Изучение скорости деградации материала состава полилактид/гидроксиапатит в зависимости от кристалличности структуры полимера
Автор: Стогов М.В., Киреева Е.А., Дубиненко Г.Е., Твердохлебов С.И.
Журнал: Гений ортопедии @geniy-ortopedii
Рубрика: Оригинальные статьи
Статья в выпуске: 6 т.29, 2023 года.
Бесплатный доступ
Введение. Изучение биологических характеристик биодеградируемых материалов на основе полилактида (PLLA) с включениями гидроксиапатита (НА) является важной задачей для определения показаний для их применения в клинической практике.Цель. Изучение кинетики высвобождения кальция и фосфата из PLLA в зависимости от кристалличности структуры полимера.
Имплантат, полилактид (plla), гидроксиапатит (на), кристалличность, гидролитическая деградация
Короткий адрес: https://sciup.org/142240028
IDR: 142240028 | УДК: 616.71-77-092.4: | DOI: 10.18019/1028-4427-2023-29-6-591-595
Текст научной статьи Изучение скорости деградации материала состава полилактид/гидроксиапатит в зависимости от кристалличности структуры полимера
В настоящее время медицинские изделия из полимерных биодеградируемых материалов на основе полилактида (PLLA) с включениями гидроксиапатита (НА) являются перспективными для клинической практики [1-4]. Наличие в объеме полимера гидроксиапатита обеспечивает повышение остеоиндуктив- ных и остеогенных характеристик изделий на основе PLLA [5]. При этом отмечено, что при увеличении доли НА в PLLA повышаются остеоиндуктивные свойства материала за счет большей элиминации из его состава кальция и фосфата [6]. Однако увеличение содержания НА в полимере может приводить к снижению биоме-
ханических характеристик материала. Поэтому к настоящему времени наряду с повышением остеогенных свойств материалов на основе PLLA важной требуемой характеристикой является их пористость и механическая прочность [7, 8]. В этом направлении в ряде исследований показано, что для обеспечения механической прочности и устойчивости PLLA к гидролизу может стать повышение степени его структурированности (кристалличности) [9-11]. Это, по мнению ряда авторов, может обеспечить еще больший потенциал применения структурированного PLLA как в области конструкционных, так и в области биоинженерных материалов, таких как искусственные кости и тканевые каркасы [12]. В этом плане определение взаимосвязи скорости гидролиза материалов состава PLLA/НА в зависимости от кристалличности полимера и содержания в нем HA является актуальной задачей.
Цель – изучение кинетики высвобождения кальция и фосфата из PLLA в зависимости от кристалличности структуры полимера.
МАТЕРИАЛЫ И МЕТОДЫ
Образцы изделий изготавливали методом экструзии из композита на основе PLLA с наполнением HA 25 % и 50 % массовых долей (мас. %). Для повышения кристалличности PLLA часть образцов подвергали отжигу при температуре 110 °C. Образцы имели форму цилиндра длиной 1 см и диаметром 2 мм.
Исследовали 4 вида материалов. Материалы 1 и 3 на основе кристаллического (после отжига) PLLA (PLLAc) содержали 25 % и 50 % массовых долей HA соответственно. Материалы 2 и 4 на основе аморфного (без отжига) PLLA (PLLAa) содержали 25 % и 50 % массовых долей HA соответственно. В каждой группе исследовали по 6 образцов.
Результаты исследования термических характеристик исследуемых материалов методом дифференциальной сканирующей калориметрии подтвердили увеличение кристалличности полимерной PLLA матрицы после отжига (табл. 1). Исследование проводили на приборе NETZSCH DSC 204 F1 Phoenix (NETZSCH, Германия).
Таблица 1 Значения степени кристалличности исследуемых материалов до и после отжига
|
Материал |
Степень кристалличности, % |
|
Материал 1 – PLLAc/HA25 % |
46,1 ± 3,0 |
|
Материал 2 – PLLAa/HA25 % |
34,3 ± 2,7 |
|
Материал 3 – PLLAc/HA50 % |
17,6 ± 3,2 |
|
Материал 4 – PLLAa/HA50 % |
12,4 ± 1,8 |
Для изучения гидролитической деградации выполняли эксперименты in vitro . Каждый образец помещали в отдельную измерительную ячейку, заполненную дистиллированной водой, объем которой определяли из расчета 4 мл на 1 см2 поверхности образца. Далее образцы инкубировали в термостате при температуре 37 °С. После недели инкубации среду меняли. На место старого раствора заливали новый. Гидролизат подвергали химическому анализу на наличие лактата, ионов кальция и неорганического фосфата. Скорость деградации PLLA оценивали по накоплению в гидролизате его мономера – лактата. Для оценки скорости гидролиза НА в гидролизате определяли концентрацию ионов кальция и ионов фосфата.
Длительность инкубации образцов всех материалов составила 52 недели (гидролизат на данном сроке не содержал обнаруживаемых веществ). Определение лактата, кальция и фосфат-ионов осуществляли с помощью реагентов фирмы BioSystems (Испания) на биохимическом анализаторе Hitachi 902 (Hitachi Ltd., Япония).
Для количественных параметров определяли среднюю арифметическую (М) и стандартное отклонение (SD). Достоверность межгрупповых различий оценивали с помощью Н-критерия Крускала – Уоллиса. Различия считали статистически значимыми при p < 0,05. Статистический анализ проводили с использованием надстройки AtteStat 13.1 к электронным таблицам Excel.
РЕЗУЛЬТАТЫ
Результаты исследования показали, что скорость гидролиза PLLA, находящегося в кристаллической фазе, с содержанием НА 25 мас. % (материал 1) была максимальна в течение первых двух недель инкубации, для образца PLLAc/НА 50 % (материал 3) – только на первой неделе инкубации (табл. 2). Гидролиз образцов PLLA, находящихся в преимущественно аморфном состоянии, с содержанием HA 25 мас. % (материал 2) был наиболее интенсивен в течение первых четырех недель инкубации, образца PLLAa/HA 50 % (материал 4) – в первые три недели.
Таблица 2
Скорость гидролиза PLLA на сроках инкубации тестируемых материалов (% от всего лактата, образованного в результате гидролиза)
|
Неделя инкубации |
Материал 1 PLLAc/HA25 % |
Материал 2 PLLAa/HA25 % |
Материал 3 PLLAc/HA50 % |
Материал 4 PLLAa/HA50 % |
|
1 |
47,9 ± 5,5 |
47,4 ± 6,0 |
90,2 ± 8,2 |
67,0 ± 4,7 |
|
2 |
20,9 ± 3,2 |
15,0 ± 3,3 |
3,3 ± 1,1 |
10,8 ± 3,6 |
|
3 |
7,5 ± 1,1 |
19,7 ± 4,9 |
3,0 ± 0,7 |
16,2 ± 2,0 |
|
4 |
9,5 ± 2,0 |
10,9 ± 3,0 |
3,5 ± 1,8 |
3,4 ± 0,9 |
|
5 |
7,2 ± 1,4 |
7,9 ± 2,0 |
0 |
1,3 ± 0,5 |
|
6 |
4,2 ± 0,9 |
4,7 ± 1,8 |
0 |
1,2 ± 0,3 |
|
7 |
2,7 ± 0,8 |
3,9 ± 0,6 |
0 |
0 |
|
8 |
0 |
2,2 ± 0,7 |
0 |
0 |
|
10 |
0 |
5,9 ± 1,0 |
0 |
0 |
|
11 |
0 |
0 |
0 |
0 |
|
Длительность гидролиза, недели |
7 |
10 |
4 |
6 |
Примечание : жирным шрифтом выделены значения >10 %.
Динамика высвобождения ионов кальция и ионов фосфата из тестируемых образцов приведена на рисунке 1. Обнаружено, что значительное высвобождение ионов кальция из тестируемых материалов начиналось с шестой недели инкубации для всех образцов, фосфат-ионов – с третьей недели. При этом суммарное количество высвобожденных ионов кальция и фосфат-ионов снижалось в ряду: материал 3 > материал 4 > материал 1 > материал 2. Ионы кальция в гидролизатах образцов всех материалов обнаруживались до 42 недели инкубации, фосфат-ионы – до 52-й недели.
Суммарное количество высвобожденного кальция и фосфата из исследуемых материалов представлено в таблице 3. Очевидно, что количество выделенных ионов было больше в образцах с большим содержанием НА (образцы № 3 и № 4) (p < 0,05). При этом доступ- ность данных ионов из материалов, в которых полимер был в кристаллической фазе, была выше относительно образцов, в которых PLLA находилась в преимущественно аморфной фазе (пары №№ 1-2 и №№ 3-4).
Результаты сопоставления кинетики гидролиза PLLA и НА из тестируемых материалов представлено в таблице 4. Показано, что за период распада PLLA (длительность гидролиза указана в таблице 1) высвобождалось не более 15 % от всего высвобожденного кальция и от 40,9 до 61,1 % фосфата.
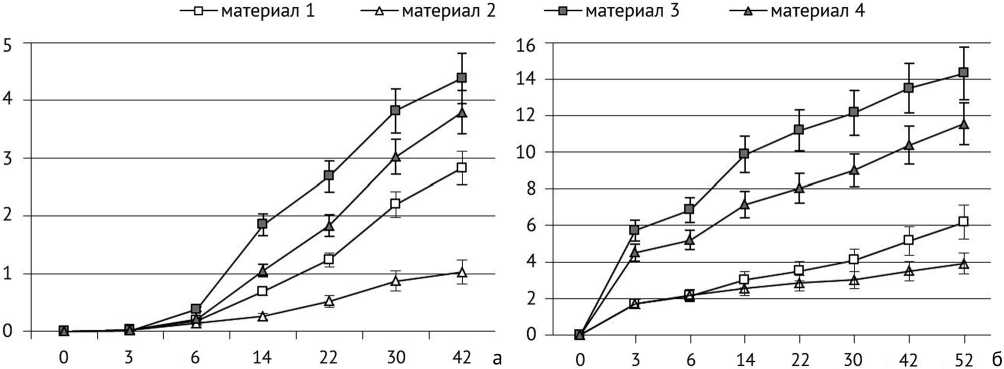
Рис. 1. График накопления: а – высвобождавшихся ионов кальция из образцов за время инкубации; б – высвобождавшихся ионов неорганического фосфата из образцов за время инкубации. По оси ОХ – недели инкубации, по оси OY – количество ионов (мкмоль/см2)
Таблица 3
Суммарное количество высвобожденного кальция и фосфата из исследуемых материалов
|
Ион |
Материал 1 |
Материал 2 |
Материал 3 |
Материал 4 |
|
Са, мкмоль/см2 |
2,83 ± 0,342,3,4 |
1,03 ± 0,211,3,4 |
4,38 ± 0,821,2 |
3,79 ± 0,461,2 |
|
Р, мкмоль/см2 |
6,18 ± 0,682,3,4 |
3,92 ± 0,231,3,4 |
14,31 ± 0,491,2,4 |
11,55 ± 1,061,2,3 |
Примечание : верхний индекс – материал, относительно которого отмечались значимые отличия при p < 0,05.
Таблица 4
Процент высвобожденного кальция и фосфата из исследуемых материалов за период полного распада PLLA
|
Ион |
Материал 1 |
Материал 2 |
Материал 3 |
Материал 4 |
|
Са, % |
11,03 ± 1,523,4 |
15,12 ± 1,813,4 |
5,46 ± 0,621,2 |
5,75 ± 0,691,2 |
|
P, % |
40,9 ± 2,82 |
61,1 ± 4,11,3,4 |
41,6 ± 3,02 |
48,2 ± 2,52 |
Примечание : верхний индекс – материал, относительно которого отмечались значимые отличия при p < 0,05.
ОБСУЖДЕНИЕ
Проведенные нами исследования показали, что степень кристалличности PLLA влияла на кинетику высвобождения НА из образцов исследуемых материалов. В частности, обнаружено, что с повышением кристалличности росла скорость гидролиза НА. Такое наблюдение можно объяснить тем, что, как показывают наши данные, полимер в кристаллической фазе подвергался гидролизу быстрее, чем в аморфной. Это и способствовало более полному гидролизу НА.
Еще одной особенностью изученной нами кинетики является то, что гидролиз PLLA и НА происходил ступенчато. Вначале гидролизовался полимер, затем НА. На это указывает то, что на момент полного гидролиза PLLA количество гидролизуемого НА состав- ляло не более 15 %. В целом такая последовательность процесса деградации PLLA и наполнителя (НА) является достаточно важной характеристикой материала для практики, т.к. подразумевает наличие лаг-периода при имплантации, связанного с гидролизом части полимера без высвобождения в окружающее пространство ионов кальция.
В целом же по результатам выполненного исследования можно говорить, что насыщение кристаллического PLLA гидроксиапатитом может являться оптимальным вариантом для создания материалов для проектирования медицинских изделий для ортопедии. При этом вопрос наиболее приемлемого содержания НА в PLLA остается открытым. С одной стороны, показана био- логическая эффективность материалов, содержащих как небольшое количество НА (до 10 мас. %), так и значительную его долю (до 80 мас. % НА) [13, 14]. С другой стороны, остеогенная активность материалов на основе PLLA повышается с увеличением содержания НА в составе полимера [6].
Наше исследование, как и исследование Zhang et al. [15], показывает, что структурированность (кристалличность) полимера наряду с содержанием НА определяет уровень деградируемости НА и, следовательно, биологические характеристики материала состава PLLA/НА. Поэтому показатель кристалличности PLLA и его насыщенность HA являются основными характеристиками материала PLLA/НА, определяющими его эффективность и показания к применению.
По результатам выполненного анализа говорить об исключительном превосходстве какого-либо из изученных нами материалов не корректно. Мы полагаем, что все изученные материалы и изделия на их основе могут быть использованы в клинической практике. Всё зависит от выбора показаний для их применения. На это указывает и клинический опыт применения изделий из материала PLLA/НА, который отмечает, что выбор биодеградируемого изделия должен осуществляться с учетом характера деградации его полимера [16].
В данном плане мы полагаем, что показаниями к применению изделий из PLLA, насыщенных HA более чем на 50 мас. %, могут стать случаи замещения крупных дефектов, когда НА в составе полимера фактически становится резервом кальция для локального образования костной ткани [17]. Кроме того, дополнительным плюсом таких изделий может стать то, что на поверхности материалов PLLA с высокой насыщенностью НА значительно снижена способность S. aureus и P. aeruginosa образовывать биопленки [18], а сам материал обладает при таком содержании НА оптимальной прочностью на сжатие.
Менее насыщенные НА материалы (< 25 мас. %), возможно, найдут своё применение для лечения небольших дефектов [19].
Очевидно, что варьирование содержания НА и степени кристалличности PLLA позволит оптимизировать и варианты применения материалов состава PLLA/HA для создания имплантатов индивидуального применения [14, 20, 21]. Перспективность такому материалу придает и то, что он совместим с другими полимерами, используемыми в ортопедии, а также может быть использован как матрица для клеточных элементов [22, 23] и лекарственных препаратов.
Отдельно нужно отметить возможные риски клинического использования материалов с высокой степенью насыщенности НА. Большая скорость деградации PLLA и HA повышает риск раннего нежелательного снижения механических свойств имплантированных изделий и риск избыточной оссификации тканей.
ЗАКЛЮЧЕНИЕ
Таким образом, проведенное исследование показало, что повышение кристалличности PLLA за счет его отжига повышает скорость гидролиза НА, включенного в PLLA матрикс. Изменения содержания НА и степени кристалличности PLLA позволяет регули- ровать биологические характеристики (механическая устойчивость, высвобождение кальция, остеогенные свойства) материалов состава PLLA/HA. Это также расширяет показания к возможному их клиническому применению.
Список литературы Изучение скорости деградации материала состава полилактид/гидроксиапатит в зависимости от кристалличности структуры полимера
- Alizadeh-Osgouei M, Li Y, Wen C. A comprehensive review of biodegradable synthetic polymer-ceramic composites and their manufacture for biomedical applications. Bioact Mater. 2018;4(1):22-36. doi: 10.1016/j.bioactmat.2018.11.003
- Bharadwaz A, Jayasuriya AC. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater Sci Eng C Mater Biol Appl. 2020;110:110698. doi: 10.1016/j.msec.2020.110698
- Fairag R, Li L, Ramirez-GarciaLuna JL, et al. A Composite Lactide-Mineral 3D-Printed Scaffold for Bone Repair and Regeneration. Front Cell Dev Biol. 2021;9:654518. doi: 10.3389/fcell.2021.654518
- Popkov AV, Popkov DA, Kononovich NA, et al. Biological activity of the implant for internal fixation. J Tissue Eng Regen Med. 2018;12(12):2248-2255. doi: 10.1002/term.2756
- Tayton E, Purcell M, Aarvold A, et al. A comparison of polymer and polymer-hydroxyapatite composite tissue engineered scaffolds for use in bone regeneration. An in vitro and in vivo study. J Biomed Mater Res A. 2014;102(8):2613-24. doi: 10.1002/jbm.a.34926
- Wozna AE, Junka A, Hoppe VW. Influence of the different composites (PLA/PLLA/HA/p-TCP) contents manufactured with the use of additive laser technology on the biocompatibility. Acta Bioeng Biomech. 2021;23(2):169-180.
- Murugan S, Parcha SR. Fabrication techniques involved in developing the composite scaffolds PCL/HA nanoparticles for bone tissue engineering applications. J Mater Sci Mater Med. 2021;32(8):93. doi: 10.1007/s10856-021-06564-0
- Ngo HX, Bai Y, Sha J, et al. A Narrative Review of u-HA/PLLA, a Bioactive Resorbable Reconstruction Material: Applications in Oral and Maxillofacial Surgery. Materials (Basel). 2021;15(1):150. doi: 10.3390/ma15010150
- Purnama P, Samsuri M, Iswaldi I. Properties Enhancement of High Molecular Weight Polylactide Using Stereocomplex Polylactide as a Nucleating Agent. Polymers (Basel). 2021;13(11):1725. doi: 10.3390/polym13111725
- Samsuri M, Iswaldi I, Purnama P. The Effect of Stereocomplex Polylactide Particles on the Stereocomplexation of High Molecular Weight Polylactide Blends. Polymers (Basel). 2021;13(12):2018. doi: 10.3390/polym13122018
- Zhao X, Liu J, Li J, et al. Strategies and techniques for improving heat resistance and mechanical performances of poly(lactic acid) (PLA) biodegradable materials. Int J Biol Macromol. 2022;218:115-134. doi: 10.1016/j.ijbiomac.2022.07.091
- He Y, Xu WH, Zhang H, Qu JP. Constructing Bone-Mimicking High-Performance Structured Poly(lactic acid) by an Elongational Flow Field and Facile Annealing Process. ACS Appl Mater Interfaces. 2020;12(11):13411-13420. doi: 10.1021/acsami.0c01528
- Bernardo MP, da Silva BCR, Hamouda AEI, et al. PLA/Hydroxyapatite scaffolds exhibit in vitro immunological inertness and promote robust osteogenic differentiation of human mesenchymal stem cells without osteogenic stimuli. Sci Rep. 2022;12(1):2333. doi: 10.1038/s41598-022-05207-w
- Pandele AM, Constantinescu A, Radu IC, et al. Synthesis and Characterization of PLA-Micro-structured Hydroxyapatite Composite Films. Materials (Basel). 2020;13(2):274. doi: 10.3390/ma13020274
- Zhang Y, Wang J, Ma Y, et al. Preparation of poly(lactic acid)/sintered hydroxyapatite composite biomaterial by supercritical CO2. Biomed Mater Eng. 2018;29(1):67-79. doi: 10.3233/BME-171713
- Kim YM, Lee JH. Clinical courses and degradation patterns of absorbable plates in facial bone fracture patients. Arch CraniofacSurg. 2019;20(5):297-303. doi: 10.7181/acfs.2019.00409
- Retegi-Carrión S, Ferrandez-Montero A, Eguiluz A, et al. The Effect of Ca2+ and Mg2+ Ions Loaded at Degradable PLA Membranes on the Proliferation and Osteoinduction of MSCs. Polymers (Basel). 2022;14(12):2422. doi: 10.3390/polym14122422
- Wozna AE, Junka AF, Szymczyk PE. The influence of different composite mixtures (PLA/HA) manufactured with additive laser technology on the ability of S. aureus and P. aeruginosa to form biofilms. Acta Bioeng Biomech. 2018;20(4):101-106.
- Zimina A, Senatov F, Choudhary R, et al. Biocompatibility and Physico-Chemical Properties of Highly Porous PLA/HA Scaffolds for Bone Reconstruction. Polymers (Basel). 2020;12(12):2938. doi: 10.3390/polym12122938
- Sakamoto A, Okamoto T, Matsuda S. Unsintered Hydroxyapatite and Poly-L-Lactide Composite Screws/Plates for Stabilizing p-Tricalcium Phosphate Bone Implants. Clin Orthop Surg. 2018;10(2):253-259. doi: 10.4055/cios.2018.10.2.253
- Wu D, Spanou A, Diez-Escudero A, Persson C. 3D-printed PLA/HA composite structures as synthetic trabecular bone: A feasibility study using fused deposition modeling. J Mech Behav Biomed Mater. 2020;103:103608. doi: 10.1016/j.jmbbm.2019.103608
- Liu Z, Chu W, Zhang L, et al. The effect of enhanced bone marrow in conjunction with 3D-printed PLA-HA in the repair of critical-sized bone defects in a rabbit model. Ann Transl Med. 2021;9(14):1134. doi: 10.21037/atm-20-8198
- Oryan A, Hassanajili S, Sahvieh S, Azarpira N. Effectiveness of mesenchymal stem cell-seeded onto the 3D polylactic acid/polycaprolactone/ hydroxyapatite scaffold on the radius bone defect in rat. Life Sci. 2020;257:118038. doi: 10.1016/j.lfs.2020.118038


