная. В свою очередь, у картофеля пластидная крахмалфосфорилаза PHO1 кодируется двумя гомологичными генами, для которых характерна тканеспецифичная экспрессия: PHO1b экспрессируется преимущественно в листьях, PHO1a — в клубнях (3, 33, 34). Несмотря на то, что крахмалфосфорилазы способны проводить реакции как разрушения, так и синтеза крахмала, показано in vitro, что пластидная форма играет более существенную роль в процессе деструкции крахмала (35, 36). Однако in vivo доказательства этого отсутствуют. Также in vitro были получены данные о способности крахмалфосфорилазы синтезировать олигосахаридную затравку, которая затем достраивается крахмал-синтазой (15, 37, 38).
Гены, определяющие качественный состав крахмала . Как уже отмечалось, сфера использования крахмала обширна, и, в зависимости от конкретных задач, необходимо получать крахмал с различными физико-химическими свойствами, которые напрямую определяются количественным соотношением амилозы и амилопектина. Крахмал с высоким содержанием амилопектина (гликемический крахмал) обладает повышенной пищевой энергетической ценностью и используется для производства детского и лечебного питания. В промышленности такой крахмал также предпочтите-456
лен (экономически выгоден) как сырье для производства глюкозно-фрук-тозных сиропов и биоэтанола. Высокоамилозный (резистентный) крахмал устойчивее к воздействию α -амилаз, в силу чего используется в производстве биопластиков. Имея низкий гликемический индекс, такой крахмал также ценится в диетологии (39). Качественный состав крахмала зависит от работы двух групп ферментов — крахмалсинтаз (в том числе гранулосвязанной крахмалсинтазы) и крахмал-ветвящих ферментов.
В геноме картофеля найдены гены шести изоформ крахмалсинтаз ( SS1 , SS2 , SS3 , SS4 , SS5 , SS6 ), а также гомологичный ген гранулосвязанной крахмалсинтазы GBSS1 (3). Крахмалсинтазы SS синтезируют полисахариды амилопектина и могут находиться либо в растворенной форме, либо присоединяться к крахмальной грануле. Генетические и биохимические данные свидетельствуют о том, что каждая изоформа крахмалсинтазы SS (starch synthase, EC 2.4.1.21) играет свою уникальную роль в процессе синтеза амилопектина. Считается, что изоформы SS1, SS2 и SS3 работают непосредственно друг за другом, осуществляя синтез соответственно короткой, средней и длинной цепи (9). При этом также известно, что 80 % крахмалсинтазной активности в клубнях картофеля приходится на SS3 (9). Для гена SS5 изоформы крахмалсинтазы характерна клубнеспецифичная экспрессия, хотя в настоящий момент отсутствует in vivo подтверждение непосредственного влияния SS5 на накопление крахмала и урожайность картофеля (3). В то же время у кукурузы гомологичный ген SS5 предположительно контролирует накопление крахмала на стадии созревания зерна (3, 40). Однако считается, что активность крахмалсинтаз (за исключением SS5) в клубнях не сильно превосходит таковую в листьях и агрономическая значимость генов, кодирующих эти ферменты, не настолько существенна, как у гомологичного гена GBSS1 (3).
Гранулосвязанная крахмалсинтаза GBSS1 (granule-bound starch synthase, EC 2.4.1.242) контролирует биосинтез амилозы в формирующихся крахмальных гранулах. Многие исследования указывают на важное хозяйственное значение этого фермента (41-44). GBSS1 присоединяется непосредственно к крахмальной грануле. Экспрессия GBSS1 в клубнях несколько выше, чем в листьях. GBSSI выявлен и охарактеризован во многих сортах картофеля (36, 45, 46). Инактивация этого гена позволяет получить картофель, клубни которого содержат преимущественно амилопектин (47-50).
Крахмал-ветвящий фермент SBE (starch branching enzyme, EC 2.4.1.18) влияет на накопление определенной формы полисахаридов крахмала. SBE катализируют образование точек α -1,6-ответвлений в полисахаридной цепи с разной частотой и длиной ответвленной цепи. Впервые активность крахмал-ветвящиего фермента была обнаружена именно в картофеле (51). Полисахаридные структуры, формируемые крахмал-ветвящим ферментом, затем модифицируются ферментами, снимающими ветвление (DBEs, debranching enzymes, EC. 3.2.1.68), и таким образом получаются нерастворимые в воде гранулы. Активность крахмал-ветвящего фермента непосредственно влияет на степень разветвленности амилопектина (52, 53).
У многих видов растений выявлены различия в экспрессии отдельных классов крахмал-ветвящего фермента (39). Мутантные растения с дефицитом активности SBE имеют характерный фенотип вследствие ингибирования синтеза крахмала и накопления большого количества сахарозы и других растворимых сахаров (39). Например, у гороха ( Pisum sativum L.) образуются морщинистые плоды и содержание крахмала уменьшается на 50 % (54); для кукурузы известна мутация amylose extender ( ae –), которой сопутствует уменьшение синтеза крахмала на 20 % (55). При этом у таких 457
растений крахмал состоит в основном из амилозы, а найденный в них амилопектин мало разветвлен. У картофеля высокоамилозный крахмал удалось получить только при ингибировании активности сразу нескольких изоформ крахмал-ветвящего фермента (56).
Гены, определяющие устойчивость к холодовому осахариванию . Во время хранения при температурах ниже +10 ° С) в клубнях картофеля накапливаются редуцирующие сахара, которые при взаимодействии с а -амино-кислотами приводят к накоплению акриламида и ухудшению вкусовых качеств (57-59). Поэтому предотвращение процесса холодового осахаривания картофеля крайне важно для пищевой промышленности (60-62). Холодовое осахаривание происходит за счет процессов гидролиза полиглика-новых цепей амилазами и разрушения сахарозы инвертазами.
Как уже отмечалось, распад крахмала может осуществляться либо гидролитически, либо фосфоролитически. Гидролитический путь катализируется а -амилазами (AMY, alpha-amylase, EC 3.2.1.1) и р -амилазами (BAM, beta-amylase, EC 3.2.1.2). Оба семейства белков включают множество изоформ. В настоящее время в геноме картофеля идентифицированы как минимум пять генов а -амилаз и как минимум 10 генов р -амилаз (3). а -Амилазы гидролизуют а -1,4-гликановые связи с образованием различных линейных и разветвленных мальтоолигосахаридов. В клубнях картофеля функционируют два гена а -амилаз — StAmyl и StAmy23 . При этом при низкотемпературном хранении активна только амилаза StAmy23 (63). р -Амилазы осуществляют гидролиз нередуцирующего конца цепей гликанов, связанных а -1,4-гликозидными связями, с образованием р -мальтозы (64). Показано, что активность р -амилаз у картофеля существенно возрастает в первую неделю хранения при +4 ° С (65). Экспрессия р -амилаз также тесно коррелирует с накоплением редуцирующих сахаров в клубнях картофеля, хранящихся при положительных температурах 3-5 ° С (66), тем самым подтверждая значимость р -амилаз в процессе холодового осахаривания. Считается, что среди известных генов р -амилаз наибольший уровень транскрипции в клубнях имеют StBAM1 и StBAM9 (63).
Гидролиз сахарозы инвертазами с образованием глюкозы и фруктозы (4) также приводит к образованию редуцирующих сахаров при хранении клубней картофеля. В настоящее время однозначно показано, что основную роль в холодовом осахаривании у картофеля играет кислая вакуо-лярная инвертаза (Pain-1) (beta-fructofuranosidase, EC 3.2.1.26), катализирующая необратимый гидролиз сахарозы. Инактивация гена Pain-1 снижает накопление редуцирующих сахаров в клубнях при низких температурах (22, 67-70). У картофеля этот ген идентифицирован, изучена его структура и экспрессия, а также найдены однонуклеотидные замены (SNPs), определяющие активность фермента (71-74).
У устойчивых к холодовому осахариванию сортов картофеля наблюдается низкая транскрипция гена вакуолярной инвертазы, но у некоторых линий обнаружена высокая экспрессия этого гена и при низкой активности фермента (69). Выяснилось, что, помимо регуляции работы вакуоляр-ной инвертазы на уровне транскрипции, происходит посттрансляционная модификация белка с участием ингибиторов (75). В этой связи следует отдельно рассмотреть группу ферментов, которые опосредованно влияют на процесс холодового осахаривания, хотя и не имеют сродства к гликозидной связи и не взаимодействуют с сахарами и полигликанами. В эту группу следует отнести ингибиторы инвертаз и амилаз.
Последовательности генов ингибиторов инвертаз были определены у различных видов растений (76). У культивируемого вида картофеля 458
обнаружены два ингибитора — St-Inh (INH1) и StInvInh2 (INH2), влияющие на инвертазную активность и, следовательно, на холодовое осахаривание клубней, что подтверждалось эффектом от их оверэкспрессии в клубнях картофеля (77). На гаплоидный геном картофеля приходится по одной копии генов INH1 и INH2 , локализованных на 12-й хромосоме в тандемной ориентации и подверженных альтернативному сплайсингу, а продукты генов ингибируют апопластную (INH1) и вакуолярную (INH2) инвертазы (76). Показано in vitro, что больший ингибирующий эффект имеет INH2 (78), что подтверждается существенно более высоким уровнем экспрессии INH2 у устойчивых к холодовому осахариванию генотипов картофеля, чем у чувствительных. Кроме того, сообщается об ассоциации некоторых сплайс-вариантов гена INH2 и вариабельности его промотор-ной области со степенью подверженности клубней картофеля холодовому осахариванию (76, 79).
Другой пример посттрансляционной регуляции генов, участвующих в процессе холодового осахаривания, связан с работой ингибитора амилаз. У картофеля активность амилаз ингибирует SbAI, ген которого впервые был клонирован у вида Solanum berthaultii (21). Рост активности SbAI приводит к подавлению амилаз и, следовательно, к уменьшению накопления редуцирующих сахаров в клубнях (21). С использованием дигибридной системы показано наличие белок-белковых взаимодействий между SbAI и белками StAmy23, StBAM1 и StBAM9 (21). Поэтому ингибитор амилаз считается ключевым регулятором процессов холодового осахаривания клубней картофеля, вызванных амилазной активностью.
Итак, молекулярные и биотехнологические подходы (маркер-опо-средованная селекция, получение трансгенных растений, геномное редактирование и т.п.) уже позволяют изменять желаемые признаки растений. Однако вне зависимости от используемого подхода основополагающий этап, определяющий успешный результат работы, — это правильный выбор гена-мишени. В настоящем обзоре из большого числа белков, участвующих в метаболизме углеводов в клубнях картофеля, выделены ключевые ферменты, которые как напрямую, так и опосредованно могут осуществлять наиболее важные этапы синтеза и распада крахмала в клубнях. Определен круг кодирующих эти ферменты генов-кандидатов, аллельные варианты которых могут быть связаны с хозяйственно ценными признаками картофеля. Дальнейшая работа требует анализа аллельных вариантов этих генов-кандидатов у широкого круга сортов, линий и образцов дикорастущих видов картофеля и выявления ассоциаций с требуемыми агрономическими признаками. Это позволит использовать их в качестве генов-мишеней для разработки молекулярных маркеров и сайтов редактирования при селекции сортов с заданными признаками.
Список литературы Использование генов углеводного обмена для улучшения качества клубней картофеля (Solanum tuberosum L.)
- Хлесткин В.К., Пельтек С.Е., Колчанов Н.А. Гены-мишени для получения сортов картофеля (Solanum tuberosum l.) с заданными свойствами крахмала. Сельскохозяйственная биология, 2017, 52(1): 25-36 ( ) DOI: 10.15389/agrobiology.2017.1.25rus
- Li L., Tacke E., Hofferbert H., Lübeck J., Strahwald J., Draffehn A. Validation of candidate gene markers for marker-assisted selection of potato cultivars with improved tuber quality. Theor. Appl. Genet., 2013, 126(4): 1039-1052 ( ) DOI: 10.1007/s00122-012-2035-z
- Van Harsselaar J., Lorenz J., Senning M., Sonnewald U., Sonnewald S. Genome-wide analysis of starch metabolism genes in potato (Solanum tuberosum L.) BMC Genomics, 2017, 18: 37 ( ) DOI: 10.1186/s12864-016-3381-z
- Duarte-Delgado D., Juyó D., Gebhardt C., Sarmiento F., Mosquera-Vásquez T. Novel SNP markers in InvGE and SssI genes are associated with natural variation of sugar contents and frying color in Solanum tuberosum Group Phureja. BMC Genet., 2017, 18: 23 ( ) DOI: 10.1186/s12863-017-0489-3
- Frommer W., Sonnewald U. Molecular analysis of carbon partitioning in solanaceous species. J. Exp Bot., 1995, 46: 587-607.
- Pfister B., Zeeman S. Formation of starch in plant cells. Cell. Mol. Life Sci., 2016, 73(14): 2781-2807 ( ) DOI: 10.1007/s00018-016-2250-x
- Martin C., Smith A. Starch biosynthesis. Plant Cell, 1995, 7(7): 971-985 ( ) DOI: 10.1105/tpc.7.7.971
- Bahaji A., Li J., Sánchez-López Á., Baroja-Fernández E., Muñoz F., Ovecka M., Almagro G., Montero M., Ezquer I., Etxeberria E., Pozueta-Romero J. Starch biosynthesis, its regulation and biotechnological approaches to improve crop yields. Biotechnol Adv., 2014, 32(1): 87-106 ( ) DOI: 10.1016/j.biotechadv.2013.06.006
- Zeeman S., Kossmann J., Smith A. Starch: its metabolism, evolution, and biotechnological modification in plants. Annu. Rev. Plant Biol., 2010, 61: 209-234 ( ) DOI: 10.1146/annurev-arplant-042809-112301
- Naeem M., Tetlow I., Emes M. Starch synthesis in amyloplasts purified from developing potato tubers. The Plant Journal, 1997, 11(5): 1095-1103 ( ) DOI: 10.1046/j.1365-313X.1997.11051095.x
- Smith A., Zeeman S., Thorneycroft D., Smith S. Starch mobilization in leaves. J. Exp. Bot., 2003, 54(382): 577-583 ( ) DOI: 10.1093/jxb/erg036
- Smith A., Zeeman S., Smith S. Starch degradation. Annu. Rev. Plant Biol., 2005, 56: 73-98 (doi: 10.1146/annurev.arplant.56.032604.144257).
- Zeeman S., Smith S., Smith A. The diurnal metabolism of leaf starch. Biochem. J., 2007, 401(1): 13-28 ( DOI: 10.1042/BJ20061393
- Orzechowski S. Starch metabolism in leaves. Acta Biochim. Pol., 2008, 55(3): 435-445.
- Dauvillée D., Chochois V., Steup M., Haebel S., Eckermann N., Ritte G., Ral J., Colleoni C., Hicks G., Wattebled F. Plastidial phosphorylase is required for normal starch synthesis in Chlamydomonas reinhardtii. Plant J., 2006, 48(2): 274-285 ( ) DOI: 10.1111/j.1365-313X.2006.02870.x
- Fettke J., Albrecht T., Hejazi M., Mahlow S., Nakamura Y., Steup M. Glucose 1-phosphate is efficiently taken up by potato (Solanum tuberosum) tuber parenchyma cells and converted to reserve starch granules. New Phytol., 2010, 185(3): 663-675 ( ) DOI: 10.1111/j.1469-8137.2009.03126.x
- Yu T., Kofler H., Häusler R., Hille D., Flügge U., Zeeman S., Smith A., Kossmann J., Lloyd J., Ritte G. The Arabidopsis sex1 mutant is defective in the R1 protein, a general regulator of starch degradation in plants, and not in the chloroplast hexose transporter. Plant Cell, 2001, 13(8): 1907-1918 ( ) DOI: 10.1105/TPC.010091
- Hejazi M., Fettke J., Haebel S., Edner C., Paris O., Frohberg C., Steup M., Ritte G. Glucan, water dikinase phosphorylates crystalline maltodextrins and thereby initiates solubilization. The Plant Journal, 2008, 55(2): 323-334 ( ) DOI: 10.1111/j.1365-313X.2008.03513.x
- Zeeman, S., Smith, S., Smith A. The breakdown of starch in leaves. New Phytol., 2004, 163(2): 247-261 ( ) DOI: 10.1111/j.1469-8137.2004.01101.x
- Liu X., Song B., Zhang H., Li X.Q., Xie C., Liu J. Cloning and molecular characterization of putative invertase inhibitor genes and their possible contributions to cold-induced sweetening of potato tubers. Mol. Genet. Genomics, 2010, 284(3): 147-159 ( ) DOI: 10.1007/s00438-010-0554-3
- Zhang H., Liu J., Hou J., Yao Y., Lin Y, Ou Y., Song B., Xie C. The potato amylase inhibitor gene SbAI regulates cold-induced sweetening in potato tubers by modulating amylase activity. Plant Biotechnol. J., 2014, 12(7): 984-993 ( ) DOI: 10.1111/pbi.12221
- Clasen B., Stoddard T., Luo S, Demorest Z., Li J., Cedrone F., Tibebu R., Davison S., Ray E., Daulhac A., Coffman A., Yabandith A., Retterath A., Haun W., Baltes N., Mathis L., Voytas D., Zhang F. Improving cold storage and processing traits in potato through targeted gene knockout. Plant Biotechnol. J., 2016, 14(1): 169-176 ( ) DOI: 10.1111/pbi.12370
- Kloosterman B., Vorst O., Hall R., Visser R., Bachem C. Tuber on a chip: differential gene expression during potato tuber development. Plant Biotechnol. J., 2005, 3(5): 505-519 ( ) DOI: 10.1111/j.1467-7652.2005.00141.x
- Appeldoorn N., de Bruijn S., Koot-Gronsveld E., Visser R., Vreugdenhil D., van der Plas L. Developmental changes of enzymes involved in conversion of sucrose to hexose phosphate during early tuberisation of potato. Planta, 1997, 202(2): 220-226 ( ) DOI: 10.1007/s004250050122
- Fu H., Kim S., Park W. High-leve1 tuber expression and sucrose inducibility of a potato Sus4 sucrose synthase gene require 5´ and 3´ flanking sequences and the leader intron. The Plant Cell, 1995, 7(9): 1387-1394.
- Viola R., Roberts G., Haupt S., Gazzani S., Hancock R., Marmiroli N., Machray G., Oparka K. Tuberization in potato involves a switch from apoplastic to symplastic phloem unloading. Plant Cell, 2001, 13(2): 385-398 ( ) DOI: 10.1105/tpc.13.2.385
- Zrenner R., Salanoubat M., Willmitzer L., Sonnewald U. Evidence of the crucial role of sucrose synthase for sink strength using transgenic potato plants (Solanum tuberosum L.). The Plant Journal, 1995, 7(1): 97-107 ( ) DOI: 10.1046/j.1365-313X.1995.07010097.x
- Avigad G. Sucrose and other disaccharides. In: Encyclopedia of plant physiology/T.A. Loewus, W. Tanner (eds.). Springer-Verlag, Heidelberg, 1982.
- Koch K. Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr. Opin. Plant Biol., 2004, 7(3): 235-246 ( ) DOI: 10.1016/j.pbi.2004.03.014
- Baroja-Fernández E., Muñoz F., Montero M., Etxeberria E., Sesma M., Ovecka M., Bahaji A., Ezquer I., Li J., Prat S., Pozueta-Romero J. Enhancing sucrose synthase activity in transgenic potato (Solanum tuberosum L.) tubers results in increased levels of starch, ADPglucose and UDPglucose and total yield. Plant Cell Physiol., 2009, 50(9): 1651-166 ( ) DOI: 10.1093/pcp/pcp108
- Rathore R., Garg N., Garg S., Kumar A. Starch phosphorylase: role in starch metabolism and biotechnological applications. Crit. Rev. Biotechnol., 2009, 29(3): 214-224 ( ) DOI: 10.1080/07388550902926063
- Nighojkar S., Kumar A. Starch phosphorylase: biochemical, molecular and biotechnological aspects. Genet. Eng. Biotechnol., 1997, 17(4): 189-202.
- Sonnewald U., Basner A., Greve B., Steup M. A second L-type isozyme of potato glucan phosphorylase: cloning, antisense inhibition and expression analysis. Plant Mol. Biol., 1995, 27(3): 567-576.
- Albrecht T., Koch A., Lode A., Greve B., Schneider-Mergener J., Steup M. Plastidic (Pho1-type) phosphorylase isoforms in potato (Solanum tuberosum L.) plants: expression analysis and immunochemical characterization. Planta, 2001, 213(4): 602-613.
- Preiss J., Levi C. Starch biosynthesis and degradation. In: The biochemistry of plants. V. 3/J.B. Pridham (ed.). Academic Press, NY, 1980.
- Newgard C., Hwang P., Fletterick R. The family of glycogen phosphorylases: structure and function. Crit. Rev. Biochem. Mol. Biol., 1989, 24(1): 69-99 ( ) DOI: 10.3109/10409238909082552
- Dai W., Deng W., Cui W., Zhao Y., Wang X. Molecular cloning and sequence of potato granule-bound starch synthase. Acta Botanica Sinica, 1996, 38(10): 777-784.
- Satoh H., Shibahara K., Tokunaga T., Nishi A., Tasaki M., Hwang S.K. Mutation of the plastidial a-glucan phosphorylase gene in rice affects the synthesis and structure of starch in the endosperm. Plant Cell, 2008, 20: 1833-1849 ( ) DOI: 10.1105/tpc.107.054007
- Tetlow I., Emes M. A review of starch-branching enzymes and their role in amylopectin biosynthesis. IUBMB Life, 2014, 66(8): 546-558 ( ) DOI: 10.1002/iub.1297
- Liu H., Yu G., Wei B., Wang Y., Zhang J., Hu Y., Liu Y., Yu G., Zhang H., Huang Y. Identification and phylogenetic analysis of a novel starch synthase in maize. Front. Plant Sci., 2015, 6: 1013 ( ) DOI: 10.3389/fpls.2015.01013
- Hovenkamp-Hermelink J., Jacobsen E., Ponstein A., Visser R., Vos-Scheperkeuter G., Bijmolt E., de Vries J., Witholt B., Feenstra W. Isolation of an amylose-free starch mutant of the potato (Solanum tuberosum L.). Theor. Appl. Genet., 1987, 75(1): 217-221 ( ) DOI: 10.1007/BF00249167
- Jacobsen E., Hovenkamp-Hermelink J., Krijgsheld H., Nijdam H., Pijnacker L., Witholt B., Feenstra W. Phenotypic and genotypic characterization of an amylose-free starch mutant of the potato. Euphytica, 1989, 44(1-2): 43-48 ( ) DOI: 10.1007/BF00022597
- Visser R., Somhorst I., Kuipers G., Ruys N., Feenstra W., Jacobsen E. Inhibition of the expression of the gene for granule-bound starch synthase in potato by antisense constructs. Mol. Gen. Genet., 1991, 225(2): 285-296.
- Van der Steege G., Nieboer M., Swaving J., Tempelaar M. Potato granule-bound starch synthase promoter-controlled GUS expression: regulation of expression after transient and stable transformation. Plant Mol. Biol., 1992, 20(1): 19-30.
- Rohde W., Becker D., Kull B., Salamini F. Structural and functional analysis of two waxy gene promoters from potato. Journal of Genetics & Breeding, 1990, 44: 311-315.
- Van de Wal M., Jacobsen E., Visser R. Multiple allelism as a control mechanism in metabolic pathways: GBSSI allelic composition affects the activity of granule-bound starch synthase I and starch composition in potato. Mol. Genet. Genomics, 2001, 265(6): 1011-1021 ( ) DOI: 10.1007/s004380100496
- Visser R., Stolte A., Jacobsen E. Expression of a chimaeric granule-bound starch synthase-GUS gene in transgenic potato plants. Plant Mol. Biol., 1991, 17(4): 691-699 ( ) DOI: 10.1007/BF00037054
- Kuipers G., Vreem J., Meyer H., Jacobsen E., Feenstra W., Visser R. Field evaluation of antisense RNA mediated inhibition of GBSS gene expression in potato. Euphytica, 1992, 59(1): 83-91 ( ) DOI: 10.1007/BF00025364
- Flipse E., Keetels C., Jacobson E., Visser R. The dosage effect of the wild type GBSS allele is linear for GBSS activity, but not for amylose content: absence of amylose has a distinct influence on the physico-chemical properties of starch. Theor. Appl. Genet., 1996, 92(1): 21-127 ( ) DOI: 10.1007/BF00222961
- Heilersig B., Loonen A., Janssen E., Wolters A., Visser R. Efficiency of transcriptional gene silencing of GBSSI in potato depends on the promoter region that is used in an inverted repeat. Mol. Genet. Genomics, 2006, 275(5): 437-449 ( ) DOI: 10.1007/s00438-006-0101-4
- Haworth W., Peat S., Bourne E. Synthesis of amylopectin. Nature, 1944, 154: 236-238 ( ) DOI: 10.1038/154236a0
- Hizukuri S. Polymodal distribution of the chain lengths of amylopectin, and its significance. Carbohyd. Res., 1986, 147(2): 342-347 ( ) DOI: 10.1016/S0008-6215(00)90643-8
- Bertoft E., Seetharaman K. Starch structure. In: Starch: origins, structure and metabolism/I.J. Tetlow (ed.). Society for Experimental Biology, London, 2002.
- Bhattacharyya M., Smith A., Ellis T., Hedley C., Martin C. The wrinkle-seeded character of peas described by Mendel is caused by a transposon-like insertion in a gene encoding starch branching enzyme. Cell, 1990, 60(1): 115-122 ( ) DOI: 10.1016/0092-8674(90)90721-P
- Boyer C., Daniels R., Shannon J. Abnormal starch granule formation in Zea mays L. endosperms possessing the amylose-extender mutant. Crop Sci., 1976, 16: 298-301.
- Schwall G., Safford R., Westcott R., Jeffcoat R., Tayal A. Production of very-high-amylose potato starch by inhibition of SBE A and B. Nature Biotechnology, 2000, 18: 551-554 ( ) DOI: 10.1038/75427
- Tareke E., Rydberg P., Karlsson P., Eriksson S., Törnqvist M. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J. Agric. Food Chem., 2002, 50(17): 4998-5006 ( ) DOI: 10.1021/jf020302f
- Shepherd L., Bradshaw J., Dale M., McNicol J., Pont S., Mottram D., Davies H. Variation in acrylamide producing potential in potato: segregation of the trait in a breeding population. Food Chem., 2010, 123(3): 568-573 ( ) DOI: 10.1016/j.foodchem.2010.04.070
- Hou J., Zhang H., Liu J., Reid S., Liu T., Xu S., Tian Z., Sonnewald U., Song B., Xie C. Amylases StAmy23, StBAM1 and StBAM9 regulate cold-induced sweetening of potato tubers in distinct ways. J. Exp. Bot., 2017, 68(9): 2317-2331 ( ) DOI: 10.1093/jxb/erx076
- Xin Z., Browse J. Cold comfort farm: the acclimation of plants to freezing temperatures. Plant, Cell & Environment, 2000, 23(9): 893-902 ( ) DOI: 10.1046/j.1365-3040.2000.00611.x
- Mottram D., Wedzicha B., Dodson A. Food chemistry: Acrylamide is formed in the Maillard reaction. Nature, 2002, 419: 448-449 ( ) DOI: 10.1038/419448a
- Halford N., Curtis T., Muttucumaru N., Postles J., Elmore J., Mottram D. The acrylamide problem: a plant and agronomic science issue. J. Exp. Bot., 2012, 63(8): 2841-2751 ( ) DOI: 10.1093/jxb/ers011
- Zhang H., Hou J., Liu J., Xie C., Song B. Amylase analysis in potato starch degradation during cold storage and sprouting. Potato Res., 2014, 57(1): 47-58, ( ) DOI: 10.1007/s11540-014-9252-6
- Weise S., Kim K., Stewart R., Sharkey T. b-Maltose is the metabolically active anomer of maltose during transitory starch degradation. Plant Physiol., 2005, 137(2): 756-761 ( ) DOI: 10.1104/pp.104.055996
- Cottrell J., Duffus C., Paterson L., Mackay G., Allison M., Bain H. The effect of storage temperature on reducing sugar concentration and the activities of three amylolytic enzymes in tubers of the cultivated potato, Solanum tuberosum L. Potato Res., 1993, 36(2): 107-117 ( ) DOI: 10.1007/BF02358725
- Wiberley-Bradford A., Busse J., Bethke P. Temperature-dependent regulation of sugar metabolism in wild-type and low-invertase transgenic chipping potatoes during and after cooling for low-temperature storage. Postharvest Biol. Tec., 2016, 115: 60-71 ( ) DOI: 10.1016/j.postharvbio.2015.12.020
- Bhaskar P., Wu L., Busse J., Whitty B., Hamernik A., Jansky S., Buell C., Bethke P., Jiang J. Suppression of the vacuolar invertase gene prevents cold-induced sweetening in potato. Plant Physiol., 2010, 154(2): 939-948 ( ) DOI: 10.1104/pp.110.162545
- Ye J., Shakya R., Shrestha P., Rommens C. Tuber-specific silencing of the acid invertase gene substantially lowers the acrylamide-forming potential of potato. J. Agric. Food Chem., 2010, 58(23): 12162-12167 ( ) DOI: 10.1021/jf1032262
- Liu X., Zhang C., Ou Y., Lin Y., Song B., Xie C., Liu J., Li X.Q. Systematic analysis of potato acid invertase genes reveals that a cold-responsive member, StvacINV1, regulates cold-induced sweetening of tubers. Mol. Genet. Genomics, 2011, 286(2): 109-118 ( ) DOI: 10.1007/s00438-011-0632-1
- Wu L., Bhaskar P., Busse J., Zhang R., Bethke P., Jiang J. Developing cold-chipping potato varieties by silencing the vacuolar invertase gene. Crop Sci., 2011, 51(3): 981-990 ( ) DOI: 10.2135/cropsci2010.08.0473
- Draffehn A., Meller S., Li L., Gebhardt C. Natural diversity of potato (Solanum tuberosum) invertases. BMC Plant Biol., 2010, 10(1): 271 ( ) DOI: 10.1186/1471-2229-10-271
- Slugina M., Snigir E., Ryzhova N., Kochieva E. Structure and polymorphism of a fragment of the Pain-1 vacuolar invertase locus in Solanum species. Mol. Biol. (Russia), 2013, 7(2): 215-221 ( ) DOI: 10.7868/S0026898413020146
- Слугина М.А., Храпалова И.А., Рыжова Н.Н., Кочиева Е.З., Скрябин К.Г. Полиморфизм гена инвертазы Pain-1 у представителей рода Solanum. Доклады Академии наук, 2014, 454(1): 100 ( ) DOI: 10.7868/S0869565214010253
- Slugina M., Shchennikova A., Kochieva E. TAI vacuolar invertase orthologs: the interspecific variability in tomato plants (Solanum section Lycopersicon). Mol. Genet. Genomics, 2017, 292(5): 1123-1138 ( ) DOI: 10.1007/s00438-017-1336-y
- Rausch T., Greiner S. Plant protein inhibitors of invertases. Biochim. Biophys. Acta, 2004, 1696(2): 253-261 ( ) DOI: 10.1016/j.bbapap.2003.09.017
- Brummell D., Chen R.K.Y., Harris J.C., Zhang H., Hamiaux C., Kralicek A.V., McKenzie M.J. Induction of vacuolar invertase inhibitor mRNA in potato tubers contributes to cold-induced sweetening resistance and includes spliced hybrid mRNA variants. J. Exp. Bot., 2011, 62(10): 3519-3534 ( ) DOI: 10.1093/jxb/err043
- Glaczinski H., Heibges A., Salamini R., Gebhardt C. Members of the Kunitz-type protease inhibitor gene family of potato inhibit soluble tuber invertase in vitro. Potato Res., 2002, 45(2-4): 163-176 ( ) DOI: 10.1007/BF02736112
- Liu X., Cheng S., Liu J., Ou Y., Song B., Zhang C., Lin Y., Li X., Xie C. The potato protease inhibitor gene, St-Inh, plays roles in the cold-induced sweetening of potato tubers by modulating invertase activity. Postharvest Biol. Tec., 2013, 86: 265-271 ( ) DOI: 10.1016/j.postharvbio.2013.07.001
- Liu Q., Guo Q., Akbar S., Zhi Y., El Tahchy A., Mitchell M., Li Z., Shrestha P., Vanhercke T., Ral J.P., Liang G., Wang M.B., White R., Larkin P., Singh S., Petrie J. Genetic enhancement of oil content in potato tuber (Solanum tuberosum L.) through an integrated metabolic engineering strategy. Plant Biotechnol. J., 2017, 15(1): 56-67 ( ) DOI: 10.1111/pbi.12590


