Исследование геропротекторных и радиопротекторных эффектов берберина и трихостатина а на модели Drosophila melanogaster
Автор: Уляшева Н.С., Прошкина Е.Н., Шапошников М.В., Москалев А.А.
Журнал: Известия Коми научного центра УрО РАН @izvestia-komisc
Рубрика: Научные статьи
Статья в выпуске: 6 (64), 2023 года.
Бесплатный доступ
Поиск препаратов, влияющих на замедление темпов старения и стимуляцию радиоустойчивости, является актуальной задачей биологии, экологии и медицины. В данной работе изучены эффекты влияния трихостатина А и берберина на продолжительность жизни, устойчивость к прооксиданту параквату и острому гамма-излучению плодовой мушки Drosophila melanogaster. Трихостатин А оказал более выраженный геропротекторный эффект, увеличив продолжительность жизни дрозофил на 3-9 %. Однако повышение радиоустойчивости наблюдали только после применения берберина у самцов. Их медианная выживаемость после действия гамма-излучения увеличилась на 25 %. Трихостатин А, напротив, повысил чувствительность мух к генотоксическому воздействию гамма-излучения, снизив выживаемость на 7-17 %.
Продолжительность жизни, стрессоустойчивость, радиоустойчивость, гамма-излучение, трихостатин а, берберин, drosophila melanogaster
Короткий адрес: https://sciup.org/149143630
IDR: 149143630 | УДК: 591.139 | DOI: 10.19110/1994-5655-2023-6-94-102
Текст научной статьи Исследование геропротекторных и радиопротекторных эффектов берберина и трихостатина а на модели Drosophila melanogaster
Старение представляет собой биологический процесс, характеризующийся снижением биологических функций организма и потерей устойчивости к стрессорам [1]. Организм перестает поддерживать постоянство внутреннего состояния, в результате чего становиться более восприимчивым к повреждающим воздействиям и развитию возрастных патологических процессов, обусловливающих ухудшение состояние здоровья и дальнейшее нарастание темпов старения. В конечном итоге это приводит к гибели
-
[2, 3]. Таким образом, продолжительность жизни (далее – ПЖ) организма тесно связана с его стрессоустойчивостью.
Ионизирующие излучения и прооксиданты оказывают значительное влияние на длительность жизни и темпы старения, вызывая изменения на всех уровнях организации, начиная с молекулярного. Они могут непосредственно повреждать макромолекулы, способствуют индукции нестабильности генома и изменению паттернов генной экспрессии, вызывают митохондриальную дисфункцию и метаболические нарушения, что приводит к выраженному токсическому эффекту на уровне тканей и органов. Реакция на эти факторы отражает общую жизнеспособность организма [4-6].
Поиск средств, замедляющих темпы старения и стимулирующих стрессо- и радиоустойчивость, является актуальной задачей в настоящее время. Наиболее доступным способом замедления темпов старения представляется применение веществ, которые могут воздействовать на скорость старения и повышать сопротивляемость организма негативным воздействиям. Ранее были описаны вещества, которые являются потенциальными геропро-текторами и антимутагенами [7]. Примерами таких веществ являются берберин и трихостатин А.
Берберин – хорошо изученный природный алкалоид, для которого описано потенциальное действие против старения и развития ряда возрастзависимых заболеваний [8-11]. Он обладает антиоксидантной активностью [12], противоопухолевыми свойствами [13-15], обезболивающим [16] и противовоспалительным [17] действиями. Его биологические эффекты опосредованы несколькими сигнальными путями, включая NRF2/KEAP1, SIRT1, AMPK, IRS/PI3K/Akt/ mTORC1, PTEN, GSK-3, NF-κB, JAK/STAT, MAPK [8, 10, 17–19], а также регуляцией метилирования ДНК и активности микроРНК [17, 19, 20].
Трихостатин А является ингибитором гистондеацетилаз (HDAC) классов I и II и способен влиять на функционирование хроматина через ацетилирование гистонов, а также регулировать экспрессию микроРНК [21, 22]. Известно, что препараты, воздействующие на эпигенетические механизмы, включая ингибиторы HDAC, могут влиять на скорость старения организма и являются потенциальными средствами для лечения возраст-зависимых заболеваний [23].
Ранее проведенные исследования показали положительное действие берберина и трихостатина А на ПЖ дрозофил и других модельных животных [24-30], но влияние данных веществ на устойчивость организма к генотоксическим факторам не описано. В данной работе мы исследовали влияние берберина и трихостатина А на продолжительность жизни и устойчивость Drosophila melano-gaster к γ-излучению и прооксиданту параквату.
Материалы и методы
Линия Drosophila melanogaster и условия содержания
Исследование проводили на мухах линии дикого типа Canton-S (#64349), полученной из Дрозофилиного центра Университета Индианы (Блумингтон, США). Для каждого варианта эксперимента отбирали по 120–150 особей каждого пола в течение 24 ч после вылупления имаго. В эксперименте использовали самцов и оплодотворенных самок.
Для содержания дрозофил применяли климатические камеры Binder KBF720-ICH (Binder, Германия). Особей содержали при температуре +25 °С, относительной влажности воздуха 60 % и 12-часовом режиме освещения. Состав питательной среды был адаптирован из работы Xia и de Belle [31]: вода – 1 л, кукурузная мука – 92 г, сухие дрожжи – 32.1 г, агар-агар – 5.2 г, глюкоза – 136.9 г.
Исследуемые вещества
В эксперименте применяли растворы берберина (B3251, Sigma-Aldrich, США) в концентрациях 1; 10; 50; 100; 500; 1000; 5000 мкмоль/л и трихостатина А (T1952, Sigma-Aldrich, США) в концентрациях 0.1; 1; 10; 100 мкмоль/л. В качестве растворителя использовали 0.2 %-ный диметилсульфоксид (DMSO, D2650, Sigma-Aldrich, США) согласно рекомендациям изготовителя. Для DMSO в такой концентрации возможен токсичный эффект, но он не приводит к острой летальности у дрозофилы [32].
При анализе ПЖ исследуемые вещества наносили на поверхность питательной среды в объеме 30 мкл на пробирку в течение всей жизни имаго. В качестве контроля использовали 0.2 % DMSO. В случае изменения устойчивости к параквату и гамма-излучению вещества давали в течение первых 15 сут жизни имаго.
Анализ продолжительности жизни
Для каждого эксперимента дрозофил собирали в течение 24 ч после вылета имаго из куколок. С использованием углекислотной анестезии (Genesee Scientific, США) мух усыпляли, сортировали по полу и рассаживали в пробирки по 30 особей. Начиная с первого дня жизни имаго ежедневно вели подсчет числа умерших особей, два раза в неделю мух переносили на свежую среду.
Результаты представляли в виде кривых выживаемости Каплана-Майера и рассчитывали медианную ПЖ (длительность жизни наиболее типичных представителей выборки) и возраст 90 % смертности (показатель максимальной ПЖ). Для сравнения функций дожития использовали критерии Колмогорова-Смирнова [33] и Мантеля-Кок-са [34]. Для оценки достоверности различий по медианной ПЖ применяли критерий Гехана-Бреслоу-Вилкоксона [35]. Для оценки статистической значимости различий возраста 90 % смертности использовали метод Ванг-Аллисона [36]. Обработку данных проводили с помощью программы Statistica, версия 6.1 (StatSoft, США), статистической среды R, версия 2.15.1 (The R Foundation) и онлайн-приложения OASIS 2 (Online application for survival analysis) [37].
Обработка паракватом
При проведении эксперимента мух содержали на среде, содержащей 2 % агар-агара и 5 % сахарозы и 20 ммоль/л параквата (#856177, Sigma-Aldrich). В эксперименте использовали мух в возрасте 15 сут. Мухи находились в стресс-индуцируемых условиях до конца жизни. Для оценки динамики гибели мушек по одной рассаживали в стеклянные капилляры диаметром 5 мм и анализировали в мониторе локомоторной активности DAM ( Drosophila Activity Monitor, Trikinetics, США). Данные активности от отдельных мух были объединены в 30-минутные периоды и проанализированы. Погибших мух идентифицировали по полному отсутствию локомоторной активности. На основании полученных данных были построены кривые выживаемости. На каждый экспериментальный вариант анализировали по 32 особи в трех биологических повторностях.
Условия облучения
В возрасте 15 сут дрозофил облучали в дозе 800 Гр с использованием γ-источника с Cs-137 «Исследователь» (СССР). При мощности дозы 0.74 Гр/мин продолжительность облучения составила 18 ч. Выбрана доза 800 Гр, так как она значительно снижает выживаемость имаго самцов и самок дрозофил без острого летального эффекта. После облучения мух помещали на стандартную питательную среду без добавления исследуемых веществ.
Анализ экспрессии генов стресс-ответа
Оценку транскрипционной активности генов стресс-от-вета проводили с применением метода ПЦР «в реальном времени» с этапом обратной транскрипции. Для каждого варианта эксперимента отбирали 10 особей каждого пола, которых предварительно содержали на среде с 0.2 % DMSO (контроль) или исследуемыми веществами в течение 15 сут.
РНК выделяли с помощью набора Aurum Total RNA Mini (Bio-Rad, США) в соответствии с инструкциями производителя. Концентрацию РНК измеряли с помощью набора для анализа РНК Quant-iT (Invitrogen, США) в соответствии с инструкциями производителя. кДНК была синтезирована в соответствии с набором для синтеза кДНК iScript (Bio-Rad, Hercules, CA, США) из полученного раствора РНК. Реакционную смесь для ПЦР-реакции готовили на основе смеси qPCRmix-HC SYBR (Евроген, Россия) и праймеров (Евроген, Россия) (табл. 1). ПЦР проводили в амплифика-торе CFX96 (Bio-Rad, США) по следующей программе: (1) 95 Выбрана °С в течение 5 мин, (2) 95 °С в течение 10 сек, (3) 60 °С в течение 10 сек, (4) второй-третий этапы повторялись 49 раз, (5) стадия плавления ДНК. Экспрессию исследуемых генов рассчитывали относительно экспрессии референсных генов β-Tubulin, RpL32, EF1α. Обработку данных осуществляли с помощью программного обеспечения CFX Manager 3.1 (Bio-Rad, США) и программы Statistica, версия 6.1 (StatSoft, США). Эксперименты проводили в трех биологических и трех технических повторностях.
Таблица 1
Праймеры генов контроля клеточного стресс-ответа
Table 1
Primers of genes controlling the cellular stress response
|
Ген |
Прямой праймер |
Обратный праймер |
|
β-Tubulin |
GCAACTCCACTGCCATCC |
CCTGCTCCTCCTCGAACT |
|
RpL32 |
GAAGCGCACCAAGCACTTCATC |
CGCCATTTGTGCGACAGCTTAG |
|
EF1α |
AGGGCAAGAAGTAGCTGGTTTGC |
GCTGCTACTACTGCGTGTTGTTG |
|
D-Gadd45 (гомолог (GADD45) |
AAGTCGCGCACAGATACTCACG |
AAGTCGCGCACAGATACTCACG |
|
Rrp1 (гомолог APE1) |
AGGATGGTCTGCAGTTGATTGACC |
CGTTTGCGCACTTGGTTTCCTG |
|
mus 210 |
AGAAGACGGTGCATTTGAGATTGC |
ATGGGATGACAAGCGCCTTGATG |
|
Brca2 |
CAACCGAAGCAAGGCAGGATTC |
TCTGCCATAGTTCCTGGACCTTCC |
|
okr |
AGTCGGCCGAGAAGCATTTCAC |
GCAGCGCTTACACTTGAGCTTG |
|
Ku80 |
AGCTTCAGAATGTCGCAACTACC |
TCGTTGAAATCGAAGAGCAGGAG |
|
Sod1 |
TGCACGAGTTCGGTGACAACAC |
TCCTTGCCATACGGATTGAAGTGC |
|
Prx5 |
CCGATGAGCTGAAGTCCAAG |
TTGCCGTTCTCCACCACCAG |
|
Hsp68 |
TGGGCACATTCGATCTCACTGG |
TAACGTCGATCTTGGGCACTCC |
|
Hsp 83 |
TCAAGTGTTTGAGGGCGAGAGG |
ACGCCAGTGAGATCGAATGTGC |
|
Atg1 |
AGACTCTTCCTCGTGCAACTAGC |
GCTTGAGATCACGATGCACAATTC |
|
Atg5 |
CTCGTCAAGCTCAACTCCAAGG |
GTTGACCAATCCCAGCCAAAGC |
|
Ire1 |
GACAGTGAGGACAGCCGAATTATC |
GCGATTGCGGATCCTTGTGTATC |
Результаты и их обсуждение
Влияние берберина и трихостатина А на продолжительность жизни дрозофил
Мы проанализировали изменение ПЖ дрозофил при применении широкого спектра концентраций берберина (1-5000 мкмоль/л) и трихостатин А (0.1-100 мкмоль/л) на протяжении всей жизни.
Трихостатин А оказал положительное влияние на длительность жизни дрозофил. Он увеличивал медианную ПЖ самцов на 2–5 % (p < 0.001) и возраст 90 % смертности на 2 % (p < 0.001) при концентрациях вещества 0.1, 1 и 100 мкмоль/л. У самок показаны более значительные изменения при добавлении 0.1, 1 и 10 мкмоль/л трихостатина А - увеличение медианной ПЖ на 3–9 % (p < 0.001) и показателя максимальной ПЖ – на 8% (p < 0.001).
Природное соединение берберин оказало положительное действие только на самок дрозофил, увеличив их медианную ПЖ на 2-3 % (p < 0.01) и возраст 90 % смертности на 1 % (p < 0.01). У самцов результаты были статистически незначимы (р > 0.05) или связаны с укорочением жизни (табл. 2, рис. 1).
Влияние берберина и трихостатина А на устойчивость дрозофил к параквату и гамма-излучению
Мы оценили влияние берберина и трихостатина А на устойчивость дрозофил к стрессорам: прооксиданту параквату в концентрации 20 ммоль/л и гамма-излучению в дозе 800 Гр. Для исследования выбрали концентрации изучаемых веществ, показавшие наибольший положительный эффект на ПЖ.
Берберин и трихостатин А преимущественно не оказывали влияния на выживаемость самок в условиях воздействия параквата. Однако берберин в концентрации 1000 мкмоль/л снизил медианную выживаемость самцов на 8–10 % (р < 0.01) и максимальную – на 6 % (р < 0.05).
Берберин в концентрациях 10 и 1000 мкмоль/л увеличил медианную выживаемость самцов на 25 % (р < 0.001) после острого действия гамма-излучения. В то же время трихостатин А повысил чувствительность самцов к генотоксическому воздействию, снизив выживаемость на 9–17 % (р < 0.001) (рис. 2). Действие берберина на радиоустойчивость самок оказалось статистически незначимым (р > 0.05), а трихостатин А снизил их выживаемость на 7 % (р < 0.01).
Изменение экспрессии генов стресс-ответа у дрозофил после обработки берберином и трихостатином А
У мух, потреблявших берберин и трихостатин А анализировали экспрессию генов, участвующих в стресс-ответе, включая гены антиоксидантной защиты ( Prx5, Sod2 ), гены ответа на повреждение ДНК и репарации ДНК ( Gadd45, Rrp1, mus210, Brca2, okr, Ku80 ), а также генов протеостаза ( Hsp68, Hsp83, Atg1, Atg5, Ire1 ).
Берберин повышал активность гена Hsp68 , кодирующего белок теплового шока 68, у самцов и самок в 2.6 и 1.7 раз (p < 0.05) соответственно. Кроме того, у самок наблюдалась активация транскрипции генов аутофагии Atg1 и Atg5
Таблица 2
Параметры продолжительности жизни самцов и самок Drosophila melanogaster при обработке берберином и трихостатином А
Table 2
Lifespan parameters of Drosophila melanogaster males treated with berberine and trichostatin A
|
Вещество |
C |
Повторность |
Самцы |
Самки |
||||
|
M |
90% |
N |
M |
90% |
N |
|||
|
0 (DMSO) |
1 |
56 |
66 |
159 |
63 |
69 |
147 |
|
|
1 |
52 |
63* |
129 |
63* |
70 |
202 |
||
|
10 |
55 |
63 |
149 |
59* |
69 |
147 |
||
|
100 |
56 |
64 |
146 |
60 |
69 |
157 |
||
|
500 |
55 |
63 |
141 |
57* |
69 |
144 |
||
|
1000 |
55* |
64 |
145 |
62 |
72** |
134 |
||
|
5000 |
56 |
63 |
113 |
60 |
69 |
126 |
||
|
0 (DMSO) |
2 |
56 |
63 |
136 |
57 |
66 |
142 |
|
|
1 |
56 |
63 |
138 |
59 |
67 |
148 |
||
|
10 |
56 |
63 |
142 |
61*** |
71*** |
150 |
||
|
100 |
53 |
63 |
126 |
56 |
65 |
144 |
||
|
500 |
53* |
63 |
144 |
59 |
67 |
146 |
||
|
1000 |
56* |
63 |
147 |
59* |
70* |
153 |
||
|
5000 |
56* |
63 |
150 |
59 |
70* |
149 |
||
|
0 (DMSO) |
3 |
57 |
64 |
154 |
60 |
71 |
148 |
|
|
1 |
67 |
64 |
141 |
61 |
70 |
145 |
||
|
10 |
56 |
64 |
133 |
61 |
71 |
144 |
||
|
100 |
56 |
64 |
138 |
64*** |
71 |
150 |
||
|
500 |
57 |
64 |
148 |
64** |
72** |
144 |
||
|
1000 |
58 |
64 |
148 |
64*** |
72** |
149 |
||
|
5000 |
56 |
64 |
151 |
64*** |
71* |
148 |
||
|
0 (DMSO) |
1 |
56 |
63 |
157 |
59 |
66 |
159 |
|
|
0.1 |
60 |
62 |
215 |
63 |
64 |
163 |
||
|
1 |
56 |
63 |
147 |
60 |
68 |
156 |
||
|
10 |
57** |
63** |
156 |
60* |
66 |
159 |
||
|
100 |
57** |
63 |
157 |
59 |
66 |
149 |
||
|
0 (DMSO) |
2 |
57 |
61 |
146 |
57 |
66 |
142 |
|
|
0.1 |
57 |
64 |
153 |
64 |
65 |
161 |
||
|
1 |
57 |
64 |
153 |
60 |
68 |
152 |
||
|
10 |
53 |
64 |
148 |
64*** |
71 |
147 |
||
|
100 |
57* |
64 |
141 |
57 |
68 |
143 |
||
Примечание. С - концентрация (мкмоль/л); М – медианная ПЖ (сут); 90 % – время 90 % смертности (сут); N – количество особей в выборке. При сравнении времени 90 % смертности использовали метод Ванг-Аллисона, для медианной выживаемости – критерии Гехана-Бреслоу-Вилкоксона и Мантеля-Кокса.
Условные обозначения. Различия с контролем достоверны при * – p < 0.05; ** – p < 0.01, *** – p < 0.001.
Note. C - concentration ( ц М/l); M - median lifespan (days); 90 % - time of 90 % mortality (days); N – a number of individuals in a sample. When comparing the time of 90 % mortality, the Wang-Allison method was used; for median survival, the Gehan-Breslow-Wilcoxon and Mantel-Cox tests were used.
Symbols. Differences with a control are significant at * – p < 0.05, ** – p < 0.01, *** – p < 0.001.
и гена ответа на стресс эндоплазматической сети Ire1 в 1.31.5 раз (p < 0.05) после потребления этого вещества.
Трихостатин А активировал ген ответа на повреждение Gadd45 у самцов и самок, а также гены репарации ДНК Mus210, Brca2, Ku80 у самок в 1.5-3.0 раза (p < 0.05). Но у самцов наблюдалось подавление экспрессии Ku80 после применения этого вещества. В то же время действие трихо-статина А на активность генов протеостаза оказалось противоположным у особей разного пола. У самок наблюдали повышение их активности, а у самцов - снижение, кроме гена Hsp68 , который активировался у всех дрозофил в 2.1-
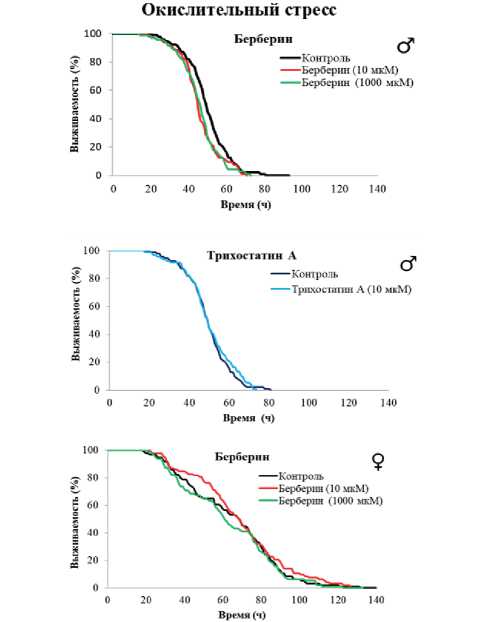
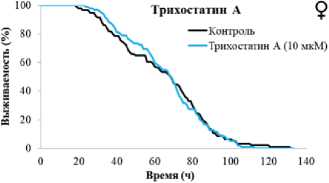
Рисунок 1. Влияние берберина и трихостатина А на продолжительность жизни Drosophila melanogaster.
Условные обозначения. * – p < 0.05; ** – p < 0.01; *** – p < 0.001.
Figure 1. Effect of berberine and trichostatin A on the lifespan of Drosophila melanogaster.
Symbols. * – p < 0.05, ** – p < 0.01, *** – p < 0.001.
-
2.2 раза (p < 0.05). Положительный эффект также был выявлен у самок в отношении гена Sod2 (p < 0.05) (рис. 3).
В данном исследовании in vivo были сопоставлены эффекты берберина и трихостатина А на ПЖ Drosophila mel-anogaster , а также впервые изучено влияние этих веществ на устойчивость дрозофил к острому гамма-излучению и прооксиданту параквату.
Положительное действие выбранных нами препаратов было показано ранее на различных модельных организмах. Берберин увеличивал длительность жизни дрожжей [27], мышей [27] и дрозофил [24, 25]. Трихостатин А также показал положительное влияние на ПЖ нематод [28], дрозофил [26] и мышей [29, 30]. В нашей работе мы подтвердили их ге-ропротекторное действие, оба препарата продлили жизнь дрозофил, но более выраженный эффект был получен для трихостатина А, который увеличил ПЖ на 3-9 %. Различия в эффектах этих двух соединений могут быть обусловлены их разной биодоступностью [38, 39].
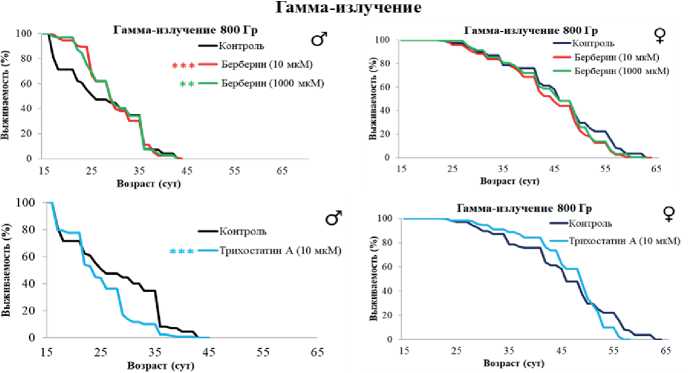
Рисунок 2. Влияние берберина и трихостатина А на устойчивость дрозофил к прооксиданту параквату и гамма-излучению.
Условные обозначения. * – p < 0.05, ** – p < 0.01, *** – p < 0.001.
Figure 2. The effect of berberine and trichostatin A on the resistance of prooxidant and gamma irradiation.
Symbols. * – p < 0.05, ** – p < 0.01, *** – p < 0.001.
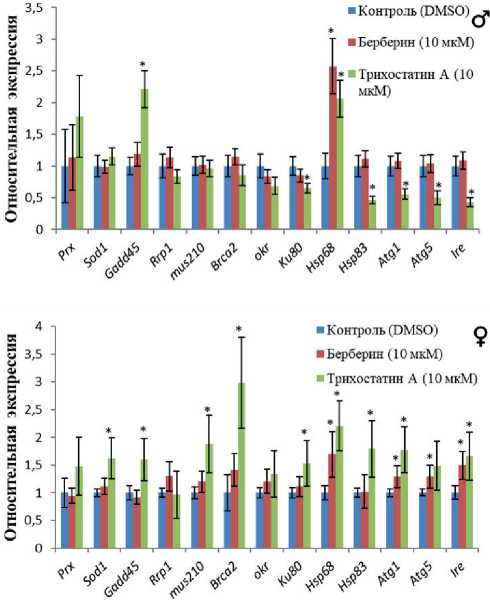
Рисунок 3. Влияние берберина и трихостатина А на экспрессию генов стресс-ответа.
Условные обозначения. * - p < 0.05 по U-критерию Манна-Уитни.
Figure 3. The effect of berberine and trichostatin A on the expression of stress response genes.
Symbols. * - p < 0.05 according to the Mann-Whitney U-test.
Ионизирующие излучения являются одним из факторов, приводящих к повреждениям ДНК, образованию промежуточных продуктов репарации, мутациям [40]. Кроме того, они вызывают спектр эпигенетических изменений, вносящих вклад в состояние генетической нестабильности (изменение экспрессии генов, ацетилирования гистонов или паттернов метилирования ДНК) [41]. Паракват также способен нарушать целостность и стабильность генома, опосредованно через индукцию свободных радикалов. Он вызывает выраженную митохондриальную токсичность, индуцирует апоптоз, перекисное окисление липидов и тяжелое вторичное воспаление [42, 43].
Согласно данным литературы, выбранные нами препараты – берберин и трихостатин А – оказывают защитное действие на клетки и организм в целом и могут стимулировать внутренние компенсаторные механизмы. В частности, трихо-статин А способен активировать внутриклеточный сигнальный путь Akt/Nrf2 и усиливать антиокси- дантную защиту в раковых клетках
Drosophila to the paraquat HeLa и HepG2 [44], а его положительное влияние на продолжительность жизни дрозофил может быть связано с активацией уровня экспрессии генов белков теплового шока [26, 45]. В исследовании на мышах трихоста-тин А оказывает кардиозащитное действие, через активацию сигнального пути FoxO3a и белков антиоксидантной защиты (SOD2, каталазы) [46]. Берберин повышал выживаемость дрозофил при высокой температуре [25]. У мышей этот алкалоид подавлял окислительный стресс и воспаление посредством сигнальных путей, включая NF-κB, AMPK и Nrf2 [47, 48]. В то же время имеются экспериментальные данные, указывающие на повышение чувствительности раковых клеток человека к ультрафиолетовому и ионизирующему излучению после обработки трихостатином [4952] и берберином [53].
Мы предположили, что оба вещества повысят выживаемость особей Drosophila melanogaster при воздействии параквата и гамма-излучения. Однако положительное действие оказал только берберин на радиоустойчивость самцов дрозофил, увеличив данный показатель на 25 %. Трихостатин А, напротив, снижал выживаемость дрозофил после действия острого гамма-излучения на 17 %.
Для выяснения возможных молекулярных механизмов, обусловливающих наблюдаемые эффекты берберина и трихостатина А на уровне организма, мы оценили изменение экспрессии генов, вовлеченных в механизмы стресс-ответа, после применения веществ. Берберин преимущественно активировал гены протеостаза, особенно Hsp68. Продукт этого гена является одним из белков теплового шока – важных регуляторов ПЖ организма и ответа на повреждающие факторы. Они противодействуют накоплению аномальных белков, последствиям окислительного стресса и модулируют апоптоз (активируют его в случае избытка поврежденных макромолекул, либо обеспечивают выживание в стрессовых условиях) [54]. Повышенная экспрессия гена Hsp68 у дрозофил связана с долгожительством [55]. Тем не менее в некоторых моделях in vitro и in vivo берберин, напротив, подавлял гены и белки теплового шока и аутофагии [56-58].
Трихостатин А повышал экспрессию гена ответа на повреждение ДНК Gadd45 и генов, отвечающих за разные механизмы репарации ДНК. Эффект в большей степени проявлялся у самок, для которых также был отмечен и больший эффект на ПЖ. В экспериментах на клеточных культурах ранее была установлена способность данного вещества стимулировать клеточный ответ на повреждение ДНК через ингибирование деацетилаз и изменение конформации хроматина [59-62]. Стоит отметить, что повышенная экспрессия генов репарации ДНК (включая Gadd45 ) может быть связана с продлением жизни организма [63, 64]. При этом она сопровождается выраженным повышение радиочувствительности [65]. Возможно, что негативный эффект трихостатина А на устойчивость к острому гамма-излучению отчасти обусловлен избыточной активацией и нарушением баланса систем ответа на повреждение ДНК.
У самок, получавших трихостатин А, также наблюдалась активация гена антиоксидантной защиты Sod1 и генов протеостаза. Ранее в исследованиях на дрозофиле было показано, что трихостатин А через регуляцию ацетилирования гистонов способен стимулировать экспрессию генов, связанных с ответом на повреждение белков, например Hsp22 и Hsp70 [26, 45], что также подтверждают полученные данные на культурах клеток человека [66].
В то же время трихостатин А оказывал разнонаправленное действие на экспрессию большинства анализируемых генов стресс-ответа у самцов и самок, и схожую тенденцию для радиоустойчивости дрозофил. Половые различия могут быть обусловлены взаимодействием ингибитора HDAC с гормонами, регулирующими размножение и развитие организма. Например, в исследованиях на грызунах описана обратная связь между эпигенетическими модификациями и половыми гормонами (эстрадиолом и лютеинизирующим гормоном), которая определяет состояние здоровье и старение [67, 68]. Также установлено, что ацетилирование и деацетилирование гистонов важны для регуляторной активности ювенильного гормона у дрозофилы, а трихостатин А способен влиять на экспрессию ряда генов, опосредующих действие этого гормона [69].
Таким образом, нами показаны потенциальные герои радиопротекторные эффекты трихостатина А и берберина на модели D. melanogaster . Трихостатин А оказал положительное влияние на длительность жизни дрозофил, но повысил их чувствительность к острому гамма-излучению. Берберин при небольшом увеличении продолжительности жизни значительно повысил выживаемость самцов при радиационном воздействии.
Список литературы Исследование геропротекторных и радиопротекторных эффектов берберина и трихостатина а на модели Drosophila melanogaster
- Da Costa, J. P. A synopsis on aging-theories, mechanisms and future prospects / J. P. da Costa, R. Vitorino, G. M. Silva [et al.] // Ageing Res Rev. – 2016. – Vol. 29. – P. 90-112.
- Moskalev, A. A. Genetics and epigenetics of aging and longevity / A. A. Moskalev, A. M. Aliper, Z. Smit-McBride [et al.] // Cell Cycle. – 2014. – Vol. 13. – № 7. – P. 1063-77.
- Dues, D. J. Aging causes decreased resistance to multiple stresses and a failure to activate specific stress responsepathways / D. J. Dues, E. K. Andrews, C. E. Schaar [et al.] // Aging (Albany NY). – 2016. – Vol. 8. – № 4. – P. 777-95.
- Marion, J. The effects of radiation on the longevity of female Drosophila subobscura / J. Marion, Lamb // Journal of Insect Physiology. –1964. –Vol. 10. – № 3. – P. 487-497.
- Gaman, L. Can ageing be slowed: Hormetic and redox perspectives / L. Gaman, I. Stoian, V. Atanasiu // J Med Life. –2011. – Vol. 4. – № 4. – P. 346-51.
- Belyi, A. A. The resistance of Drosophila melanogaster to oxidative, genotoxic, proteotoxic, osmotic stress, infection, and starvation depends on age according to the stress factor / А. А. Belyi, A. A. Alekseev, A. Y. Fedintsev [et al.] // Antioxidants (Basel). – 2020. – Vol. 9. – № 12. – P. 1239.
- Прошкина, Е. Н. Ключевые молекулярные механизмы старения, биомаркеры и потенциальные интервенции / Е. Н. Прошкина, И. А. Соловьев, М. В. Шапошников, А. А. Москалев // Молекулярная биология. – 2020. – Т. 54, № 6. – С. 883-921.
- McCubrey, J. A. Regulation of GSK-3 activity by curcumin, berberine and resveratrol : Potential effects on multiple diseases / J. A. McCubrey, K. Lertpiriyapong, L. S. Steelman [et al.] // Adv Biol Regul. – 2017. – Vol. 65. – P. 77-88.
- Xu, Z. Rhizoma coptidis and berberine as a natural drug to combat aging and aging-related diseases via anti-oxidation and AMPK activation / Z. Xu, W. Feng, Q. Shen [et al.] // Aging Dis.– 2017.–Vol. 8. –№ 6.– P. 760-777.
- Kooshki, L. The pivotal role of JAK/STAT and IRS/PI3K signaling pathways in neurodegenerative diseases: Mechanistic approaches to polyphenols and alkaloids / L. Kooshki, S.N Zarneshan, S. Fakhri [et al.] // Phytomedicine. – 2023. – Vol. 112. – P. 154686.
- Gjorgieva Ackova, D. Alkaloids as natural NRF2 inhibitors: Chemoprevention and cytotoxic action in cancer / D. Gjorgieva Ackova, V. Maksimova, K. Smilkov [et al.] // Pharmaceuticals (Basel). – 2023. –Vol. 16. – № 6. – P. 850.
- Vuddanda, P. R. Berberine: a potential phytochemical with multispectrum therapeutic activities / P. R. Vuddanda, S. Chakraborty, S. Singh // Expert Opin Investig Drugs. – 2010. – Vol. 19. –№ 10. – P. 1297-307.
- Xiong, R.G. Anticancer effects and mechanisms of berberine from medicinal herbs: An update review / R. G. Xiong, S. Y. Huang, S. X. Wu [et al.] // Molecules. – 2022. – Vol. 27.– № 14. – P. 4523.
- Lui, D. Natural isoquinoline alkaloid with antitumor activity: studies of the biological activities of berberine / D. Liu, X. Meng, D. Wu [et al.] // Front Pharmacol. – 2019. – Vol. 10. – № 9.
- Rauf, A. Berberine as a potential anticancer agent: A comprehensive review / A. Rauf, T. Abu-Izneid, A. A. Khalil [et al.] // Molecules. - 2021. – Vol. 26. – № 23. – P. 7368.
- Hashemzaei, M. A review on pain-relieving activity of berberine / M. Hashemzaei, R. Rezaee // Phytother Res. – 2021. – Vol. 35. – № 6. – P. 2846-2853.
- Haftcheshmeh, S. M. Berberine as a natural modulator of inflammatory signaling pathways in the immune system: Focus on NF-κB, JAK/STAT, and MAPK signaling pathways / S.M. Haftcheshmeh, M. Abedi, K. Mashayekhi [et al.] // Phytother Res. – 2022. – Vol. 36. – № 3. – P. 116-123.
- Gjorgieva Ackova, D. Alkaloids as natural NRF2 inhibitors: Chemoprevention and cytotoxic action in cancer / D. Gjorgieva Ackova, V. Maksimova, K. Smilkov [et al.] // Pharmaceuticals (Basel). – 2023. – Vol. 16. – № 6. – P. 850.
- DiNicolantonio, J. J. Ferulic acid and berberine, via Sirt1 and AMPK, may act as cell cleansing promoters of healthy longevity / J. J. DiNicolantonio, M. F McCarty, S. I. Assanga [et al] // Open Heart. – 2022. – Vol. 9. – № 1. – P. e001801.
- McCubrey, J. A. Effects of resveratrol, curcumin, berberine and other nutraceuticals on aging, cancer development, cancer stem cells and microRNAs / J. A. McCubrey, K. Lertpiriyapong, L. S. Steelman [et al.] // Aging (Albany NY). – 2017. – Vol. 9. – № 6. – P. 1477-1536.
- Rhodes, L.V. The histone deacetylase inhibitor trichostatin A alters microRNA expression profiles in apoptosis-resistant breast cancer cells / L. V. Rhodes, A. M. Nitschke, H. C. Segar [et al.] // Oncol Rep. – 2012. – Vol. 27. – № 1. – Р. 6-10.
- Dekker, F. J. Small molecule inhibitors of histone acetyltransferases and deacetylases are potential drugs for inflammatory diseases / F. J. Dekker, T. Bosch, N. I. Martin // Drug Discov Today. – 2014. – Vol. 19. – № 5. – P. 654-60.
- Pasyukova, E. G. Epigenetic enzymes: A role in aging and prospects for pharmacological targeting / E. G. Pasyukova, A. V. Symonenko, O. Y. Rybina, A. M.Vaiserman // Ageing Research Reviews. – 2021. – Vol. 67. – P. 1568-1637.
- Navrotskaya, V. V. Berberine prolongs lifespan and stimulates locomotor activity of Drosophila melanogaster / V. V. Navrotskaya, G. Oxenkrug, L. I. Vorobyova, P. Summergrad // Am J Plant Sci. – 2012. – Vol. 3. – № 7A. – P. 1037-1040.
- Navrotskaya, V. Berberine attenuated aging-accelerating effect of high temperature in Drosophila model / V. Navrotskaya, G. Oxenkrug, L.Vorobyova, P. Summergrad // Am J Plant Sci. – 2014. – Vol. 5. – № 3. – P. 275-278.
- Tao, D. Trichostatin A extends the lifespan of Drosophila melanogaster by elevating hsp22 expression / D. Tao, J. Lu, H. Sun [et al.] // Acta Biochim Biophys Sin (Shanghai). – 2004. – Vol. 36. – № 9. – P. 618-622.
- Dang, Y. Berberine ameliorates cellular senescence and extends the lifespan of mice via regulating p16 and cyclin protein expression / Y. Dang, Y. An, J. He [et al.] // Aging Cell. – 2020. – Vol. 19. – № 81.
- Calvert, S. A network pharmacology approach reveals new candidate caloric restriction mimetics in C. elegans / S. Calvert, R. Tacutu, S. Sharifi [et al.] // Aging Cell. – 2016.–Vol. 15. – № 2. – P. 256-266.
- Avila, A. M. Trichostatin A increases SMN expression and survival in a mouse model of spinal muscular atrophy / A. M. Avila, B. G. Burnett, A. A. Taye [et al.] // J Clin Invest. – 2007. – Vol. 117. – № 3. – P. 659-71.
- Lui, H. The Smn-independent beneficial effects of trichostatin A on an intermediate mouse model of spinal muscular atrophy / H. Liu, A. Yazdani, L. M. Murray [et al.] // PLoS One. – 2014. – Vol. 9. – № 7. – P. e101225
- Xia, B. Transgenerational programming of longevity and reproduction by post-eclosion dietary manipulation in Drosophila / B. Xia, J.S. de Belle // Aging. – 2016. – Vol. 8. – № 5. – P. 1115–1134.
- Solovev, I.A. Chronobiotics KL001 and KS15 extend lifespan and modify circadian rhythms of Drosophila melanogaster / I.A. Solovev, M.V. Shaposhnikov, A.A. Moskalev // Clocks Sleep. – 2021. – Vol. 3. – № 3. – P. 429-441.
- Hilton, J. F. An algorithm for conducting exact Smirnov tests / J. F. Hilton, C. R. Mehta, N. R. Patel // Computational Statistics & Data Analysis. – 1994. – Vol. 17. – № 4. – P. 351–361.
- Mantel, N. Evaluation of survival data and two new rank order statistics arising in its consideration / N. Mantel // Cancer Chemotherapy Reports. Part 1. – 1966. – Vol. 50. – № 3. – P. 163–170.
- Martinez, R. L. Pretest for choosing between logrank and wilcoxon tests in the two-sample problem / R. L. Martinez, D.A. NaranjoJ // Metron. – 2012. – Vol. 68. – № 2. – P. 111–125.
- Wang, C. Statistical methods for testing effects on “maximum lifespan” / C. Wang, Q. Li, D. Redden [et al.] // Mechanisms of Ageing and Development. – 2004. – Vol. 125. – № 9. – P. 629–632.
- Han, S. K. OASIS2: online application for survival analysis 2 with features for the analysis of maximal lifespan and healthspan in aging research / S. K. Han, D. Lee, H. Lee [et al.] // Oncotarget. – 2016. – Vol. 7. – № 35. – P. 56147–56152.
- Tambunan, U. S. Identification of a better Homo sapiens Class II HDAC inhibitor through binding energy calculations and descriptor analysis / U. S. Tambunan, E. K. Wulandari // BMC Bioinformatics. – 2010. – Vol. 11. – Suppl. 7(S16).
- Gasmi, A. Berberine: Pharmacological features in health, disease and aging / A. Gasmi, F. Asghar, S. Zafar [et al.] // Curr Med Chem. – 2023.
- Vaiserman, A. M. Cross-life stage and cross-generational effects of gamma irradiations at the egg stage on Drosophila melanogaster life histories / A. M. Vaiserman, N. M. Koshel, L. V. Mechova [et al.] // Biogerontology. – 2004. – Vol. 5. – № 5. – P. 327-37.
- Vaisnav, M. Genome-wide association analysis of radiation resistance in Drosophila melanogaster / M. Vaisnav, C. Xing, H.C. Ku [et al.] // PLoS One. – 2014. – Vol. 9. – № 8. – P. e104858.
- Gawarammana, I. B. Medical management of paraquat ingestion / I. B. Gawarammana, N. A Buckley // Br J Clin Pharmacol. – 2011. – Vol. 72. – № 5. – P. 745-57.
- Gao, L. Toxicology of paraquat and pharmacology of the protective effect of 5-hydroxy-1-methylhydantoin on lung injury caused by paraquat based on metabolomics / L. Gao, H. Yuan, E. Xu [et al.] // Sci Rep. – 2020. – Vol. 10. – № 1. – P. 1790.
- Zhang, F. Low dose of trichostatin A improves radiation resistance by activating Akt/Nrf2-dependent antioxidation pathway in cancer cells / F. Zhang, C. Shao, Z. Chen [et al.] // Radiat Res. – 2021. – Vol. 195. – № 4. – P. 366-367.
- Zhao, Y. Lifespan extension and elevated hsp gene expression in Drosophila caused by histone deacetylase inhibitors / Y. Zhao, H. Sun, J. Lu [et al.] // J Exp Biol. – 2005. – Vol. 208 (Pt 4). – P. 697-705.
- Guo, Y. Trichostatin A attenuates oxidative stress-mediated myocardial injury through the FoxO3a signaling pathway / Y. Guo, Z. Li, C. Shi [et al.] // Int J Mol Med. – 2017. – Vol. 40. – № 4. – P. 999-1008.
- Lee, D. Inhibitory effects of berberine on lipopolysaccharide- induced inducible nitric oxide synthase and the high-mobility group box 1 release in macrophages / D. Lee, J. Bae, Y. K. Kim [et al.] // Biochem Biophys Res Commun. – 2013. – Vol. 431. – № 3. – P. 506-11.
- Ma, X. The pathogenesis of Diabetes mellitus by oxidative stress and inflammation: Its inhibition by berberine / X. Ma, Z. Chen, L. Wang [et al] // Front Pharmacol. – 2018. – Vol. 9. – P. 782.
- Wang, S. Trichostatin A enhances radiosensitivity and radiation-induced DNA damage of esophageal cancer cells / S. Wang, M. Song, B.Zhang // J Gastrointest Oncol. – 2021. – Vol. 12. – № 5. – P. 1985-1995.
- Nagarajan, D. Trichostatin A inhibits radiation-induced epithelial-to-mesenchymal transition in the alveolar epithelial cells / D. Nagarajan, L. Wang, W. Zhao, X. Han // Oncotarget. – 2017. – Vol. 8. – № 60. – P. 101745-101759.
- Kim, J.H. Sequence-dependent radiosensitization of histone deacetylase inhibitors trichostatin A and SK-7041 / J.H. Kim, I.H. Kim, J.H. Shin [et al.] // Cancer Res Treat. – 2013. – Vol. 45. – № 4. – P. 334-42.
- Qiu, X. Evaluation of the antioxidant effects of different histone deacetylase inhibitors (HDACis) on human lens epithelial cells (HLECs) after UVB exposure / X. Qiu, X. Rong, J. Yang, Y. Lu // BMC Ophthalmol. – 2019. – Vol. 19. – № 1. – P. 42.
- Peng, P. L. Synergistic tumor-killing effect of radiation and berberine combined treatment in lung cancer: the contribution of autophagic cell death / P. L. Peng, W. H. Kuo, H. C. Tseng, F. P. Chou // Int J Radiat Oncol Biol Phys. – 2008. – Vol. 70. – № 2. – P. 529-542.
- Tower, J. Heat shock proteins and Drosophila aging / J. Tower // Exp Gerontol. – 2011. – Vol. 46. – № 5. – P. 355-62.
- Wang, M. C. JNK signaling confers tolerance to oxidative stress and extends lifespan in Drosophila / M. C. Wang, D. Bohmann, H. Jasper // Dev Cell. – 2003. – Vol. 5. – № 5. – P. 811-6.
- La, X. Berberine-induced autophagic cell death by elevating GRP78 levels in cancer cells / X. La, L. Zhang, Z. Li [et al.] // Oncotarget. – 2017. – Vol. 8. – № 13. – P. 20909-20924.
- Jiang, J. F. Mechanism underlying berberine’s effects on HSP70/TNFα under heat stress: Correlation with the TATA boxes / J. F. Jiang, F. Lei, Z. X. Yuan [et al.] // Chin J Nat Med. – 2017. – Vol. 15. – № 3. – P. 178-191.
- Jiang, J. F. Novel effect of berberine on thermoregulation in mice model induced by hot and cold environmental stimulation / J. F. Jiang, Y. G. Wang, J. Hu [et al.] // PLoS One. – 2013. – Vol. 8. – № 1. – P. e54234.
- Gao, L. Histone deacetylase inhibitor trichostatin A and autophagy inhibitor chloroquine synergistically exerts anti-tumor activity in H-ras transformed breast epithelial cells / L. Gao, X. Sun, Q. Zhang [et al.] // Mol Med Rep. – 2018. – Vol. 17. – № 3. – P. 4345-4350.
- Zhang, Y. Attenuated DNA damage repair by trichostatin A through BRCA1 suppression / Y. Zhang, T. Carr, A. Dimtchev [et al.] // Radiat Res. – 2007. – Vol. 168. – № 1. – P. 115-24.
- Campanero, M. R. The histone deacetylase inhibitor trichostatin A induces GADD45 gamma expression via Oct and NF-Y binding sites / M. R. Campanero, A. Herrero, V. Calvo // Oncogene. – 2008. – Vol. 27.–№ 9. – P. 1263-72.
- Egidi, F. Modulation of chromatin conformation by the histone deacetylase inhibitor trichostatin A promotes the removal of radiation-induced lesions in ataxia telangiectasia cell lines / F. Egidi, S. Filippi, F. Manganello [et al.] // Mutat Res Genet Toxicol Environ Mutagen. – 2018. – Vol. 836(Pt A). – P. 109-116.
- Plyusnina, E.N. Increase of Drosophila melanogaster lifespan due to D-GADD45 overexpression in the nervous system / E. N. Plyusnina, M. V. Shaposhnikov. A. A. Moskalev // Biogerontology. – 2011. – Vol. 12. – № 3. – P. 211-226.
- Shaposhnikov, M. Lifespan and stress resistance in Drosophila with overexpressed DNA repair genes / M. Shaposhnikov, E. Proshkina. L. Shilova [et al.] // Sci Rep.– 2015. – Vol. 5. – P. 15299.
- Koval, L. The role of DNA repair genes in radiation-induced adaptive response in Drosophila melanogaster is differential and conditional / L. Koval, E. Proshkina, M. Shaposhnikov, A. Moskalev // Biogerontology. – 2020. – Vol. 21. – № 1.– P. 45-56.
- Gao, L. Histone deacetylase inhibitor trichostatin A and autophagy inhibitor chloroquine synergistically exerts anti-tumor activity in H-ras transformed breast epithelial cells / L. Gao, X. Sun, Q. Zhang [et al.] // Mol Med Rep. – 2018. – Vol. 17. – № 3. – P. 4345-4350.
- Kovacs, T. Estradiol-induced epigenetically mediated mechanisms and regulation of gene expression / T. Kovacs, E. Szabo-Meleg, I. M. Abraham // Int J Mol Sci. – 2020. –Vol. 21. – № 9. – P. 3177.
- Dai, R. Epigenetic modification of Kiss1 gene expression in the AVPV is essential for female reproductive aging / R. Dai, W. Xu, W. Chen [et al.] // Biosci Trends. – 2022. – Vol. 16 – № 5. – P. 346-358.
- Roy, A. Epigenetic modifications acetylation and deacetylation play important roles in juvenile hormone action / A. Roy, S. R. Palli // BMC Genomics. – 2018. – Vol. 19. – № 1. – P. 934.


