Эффективность неплатиновой монохимиотерапии в лечении платинорезистентных рецидивов рака яичников: мета-анализ
Автор: Румянцев А.А., Тюляндина А.С., Исраелян Э.Р., Покатаев И.А., Федянин М.Ю., Глазкова Е.В., Сергеев Ю.С., Тюляндин С.А.
Журнал: Злокачественные опухоли @malignanttumors
Рубрика: Собственные исследования
Статья в выпуске: 1 т.12, 2022 года.
Бесплатный доступ
Актуальность: Ключевую роль в лечении рецидивов РЯ играет правильное планирование стратегии лечения с последовательным назначением наиболее эффективных терапевтических опций. Неплатиновая химиотерапия может быть единственным вариантом лечения для пациенток с непереносимостью платины или платино-рефрактерным течением заболевания. Нами проведен мета-анализ, посвященный сравнению эффективности различных вариантов неплатиновой химиотерапии в лечении ранних рецидивов РЯ. Материалы и методы: Был произведен поиск исследований в базе данных PubMed (с 01.01.2000 по 01.07.2019 гг.). Критерии отбора включали: 1) рецидивы эпителиального рака яичников, 2) бесплатиновый интервал ≤6 мес., 3) стандартная химиотерапия без включения таргетных, экспериментальных препаратов, высокодозной химиотерапии, 4) опубликованная частота объективного ответа и критерии оценки. Первичной конечной точкой анализа была частота объективного ответа (ЧОО). Статистический анализ данных был проведен методом мета-анализа (модель случайных эффектов) с использованием пропорций и бета-регрессионного анализа с помощью программного обеспечения R и RStudio (пакеты meta и betareg). Результаты: Проанализировано 7156 исследований, для дальнейшего анализа было отобрано 157 исследований, соответствующих критериям включения, из них эффективность платиновой и неплатиновой химиотерапии была оценена в 44 (n = 1055) и 113 (n = 5272) исследованиях соответственно, в данный анализ включены работы с неплатиновой химиотерапией. По результатам мета-анализа показатель ЧОО в группе монотерапии таксанами, этопозидом, доксорубицином/ПЛД, топотеканом и гемцитабином составил 27% (95% ДИ 0,21-0,34), 19% (95% ДИ 0,13-0,27), 15% (95% ДИ 0,11-0,19), 13% (95% ДИ 0,10-0,18) и 10% (95% ДИ 0,07-0,14), соответственно. Бета-регрессионный анализ, проведенный с учетом количества линий предшествующей терапии, метода оценки эффекта и других факторов продемонстрировал схожие результаты. Выводы: Монотерапия таксанами может быть наиболее эффективной неплатиновой химиотерапевтической опцией для пациентов, не являющихся кандидатами для назначения препаратов платины.
Рак яичников, платинорезистентый рак яичников, рецидив, неплатиновая химиотерапия, мета-анализ
Короткий адрес: https://sciup.org/140293848
IDR: 140293848
Текст научной статьи Эффективность неплатиновой монохимиотерапии в лечении платинорезистентных рецидивов рака яичников: мета-анализ
Рак яичников (РЯ) ежегодно регистрируется у 13000 женщин, а более 7000 пациенток умирает от этого заболевания. Вследствие отсутствия эффективных программ ранней диагностики РЯ, биологических особенностей течения болезни, в 58% случаев РЯ выявляется на III–IV стадии, и показатели смертности остаются стабильно высокими [1].
Несмотря на последние достижения в первоначальном лекарственном лечении РЯ, главным образом, связанные с внедрением в клиническую практику ингибиторов PARP, у большинства пациенток развиваются рецидивы заболевания.
Рецидивы РЯ по-прежнему остаются неизлечимым состоянием, цели лечения пациенток включают в себя продление жизни, улучшение её качества и контроль сим- птомов болезни. Для «платинорезистентных» рецидивов РЯ существующие клинические рекомендации различных профессиональных сообществ включают в себя указания на возможность применения различных цитотоксических опций, включая таксаны (паклитаксел или доцетаксел), антрациклины, топотекан, гемцитабин и некоторые другие препараты [2,3] в самостоятельном варианте или в сочетании с антиангиогенными препаратами. В то же время данные рандомизированных исследований, посвященных сравнению эффективности многих указанных опций, отсутствуют.
Нами был проведен систематический обзор и метаанализ результатов опубликованных исследований для обеспечения возможности количественной оценки эффективности различных вариантов лечения «платинорезистентных» рецидивов рака яичников. В данной публикации проводится анализ сопоставления эффективности различных опций неплатиновой химиотерапии.
МАТЕРИАЛЫ И МЕТОДЫ
Отбор исследований и стратегия поиска
Был произведен поиск в медицинской информационной базе данных PubMed/MedLine. Критерии включали все проспективные и ретроспективные исследования, опубликованные в период с 01.01.2000 по 01.07.2019 гг. Доклинические исследования и клинические работы, не содержащие оригинальных данных, исключались. Поисковый запрос для экстракции данных включал: (ovarian* [Title] OR OVARIAN NEOPLASMS [MESH]) AND (RESISTANT OR RECURRENT OR PLATINUM-RESISTANT OR REFRACTORY OR PLATINUM-REFRACTORY). Если в опубликованном исследовании результаты применения того или иного агента были представлены в виде двух различных групп, они включались в систематический обзор и последующий мета-анализ как самостоятельные исследования. Для обеспечения прозрачности репортирования результатов систематического обзора был использован инструмент PRISMA (The Preferred Reporting Items for Systematic Reviews and Meta-Analyses).
Критерии включения
-
1. Морфологически подтвержденный диагноз эпителиального рака яичников;
-
2. Рецидивирующий платинорезистентный рак яичников (интервал ≤6 мес);
-
3. Стандартная платиносодержащей или бесплатиновая химиотерапия;
-
4. Отсутствие сопутствующей терапии таргетными препаратами, исследовательскими препаратами или высоко-дозной химиотерапии;
-
5. Наличие опубликованных данных по частоте достижения объективного ответа и критериев, использованных для оценки (RECIST [4,5], ВОЗ [6] или CA-125 [7,8]);
-
6. Наличие опубликованного полнотекстового варианта статьи.
Критерии исключения для исследований:
-
1. «Редкие» варианты РЯ как основная популяция исследования;
-
2. Нестандартные или неопределенные критерии пла-тинорезистентности;
-
3. Комбинированная неплатиновая химиотерапия («неплатиновые дуплеты»);
-
4. Проведение эндокринотерапии;
-
5. Монотерапия платиновыми препаратами;
-
6. Отсутствие данных по ЧОО или об использованных критериях оценки;
-
7. Клинические случаи, обзорные статьи, доклинические исследования.
Из отобранных исследований производилась экстракция данных об авторах и годе публикации работы, режиме терапии, использовании платиновых агентов, количестве пациенток, критериях оценки ответа, ЧОО, медиане возраста, линий ранее проведенной химиотерапии, процент пациенток с серозным раком.
Статистический анализ данных
Статистический анализ данных был проведен при помощи программного обеспечения R и RStudio с использованием дополнительных пакетов meta и betareg для проведения бета-регрессионного анализа и мета-анализа с использованием пропорций. Дизайн исследования предполагал наличие выраженной гетерогенности результатов вследствие влияния ряда факторов (количество курсов/ линий предшествующей терапии, различия в используемых и назначенных ранее платиновых и неплатиновых агентов, мутационный статус генов BRCA1/2 и другие), соответственно, была использована модель со случайными эффектами (random-effect model).
Для проведения анализа было использовано преобразование исходных данных по частоте объективного ответа на терапию, так как при оценке распределения ЧОО, выраженной в процентах, нами было отмечено значительное смещение влево в сторону 0. Преобразование исходных данных осуществлялось по следующей формуле:
logit (p) = ln(p/(1–p)), где p — исходное значение частоты объективного ответа, logit (p) — преобразованное значение, ln — логарифм. То есть, logit (p) представляет собой логарифм отношения вероятности p того, что событие произойдет, к вероятности того, что оно не произойдет. Дизайном исследования было предусмотрена проведение регрессионного анализа для уточнения факторов, влияющих на вероятность достижения объективного ответа на платиносодержащую и неплатиновую химиотерапию. С учетом природы и характера зависимой переменной, диапазоном возможных значений (0,1) было принято решение использовать бета-регрессию. При ЧОО в 0% в качестве минимального уровня ЧОО был установлен показатель в 5%.
Таблица 1. Исследования по изучению гемцитабина при платинорезистентных рецидивах рака яичников
|
Автор и год |
Серозный рак 1 |
Линии терапии2 |
N |
Оценка эффекта3 |
Объективный ответ, % |
|
Safra T, 2006 [9] |
79% |
3 |
21 |
Сочетанная |
4,8% |
|
Chanpanitkitchot S, 2014 [10] |
НД |
3 |
20 |
Сочетанная |
5,0% |
|
Kurzeder, 2016 C [11] |
77% |
1 |
25 |
Сочетанная |
0% |
|
Yoshino K, 2012 [12] |
48% |
2 |
27 |
RECIST/ВОЗ |
18,5% |
|
Ojeda Gonzales B, 2008 [13] |
56% |
1 |
41 |
Сочетанная |
22% |
|
D’Agostino G, 2003 [14] |
80% |
2 |
41 |
RECIST/ВОЗ |
17,1 % |
|
Markman M, 2003 [15] |
НД |
3 |
51 |
Сочетанная |
16% |
|
Prasad M, 2004 [16] |
НД |
3 |
56 |
Сочетанная |
17,8% |
|
Makhija S, 2010 [17] |
86% |
2 |
65 |
RECIST/ВОЗ |
5% |
|
Takei Y, 2017 [18] |
71% |
3 |
65 |
RECIST/ВОЗ |
4,6% |
|
Suprasert P, 2012 [19] |
50% |
2 |
66 |
Сочетанная |
12,1% |
|
Mutch D, 2007 [20] |
НД |
1 |
99 |
RECIST/ВОЗ |
6,1% |
Процент пациенток, у которых гистологическим подтипом опухоли была серозная аденокарцинома, без учета степени злокачественности.
Медиана линий ранее проведенной терапии.
Сочетанная оценка — использование динамики концентрации CA-125 в качестве самостоятельного метода оценки эффективности или в сочетании с различными методами визуализации; НД — нет данных.
РЕЗУЛЬТАТЫ
Суммарно за вышеуказанный период времени нами было проанализировано 7156 исследований, для дальнейшего анализа было отобрано 157 исследований, соответствующих критериям включения, из них эффективность платиновой и неплатиновой химиотерапии была оценена в 44 (n = 1055) и 113 (n = 5272) исследованиях соответственно, в данный анализ включены работы с неплатиновой химиотерапией.
Гемцитабин
Эффективность монотерапии гемцитабином была изучена в 12 исследованиях (n = 594; у 9 пациенток отсутствовали данные по оценке эффекта). Популяция для проведения данной части анализа составила 586 пациенток (табл. 1). В 75% исследований показатель медианы линий ранее проведенной терапии составила ≥2 линий. По результатам анализа показатель ЧОО составил 10% (95% ДИ 0,07–0,14) (рис. 1).
Source
D'Agoeww G . el * 2003 Mailman M at al. 2003
Prasad M el al. 2004
Saha T M al. 2008 Mulch D el* 2007
Ofeda Gonzales 8 el H. 2008 Makh»a S el al. 2010 ItiahnoK at al 2012 Srjprasart P el al 2012 CharpanoMchol S el * 2014 Kizzeder C el al. 2018 та**™* 2017 T«N(III«1 DIM) Tow (random еЛе<Ы
Proportion (ts* CD 0.17 p.07: 0 32) 0 10 p 07; 029) 010 P OO. 0 30) 0 OOP 00.024) COOP 02.0131 0 22pi1;038| 006 p01;013| 0 19)0 00 0 38) 0l2p05 0 22| 0OOP00: 025) О ОО P OO O U) 0.08 PM; 0.131 o il poo: 013) 0 10 P 07. 014)
Hrteroominly И,-1»06(Р- 0вкГ«42%
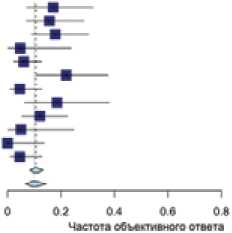
Рисунок 1. Эффективность монотерапии гемцитабином в лечении платинорезистентных рецидивов рака яичников.
Доксорубицин/пегилированный липосомальный доксорубицин
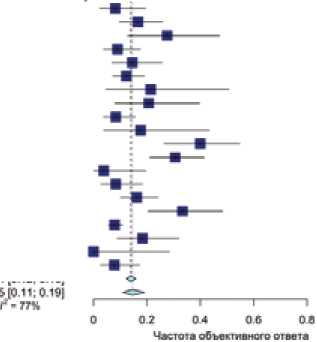
Эффективность монотерапии ПЛД/доксорубицином в лечении платинорезистентных рецидивов рака яичников была изучена в 20 клинических исследованиях (n = 1521; количество пациенток с потерей данных — 23). Краткие данные по исследованиям доксорубицина/ПЛД суммированы в табл. 2. По результатам проведенного анализа показатель ЧОО 15% (95% ДИ 0,11–0,19), данные суммированы на рис. 2.
Этопозид
Изучению эффективности монотерапии этопозидом (пероральная форма) при платинорезистентных рецидивах РЯ было посвящено 3 исследования (n = 125, без потери данных по пациенткам). Краткие данные по исследования суммированы в табл. 3. По результатам мета-анализа показатель совокупной частоты объективного ответа 19% (95% ДИ 0,13–0,27), данные суммированы на рис. 3. Вследствие небольшого количества исследований, включенных в данную часть работы, обращают на себя внимание широкие доверительные интервалы.
Топотекан
Наибольшее количество исследований, идентифицированных в рамках проведения систематического обзора и включенных в данный метаанализ, было посвящено изучению топотекана (n = 37). Суммарно в исследованиях топотекана приняло участие 1401 пациентка (данные потеряны по 71 (5%) пациентке). Использовалась как традиционная внутривенная, так и пероральная лекар-
А.А. Румянцев, А.С. Тюляндина, Э.Р. Исраелян, И.А. Покатаев, М.Ю. Федянин, Е.В. Глазкова, Ю.С. Сергеев, С.А. Тюляндин
ЭФФЕКТИВНОСТЬ НЕПЛАТИНОВОЙ МОНОХИМИОТЕРАПИИ В ЛЕЧЕНИИ ПЛАТИНОРЕЗИСТЕНТНЫХ
24 РЕЦИДИВОВ РАКА ЯИЧНИКОВ: МЕТА-АНАЛИЗ
Собственные исследования
Таблица 2. Исследования по изучению доксорубицина/пегилированного доксорубицина при платинорезистентных рецидивах РЯ
|
Автор |
Год |
Серозный рак, %1 |
Линии терапии2 |
N |
Оценка эффекта3 |
Объективный ответ, % |
|
Ichikawa R [21] |
2014 |
63 |
3 |
11 |
RECIST/ВОЗ |
0% |
|
Wilailak S [22] |
2004 |
42 |
3 |
13 |
RECIST/ВОЗ |
23% |
|
Gorumlu G [23] |
2008 |
72 |
3 |
17 |
RECIST/ВОЗ |
17% |
|
Dear R [24] |
2010 |
НД |
2 |
26 |
Сочетанная |
3,85% |
|
Chou H [25] |
2006 |
48 |
2 |
26 |
Сочетанная |
23% |
|
Markman M [26] |
2000 |
НД |
2 |
44 |
Сочетанная |
9% |
|
Strauss H [27] |
2008 |
НД |
3 |
50 |
Сочетанная |
40% |
|
Campos S [28] |
2001 |
81 |
2 |
28 |
Сочетанная |
29% |
|
Naumann R [29] |
2013 |
67 |
НД |
49 |
Сочетанная |
19% |
|
Vergote I [30] |
2010 |
67 |
1 |
60 |
RECIST/ВОЗ |
8,30% |
|
Pujade-Lauraine E [31] |
2014 |
77 |
1 |
64 |
RECIST/ВОЗ |
8% |
|
Adams S [32] |
2011 |
72 |
3 |
48 |
Сочетанная |
33% |
|
Rose P [33] |
2001 |
НД |
НД |
78 |
RECIST/ВОЗ |
8,90% |
|
Steppan I [34] |
2009 |
49 |
1 |
74 |
RECIST/ВОЗ |
35,10% |
|
Gordon A [35] |
2000 |
НД |
2 |
89 |
RECIST/ВОЗ |
16,85% |
|
Hensley M [36] |
2001 |
НД |
4 |
62 |
Сочетанная |
14,50% |
|
Mutch D [20] |
2007 |
НД |
1 |
96 |
RECIST/ВОЗ |
8,33% |
|
Monk B [37] |
2010 |
69 |
НД |
117 |
RECIST/ВОЗ |
16% |
|
Gordon A [38] |
2001 |
НД |
НД |
130 |
RECIST/ВОЗ |
12,30% |
|
Colombo N [39] |
2012 |
НД |
1 |
416 |
RECIST/ВОЗ |
8% |
Процент пациенток, у которых гистологическим подтипом опухоли была серозная аденокарцинома, без учета степени злокачественности.
Медиана линий ранее проведенной терапии.
Сочетанная оценка — использование динамики концентрации CA-125 в качестве самостоятельного метода оценки эффективности или в сочетании с различными методами визуализации; НД — нет данных.
ственная формы препарата. В 7 исследованиях данных по ранее проведенной терапии представлены не были, в остальных работах в большинстве исследований (63%) показатель медианы линий ранее проведенной терапии
MarKman M el al. 2000 Gordon A e< < 2000 Campo* S e(N. 2001 Rm* P et al. 2001 Hemley M el el. 2001 Cordon A. at M 2001 WiaMak S at el. 2004 Chou H el al . 2006 Mulch 0 et N, 2007 GoiumkiC.et al. 2008 Slrwm M et al. 2008 Sfeppan I . e< M 2009 Dear R et al, 2010 tfergotel etal, 2010 Mook В el al. 2010 Adams 8 К al. 2011 Colombo N et al. 2012 Naumann R. et ai, 2013 khkawaR el al, 2014
Total (fixed effect I Tout (random effect»)
Proportion (95% Cl) 0 08 P02. 0 20| 0 17 p 10: 0261 0 28[013:047J 0 09(0 04:0181 0 15 p 07. 0 28| 0.12 P.07.0191 0 21 p.05:051) 0 21 p.08.0 40| 0 06P04;016l 0.18 p.04.0 431 0.40 P26; 0.551 0 31 p.21:042] 0.04 p 00: 020| 0OOP03; 0 181 0 10P1O. 024] 0.33P2O.0 481 0.08 P 06;011| 0.18 P OO. 0 32| ООО poo. 0 281 2014 0 06 P.03; 0.17|
0.14 p.12; O18| 0/
Hreeroeenety rig • <5 73 (A < OOI [.
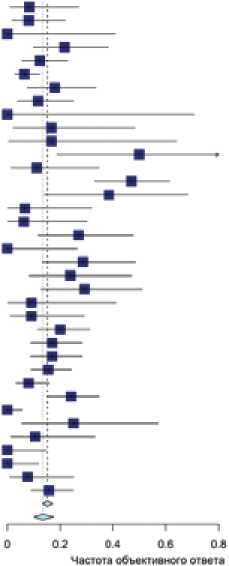
составил ≥2 линий. В табл. 4 суммированы краткие данные по исследованиям топотекана в лечении платинорезистет-ных рецидивов рака яичников. По результатам мета-анализа показатель совокупной частоты объективного ответа составил в соответствии с моделью случайных эффектов 13% (95% ДИ 0,10–0,18) (рис. 4).
Таксаны (паклитаксел и доцетаксел)
Эффективность терапии паклитакселом, наб-паклитак-селом и доцетакселом была изучена в 21, 6 и 1 исследовании, соответственно. В связи со схожим механизмом дей- source Proportion [15% Cl
AidS e«al.2OO3 0 23(0 06 0
Meleropeneity x? • 0 25 (^ • «К ? ■ 04

0 02 0.4 06 0.8
Частота объеетиемого ответа
Рисунок 2. Эффективность монотерапии доксорубицином/ пегилированным липосомальным доксорубицином в лечении платинорезистентных рецидивов рака яичников.
Рисунок 3. Эффективность монотерапии пероральным этопозидом в лечении платинорезистентных рецидивов рака яичников.
Таблица 3. Исследования по изучению этопозида при платинорезистентных рецидивах РЯ
|
Автор |
Год |
Серозный рак, %1 |
Линии терапии2 |
N |
Оценка эффекта3 |
Объективный ответ, % |
|
Alici S [40] |
2003 |
Нет данных |
2 |
22 |
Сочетанная |
22,70% |
|
Kucukoner M [41] |
2012 |
90 |
3 |
51 |
Сочетанная |
17,60% |
|
Bozkaya Y [42] |
2017 |
73 |
3 |
52 |
RECIST/ВОЗ |
19,20% |
Процент пациенток, у которых гистологическим подтипом опухоли была серозная аденокарцинома, без учета степени злокачественности.
Медиана линий ранее проведенной терапии.
Сочетанная оценка — использование динамики концентрации CA-125 в качестве самостоятельного метода оценки эффективности или в сочетании с различными методами визуализации; НД — нет данных.
ствия перечисленных препаратов они были объединены в одну группу. Суммарно терапию таксанами получили 1003 пациентки, потеря данных — по 83 (7,6%) пациенткам. Характеристики исследований перечислены в табл. 5. По результатам мета-анализа показатель ЧОО составил 27% (95% ДИ 0,21–0,34) — в соответствии с моделью случайных эффектов (рис. 5).
Многофакторный регрессионный анализ
С целью уточнения влияния дополнительных факторов, обозначенных в таблицах выше на непосредственную эффективность противоопухолевой терапии (метод оценки эффективности терапии, количество линий ранее проведенной терапии, процент пациенток с серозным гистотипом в исследованиях) нами был проведен много-
факторный бета-регрессионный анализ. Результаты суммированы в табл. 6.
Как следует из данных, представленных в таблице, в многофакторном анализе, проведенном с поправкой на факторы, перечисленные выше, монотерапия таксанами продемонстрировала наибольшую эффективность с точки зрения частоты достижения объективного ответа. Применение монотерапии топотеканом или гемцитабином, напротив, характеризовалось минимальной непосредственной эффективностью.
Source
Row. P et al 2000 Mamman M d N. 2000 Kakcfyns $ et M 2001 Rodriguez M *M 2001 CUirkn-Pearson D et al. 2001 Gorden A dN. 2001 Montazen A et al, 2002 Groniund В et al. 2007 Ыим J etal, 2003 Brown J et al, 2003 Oenachlag D el al. 2004
Anand A el al. 2004 Prasad M el al. 2004 Meched 8 et ML 2005 Pfera В et an 2005 O'Malley0 etN 2005 KafatUix 8 et al. 2005 vandeputi UM 2007 SairaT dal2007 larmier R d al. 2007 I arpiber R el al. 2007 DomraL etN. 2008
Atxiihahin F el al, 2006 Meter W et M 2009
Colcagno M et al. 2009 SehodiJ NN.2011 Sehouli J d al. 2011 Safia T NN.2013
Proportion (854 Cl) 0 08(0.01.027) 0 06(0 02.0 22) 0 00(0 00.0 41] 022(010.0381 012(005:0231 006(003:012] 018(0 08:034] 0 12(0 04:0 251 0.00(0.00; 0.71] 0.17(0.02; 0 48) 017(0 00.064| 0 30(0.10.0811 0.11(0.01.0 351 0.47 (0 33; 0.82] 038(0.14.0 681 0 07 (0 00. 0 32| 006(0 00.0 30] 027(0.12.048] 0 00(0 00.0 26) 029(0.13:0 49) 0 24(0 08.0 47] 0 29(013.051) 0 08(0 0010 41] 0 OS (0 01; 029| 020(0.11; 031] 0 17(0.09 028] 017(0 09.0281 015(0 09.024| 0 08(0.03.0.16) 0 24(0.15.0 35]
Ru^xfe-L*j«*ne 6 dal. 2014 0 00(0 00 006)
Ни С dN. 2015 BrucNml dal. 2016 KuryederC MN 2015 Poveda A *i al. 2017 Ccrteduca V et * 2018 ChakaravR etN. 2018 Total (tired effect) Total (random effects)
О 2$ |005:057] 0111001:033] 0001000:015] 000(000:0121 0 08(0 01:0 25] 016(0 00:0251 О 15(0.13; 0 17]
. ,. . ., 0.13(0.10; 0 18]
Heterogeneity x» ■ 7234 (A < 0ОЦ f ■ 80%
ОБСУЖДЕНИЕ
Современная концепция лечения рецидивов РЯ базируется на архаичном их делении на «платиночувствительные» и «платинорезистентые» в зависимости от промежутка времени, прошедшего между завершением противоопухолевой терапии и развитием рецидива заболевания [2,3]. Представляется, что это является произвольным упрощением — бесплатиновый интервал является продолжительной переменной, а «концепция шести месяцев» — бинарной, что крайне отрицательно сказывается на результатах применения этого классификационного критерия [92]. Результаты многих исследований свидетельствуют об эффективности комбинаций с включением
Zenolli K.«M 2000 KaUummNM «1.2000 tow» Y «111. 201» GfieitwKle 3 « « 2003 Markman M el al. 2003 R«e P el al. 2003 Omuras etui.2003 Omura G el al. 2003 ku T et al. 200< Baiwtoi* «»i 2004
Markman M et N. 2006 LincfiM dal 2008 Cdeman R et al. 2011
LorthelaryA dN. 2012 MeNetah I M N. 2014 Pujade-laurar* E et N. 2014
Ceieman R d al. 2014
Ptgnala S et al, 2015
Kutzedw C et al. 2018
Liu J el al 2016 Total (Awed effect) Total (random effect* |
HeWiyoety Д ■ re И Г Proportion <95% Cl) 033 10.12.0.62] 0251012.042] 0 1210 03.0.32] 0 50 10 31.0 69] 0.10 |0 02.0.27] 022 |013.0 35] 0 30)0 23:0 39] 0181012.0 26] 0 29)0 06 0 58] 0 06)001.0 21] 059)041:0 75] 0211010:0 35) 0 70)0 50:0 86] 023)017:0 38] 033)021:047] 0 42 |0 76:0 59] 031 |D 19; 0 45] 0 12)0 06:0 23] 033 |0 17; 0 53] 025 |0 11,0 45] 0 17 |0 10,0.27] 0 27)0 24,0 29] 027)0.21.0,34] Рисунок 4. Эффективность монотерапии топотеканом в лечении платинорезистентных рецидивов рака яичников. Рисунок 5. Эффективность монотерапии таксанами в лечении платинорезистентных рецидивов рака яичников. Таблица 4. Исследования по изучению топотекана при платинорезистентных рецидивах РЯ Автор Год Серозный рак, %1 Линии терапии2 N Оценка эффекта3 Объективный ответ, % Elkas J [43] 2003 НД 1 3 RECIST/ВОЗ 0% Denschlag D [44] 2004 100 2 6 RECIST/ВОЗ 16,60% Kakolyris S [45] 2001 НД 2 7 RECIST/ВОЗ 0% Levy T [46] 2004 НД 1 10 Сочетанная 52,20% Downs L [47] 2008 НД НД 11 Сочетанная 9% Vandeput I [48] 2007 НД 3 12 Сочетанная 0% Brown J [49] 2003 НД 1 12 Сочетанная 16,70% Hu C [50] 2015 69 2 12 Сочетанная 25% Mitchell S [51] 2005 НД НД 13 Сочетанная 38% Piura B [52] 2005 79 2 15 RECIST/ВОЗ 6,70% O’Malley D [53] 2005 НД 2 15 Сочетанная 7% Anand A [54] 2004 НД 1 18 Сочетанная 11% Bruchim I [55] 2016 НД 1 19 RECIST/ВОЗ 10,50% Le T [56] 2008 64 1 22 RECIST/ВОЗ 9% Karabulut B [57] 2005 НД 1 24 Сочетанная 30% Rose P [58] 2000 НД НД 23 Сочетанная 8,70% Kurzeder C [11] 2016 77 1 23 RECIST/ВОЗ 0% Safra T [59] 2007 60 1 28 Сочетанная 29% Markman M [60] 2000 НД 2 29 RECIST/ВОЗ 10% Poveda A [61] 2017 66 2 29 Сочетанная 0% Conteduca V [62] 2018 85 3 26 RECIST/ВОЗ 7,70% Montazeri A [63] 2002 НД 2 33 RECIST/ВОЗ 21% Rodriguez M [64] 2001 НД 3 36 Сочетанная 22% Gronlund B [65] 2002 НД 1 43 Сочетанная 11,60% Largillier R [66] 2007 90 4 21 Сочетанная 23,81% Largillier R [66] 2007 63 3 24 Сочетанная 29,17% Chekerov R [67] 2018 75 2 76 Сочетанная 18% Prasad M [16] 2004 НД 2 50 Сочетанная 48% Meier W [68] 2009 77 1 57 RECIST/ВОЗ 19,30% Pujade-Lauraine E [31] 2014 87 1 63 RECIST/ВОЗ 0% Calcagno M [69] 2009 НД 2 64 Сочетанная 17% Clarke-Pearson D [70] 2001 59 НД 65 RECIST/ВОЗ 12,31% Abushahin F [71] 2008 50 3 69 Сочетанная 20% Safra T [72] 2013 71 2 83 Сочетанная 24% Gordon A [38] 2001 НД НД 124 RECIST/ВОЗ 6,50% Sehouli J [73] 2011 75 НД 80 Сочетанная 18,75% Sehouli J [73] 2011 80 НД 76 Сочетанная 9,21% Elkas J [43] 2003 НД 1 3 RECIST/ВОЗ 0% Denschlag D [44] 2004 100 2 6 RECIST/ВОЗ 16,60% Процент пациенток, у которых гистологическим подтипом опухоли была серозная аденокарцинома, без учета степени злокачественности. Медиана линий ранее проведенной терапии. Сочетанная оценка — использование динамики концентрации CA-125 в качестве самостоятельного метода оценки эффективности или в сочетании с различными методами визуализации; НД — нет данных. платиновых производных при «резистентных» к платине рецидивах РЯ. Данная концепция нашла свое отражение в консенсусе ESMO-ESGO 2019 года: в соответствии с документом, при выборе терапии следует учитывать ряд других факторов, включающих токсичность ранее проведенной терапии, наличие противопоказаний к назначению платиновых агентов, необходимость контроля симптомов заболевания Таблица 5. Монотерапия таксанами в лечении платинорезистентных рецидивов РЯ Автор Год Серозный рак, %1 Линии терапии2 N Оценка эффекта3 Объективный ответ, % Kita T [74] 2004 70 1 14 Сочетанная 29,0% Zanotti K [75] 2000 70 2 15 Сочетанная 33% Niwa Y [76] 2003 83 2 20 RECIST/ВОЗ 15% Ghamande S [77] 2003 89 4 28 Сочетанная 50% Linch M [78] 2008 НД 3 27 Сочетанная 70% Kurzeder C [11] 2016 77 1 28 RECIST/ВОЗ 24% Berkenblit A [79] 2004 78 3 29 RECIST/ВОЗ 6,90% Markman M [80] 2003 НД 2 30 Сочетанная 10% Le T [81] 2006 85 2 34 Сочетанная 59,0% Katsumata N [82] 2000 63 НД 36 Сочетанная 25% McNeish I [83] 2014 81 2 35 Сочетанная 43% Pignata S [84] 2015 67 2 30 Сочетанная 35% Coleman R [85] 2011 72 1 47 RECIST/ВОЗ 23% Markman M [86] 2006 75 НД 47 RECIST/ВОЗ 21,30% Pujade-Lauraine E [31] 2014 87 2 55 RECIST/ВОЗ 30,20% Coleman R [87] 2014 НД НД 57 RECIST/ВОЗ 14,0% Lortholary A [88] 2012 76 1 41 Сочетанная 46% Rose P [89] 2003 74 1 58 RECIST/ВОЗ 22,40% Liu J [90] 2016 66 3 76 RECIST/ВОЗ 18,10% Omura G [91] 2003 60 НД 109 RECIST/ВОЗ 37% Omura G [91] 2003 63 НД 104 RECIST/ВОЗ 22% Процент пациенток, у которых гистологическим подтипом опухоли была серозная аденокарцинома, без учета степени злокачественности. Медиана линий ранее проведенной терапии. Сочетанная оценка — использование динамики концентрации CA-125 в качестве самостоятельного метода оценки эффективности или в сочетании с различными методами визуализации; НД — нет данных. и другие факторы. В то же время, значительное количество пациенток по-прежнему получает неплатиновую химиотерапию на различных этапах своего лечения, что определяет актуальность проведения данной работы. Нами был проведен анализ большого количества исследований, посвященных изучению эффективности применения различных неплатиновых агентов. По нашим данным, эта работа является наиболее крупным исследованием, в рамках которого было проведено сравнение различных опций неплатиновой химиотерапии в контексте лечения «платинорезистентных» рецидивов РЯ. Результаты работы показали, что терапия таксанами характеризуется наиболее высокими показателями непосредственной эффективности терапии, что согласуется с результатами рандомизированных исследований [31]. Напротив, монотерапия гемцитабином и топотеканом была ассоциирована с наихудшими результатами лечения и минимальной вероятностью достижения объективного ответа на проводимую терапию. Любопытно, что при проведении основной части метаанализа показатель ЧОО на фоне терапии топотеканом, гемцитабином и доксорубицином/ПЛД практически не различался и составил, в соответствии с моделью случайных эффектов, 13% (95% ДИ 0,10–0,18), 10% (95% ДИ 0,07–0,14) и 15% (95% ДИ 0,11–0,19), соответственно. В то же время, проведенный многофакторный бета-регрессионный анализ позволил выявить, что с учетом методов оценки объективного ответа и других факторов, прогнозируемая ЧОО на фоне применения таксанов, доксорубицина/ПЛД, топотекана и гемцитабина соответствует 27,8%, 19,2%, 13,2% и 9,9% соответственно. Наше исследование имеют ряд изначальных ограничений и недостатков, и в первую очередь — ретроспективный характер проводимого сравнения эффективности терапии. Отметим, что систематический характер отбора исследований, использование модели случайных эффектов и проведение многофактороного анализа снижают вероятность преднамеренных и непреднамеренных искажений полученных результатов. В то же время, необходимо отметить, что, как и при проведении любого другого непрямого сравнения, нельзя полностью исключить влияние ряда нераспознанных факторов, которые могли оказать влияние на полученные результаты. В качестве первичной конечной точки исследования была выбрана частота объективного ответа — суррогатный показатель эффективности терапии, который не всегда коррелирует с отдаленными результатами лечения. Эта конечная точка была выбрана в связи с тем, что она репор- Таблица 6. Многофакторный бета-регрессионный анализ (объективный ответ как зависимая переменная) Фактор Расчетное влияние (logit) 1 Значение p Intercept (исходная точка) –1,779 <0,001 Таксаны 0,820 <0,001 ПЛД/доксорубицин 0,333 <0,001 Топотекан –0,112 0,001 Гемцитабин –0,433 <0,001 Этопозид 0,159 0,01 Биологический ответ2 0,743 <0,001 Медиана линий терапии3 –0,0214 0,081 Серозный гистотип3 –0,0019 0,076 Phi = 20,123 (p < 0,0001); R2 (псевдо) = 0,44; вес исследований устанавливался в соответствии с количеством включенных пациенток. 1Обратное преобразование можно осуществить в соответствии с формулой exp (x)/ (exp (x) + 1), гдеx сумма факторов. 2 Оценка ответа по CA-125 или с использованием комбинированных моделей оценки эффективности терапии. 3За каждый процентный пункт/линию терапии.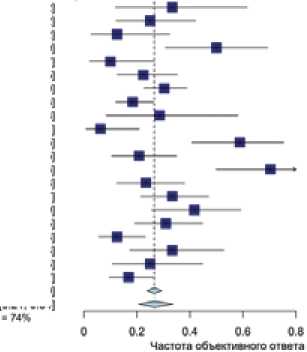
Список литературы Эффективность неплатиновой монохимиотерапии в лечении платинорезистентных рецидивов рака яичников: мета-анализ
- Каприн АД, Старинский ВВ, Шахзадова АО. Злокачественные новообразования в России в 2019 году: заболеваемость и смертность году - М.: МНИОМ им. П. А. Герцена - филиал ФГБУ «НМИЦ радиологии» Минздрава России, 2020. - илл. - 252 с. ISBN 978-5-85502-260-5.
- Тюляндина АС, Коломиец ЛА, Морхов КЮ, et al. Практические рекомендации по лекарственному лечению рака яичников, первичного рака брюшины и рака маточных труб. Злокачественные Опухоли. 2021;11 (3s2):158-171.
- Электронный ресурс: NCCN Clinical practice guidelines - Ovarian cancer, 2022. Последний доступ: 01.02.2022. Доступно онлайн на веб-странице: https://www. nccn. org/professionals/physician_gls/pdf/ovarian. pdf.
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New Guidelines to Evaluate the Response to Treatment in Solid Tumors. J Natl Cancer Inst. 2000;92 (3):12.
- Schwartz LH, Litière S, de Vries E, et al. RECIST 1.1—Update and clarification: From the RECIST committee. Eur J Cancer. 2016;62:132-137. doi:10.1016/j. ejca. 2016.03.081
- Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47 (1):207-214. doi:10.1002/1097-0142 (19810101) 47:1<207::AID-CNCR2820470134-3.0. CO;2-6
- Rustin GJS, Timmers P, Nelstrop A, et al. Comparison of CA-125 and Standard Definitions of Progression of Ovarian Cancer in the Intergroup Trial of Cisplatin and Paclitaxel Versus Cisplatin and Cyclophosphamide. J Clin Oncol. 2006;24 (1):45-51. doi:10.1200/JCO. 2005.01.2757
- Rustin GJS, Vergote I, Eisenhauer E, et al. Definitions for Response and Progression in Ovarian Cancer Clinical Trials Incorporating RECIST 1.1 and CA 125 Agreed by the Gynecological Cancer Intergroup (GCIG). Int J Gynecol Cancer. 2011;21 (2):419-423. doi:10.1097/IGC. 0b013e3182070f17
- Safra T, Ron I, Boaz M, et al. Heavily pretreated ovarian cancer patients treated by single-agent gemcitabine. A retrospective outcome comparison between platinum-sensitive and platinum-resistant patients. Acta Oncol. 2006;45 (4):463-468. doi:10.1080/02841860500509035
- Chanpanitkitchot S, Tangjitgamol S, Khunnarong J, Thavaramara T, Pataradool K, Srijaipracharoen S. Treatment Outcomes of Gemcitabine in Refractory or Recurrent Epithelial Ovarian Cancer Patients. Asian Pac J Cancer Prev. 2014;15 (13):5215-5221. doi:10.7314/APJCP. 2014.15.13.5215
- Kurzeder C, Bover I, Marmé F, et al. Double-Blind, Placebo-Controlled, Randomized Phase III Trial Evaluating Pertuzumab Combined With Chemotherapy for Low Tumor Human Epidermal Growth Factor Receptor 3 mRNA-Expressing Platinum-Resistant Ovarian Cancer (PENELOPE). J Clin Oncol. 2016;34 (21):2516-2525. doi:10.1200/JC0. 2015.66.0787
- Yoshino K, Hiramatsu K, Enomoto T, et al. Salvage Chemotherapy Using Gemcitabine for Taxane/ Platinum-resistant Recurrent Ovarian Cancer: A Single Institutional Experience. ANTICANCER Res. Published online 2012:5.
- Gonzalez BO, Martin AG, Barcelo IB, et al. A Phase II Trial of Fixed-Dosed Rate Gemcitabine in Platinum-Resistant Ovarian Cancer: A GEICO (Grupo Español de Investigación en Cáncer de Ovario) Trial. Am J Clin Oncol. 2008;31 (5):481-487. doi:10.1097/COC. 0b013e31816d1c7b
- D'Agostino G, Ferrandina G, Ludovisi M, et al. Phase II study of liposomal doxorubicin and gemcitabine in the salvage treatment of ovarian cancer. Br J Cancer. 2003;89 (7):1180-1184. doi:10.1038/sj. bjc. 6601284
- Markman M, Webster K, Zanotti K, Kulp B, Peterson G, Belinson J. Phase 2 trial of single-agent gemcitabine in plat-inum-paclitaxel refractory ovarian cancer. Gynecol Oncol. 2003;90 (3):593-596. doi:10.1016/S0090-8258 (03) 00399-8
- Prasad M, Ben-Porat L, Hoppe B, et al. Costs of treatment and outcomes associated with second-line therapy and greater for relapsed ovarian cancer. Gynecol Oncol. 2004;93 (1):223-228. doi:10.1016/j. ygyno. 2004.01.014
- Makhija S, Amler LC, Glenn D, et al. Clinical Activity of Gemcitabine Plus Pertuzumab in Platinum-Resistant Ovarian Cancer, Fallopian Tube Cancer, or Primary Peritoneal Cancer. J Clin Oncol. 2010;28 (7):1215-1223. doi:10.1200/JCO. 2009.22.3354
- Takei Y, Takahashi Y, Machida S, et al. Response to and toxicity of gemcitabine for recurrent ovarian cancer according to number of previous chemotherapy regimens: GEM and number of previous regimens. J Obstet Gynaecol Res. 2017;43 (2):358-364. doi:10.1111/jog. 13203
- Suprasert P, Cheewakriangkrai C, Manopunya M. Outcome of Single Agent Generic Gemcitabine in Platinum-Resistant Ovarian Cancer, Fallopian Tube Cancer and Primary Peritoneal Adenocarcinoma. Asian Pac J Cancer Prev. 2012;13 (2):517-520. doi:10.7314/APJCP. 2012.13.2.517
- Mutch DG, Orlando M, Goss T, et al. Randomized Phase III Trial of Gemcitabine Compared With Pegylated Liposomal Doxorubicin in Patients With Platinum-Resistant Ovarian Cancer. J Clin Oncol. 2007;25 (19):2811-2818. doi:10.1200/ JCO. 2006.09.6735
- Ichikawa R, Torii Y, Oe S, et al. Retrospective comparative study of irinotecan and pegylated liposomal doxorubicin for platinum-resistant or -refractory epithelial ovarian and primary peritoneal carcinoma. Arch Gynecol Obstet. 2014;290 (5):979-984. doi:10.1007/s00404-014-3268-7
- Wilailak S, Linasmita V. A Study of Pegylated Liposomal Doxorubicin in Platinum-Refractory Epithelial Ovarian Cancer. Oncology. 2004;67 (3-4):183-186. doi:10.1159/000081315
- Gorumlu G, Kucukzeybek Y, Kemal-Gul M, et al. Pegylated liposomal doxorubicin in heavily pretreated epithelial ovarian cancer patients.:4.
- Dear RF, Gao B, Harnett P. Recurrent ovarian cancer: Treatment with pegylated liposomal doxorubicin; a Westmead Cancer Care Centre experience. Asia Pac J Clin Oncol. 2010;6 (1):66-73. doi:10.1111/j. 1743-7563.2009.01263. x
- Chou HH, Wang KL, Chen CA, et al. Pegylated liposomal doxorubicin (Lipo-Dox®) for platinum-resistant or refractory epithelial ovarian carcinoma: A Taiwanese gynecologic oncology group study with long-term follow-up. Gynecol Oncol. 2006;101 (3):423-428. doi:10.1016/j. ygyno. 2005.10.027
- Markman M, Kennedy A, Webster K, Peterson G, Kulp B, Belinson J. Phase 2 Trial of Liposomal Doxorubicin (40 mg/m2) in Platinum/Paclitaxel-Refractory Ovarian and Fallopian Tube Cancers and Primary Carcinoma of the Peritoneum. Gynecol Oncol. 2000;78 (3):369-372. doi:10.1006/gyno. 2000.5921
- Strauss HG, Hemsen A, Karbe I, Lautenschläger C, Persing M, Thomssen C. Phase II trial of biweekly pegylated liposomal doxorubicin in recurrent platinum-refractory ovarian and peritoneal cancer: Anticancer Drugs. 2008;19 (5):541-545. doi:10.1097/CAD. 0b013e3282fcbbf7
- Campos SM, Penson RT, Mays AR, et al. The Clinical Utility of Liposomal Doxorubicin in Recurrent Ovarian Cancer. Gynecol Oncol. 2001;81 (2):206-212. doi:10.1006/gyno. 2000.5980
- Naumann RW, Coleman RL, Burger RA, et al. PRECEDENT: A Randomized Phase II Trial Comparing Vintafolide (EC145) and Pegylated Liposomal Doxorubicin (PLD) in Combination Versus PLD Alone in Patients With Platinum-Resistant Ovarian Cancer. J Clin Oncol. 2013;31 (35):4400-4406. doi:10.1200/JCO. 2013.49.7685
- Vergote I, Finkler NJ, Hall JB, et al. Randomized Phase III Study of Canfosfamide in Combination With Pegylated Liposomal Doxorubicin Compared With Pegylated Liposomal Doxorubicin Alone in Platinum-Resistant Ovarian Cancer. Int J Gynecol Cancer. 2010;20 (5):772-780. doi:10.1111/IGC. 0b013e3181daaf59
- Pujade-Lauraine E, Hilpert F, Weber B, et al. Bevacizumab Combined With Chemotherapy for Platinum-Resistant Recurrent Ovarian Cancer: The AURELIA Open-Label Randomized Phase III Trial. J Clin Oncol. 2014;32 (13):1302-1308. doi:10.1200/JCO. 2013.51.4489
- Adams SF, Marsh EB, Elmasri W, et al. A high response rate to liposomal doxorubicin is seen among women with BRCA mutations treated for recurrent epithelial ovarian cancer. Gynecol Oncol. 2011;123 (3):486-491. doi:10.1016/j. ygyno. 2011.08.032
- Rose PG, Hawthorne Maxson J, Fusco N, Mossbruger K, Rodriguez M. Liposomal Doxorubicin in Ovarian, Peritoneal, and Tubal Carcinoma: A Retrospective Comparative Study of Single-Agent Dosages. Gynecol Oncol. 2001;82 (2):323-328. doi:10.1006/gyno. 2001.6272
- Steppan I, Reimer D, Sevelda U, Ulmer H, Marth C, Zeimet AG. Treatment of Recurrent Platinum-Resistant Ovarian Cancer with Pegylated Liposomal Doxorubicin - An Evaluation of the Therapeutic Index with Special Emphasis on Cardiac Toxicity. Chemotherapy. 2009;55 (6):391-398. doi:10.1159/000262452
- Gordon AN, Granai CO, Rose PG, et al. Phase II Study of Liposomal Doxorubicin in Platinum- and Paclitaxel-Re-fractory Epithelial Ovarian Cancer. J Clin Oncol. 2000;18 (17):3093-3100. doi:10.1200/JCO. 2000.18.17.3093
- Hensley ML, Hoppe B, Leon L, et al. The Costs and Efficacy of Liposomal Doxorubicin in Platinum-Refractory Ovarian Cancer in Heavily Pretreated Patients. Gynecol Oncol. 2001;82 (3):464-469. doi:10.1006/gy no. 2001.6299
- Monk BJ, Herzog TJ, Kaye SB, et al. Trabectedin Plus Pegylated Liposomal Doxorubicin in Recurrent Ovarian Cancer. J Clin Oncol. 2010;28 (19):3107-3114. doi:10.1200/Jœ. 2009.25.4037
- Gordon AN, Fleagle JT, Guthrie D, Parkin DE, Gore ME, Lacave AJ. Recurrent Epithelial Ovarian Carcinoma: A Randomized Phase III Study of Pegylated Liposomal Doxorubicin Versus Topotecan. J Clin Oncol. 2001;19 (14):3312-3322. doi:10.1200/JCO. 2001.19.14.3312
- Colombo N, Kutarska E, Dimopoulos M, et al. Randomized, Open-Label, Phase III Study Comparing Patupilone (EPO906) With Pegylated Liposomal Doxorubicin in Platinum-Refractory or -Resistant Patients With Recurrent Epithelial Ovarian, Primary Fallopian Tube, or Primary Peritoneal Cancer. J Clin Oncol. 2012;30 (31):3841-3847. doi:10.1200/JCO. 2011.38.8082
- Alici S, Saip P, Eralp Y, Aydiner A, Topuz E. Oral Etoposide (VP16) in Platinum-Resistant Epithelial Ovarian Cancer (EOC): Am J Clin Oncol. 2003;26 (4):358-362. doi:10.1097/01. COC. 0000020590.62677. E0
- Kucukoner M, Isikdogan A, Yaman S, et al. Oral Etoposide for Platinum-Resistant and Recurrent Epithelial Ovarian Cancer: a Study by the Anatolian Society of Medical Oncology. Asian Pac J Cancer Prev. 2012;13 (8):3973-3976. doi:10.7314/APJCP. 2012.13.8.3973
- Bozkaya Y, Dogan M, Umut Erdem G, et al. Effectiveness of low-dose oral etoposide treatment in patients with recurrent and platinum-resistant epithelial ovarian cancer. J Obstet Gynaecol. 2017;37 (5):649-654. doi:10.1080/014436 15.2017.1290056
- Elkas JC, Holschneider CH, Katz B, et al. The use of continuous infusion topotecan in persistent and recurrent ovarian cancer. Int J Gynecol Cancer. 2003;13 (2):138-141. doi:10.1046/j. 1525-1438.2003.13020. x
- Denschlag D, Watermann D, Hörig K, Kissel C, Tempfer C, Gitsch G. Topotecan as a Continuous Infusion Over 14 Days in Recurrent Ovarian Cancer Patients.:3.
- Kakolyris S, Kouroussis Ch, Souglakos J, et al. A Phase I Clinical Trial of Topotecan Given Every 2 Weeks in Patients with Refractory Solid Tumors. Oncology. 2001;61 (4):265-270. doi:10.1159/000055332
- Levy T, Inbar M, Menczer J, Grisaru D, Glezerman M, Safra T. Phase II study of weekly topotecan in patients with recurrent or persistent epithelial ovarian cancer. Gynecol Oncol. 2004;95 (3):686-690. doi:10.1016/j. ygyno. 2004.09.005
- Downs LS, Judson PL, Argenta PA, et al. A prospective randomized trial of thalidomide with topotecan compared with topotecan alone in women with recurrent epithelial ovarian carcinoma. Cancer. 2008;112 (2):331-339. doi:10.1002/ cncr. 23164
- Vandenput I, Amant F, Neven P, Berteloot P, Leunen K, Vergote I. Effectiveness of weekly topotecan in patients with recurrent epithelial ovarian cancer. Int J Gynecol Cancer. 2007;17 (1):83-87. doi:10.1111/j. 1525-1438.2007.00789. x
- Brown JV, Peters WA, Rettenmaier MA, et al. Three-consecutive-day topotecan is an active regimen for recurrent epithelial ovarian cancer. Gynecol Oncol. 2003;88 (2):136-140. doi:10.1016/S0090-8258 (02) 00021-5
- Hu CF, Ou YC, Fu HC, et al. The use of weekly topotecan in the treatment of heavily pretreated recurrent epithelial ovarian and primary peritoneal cancer: The Kaohsiung Chang Gung experience. Taiwan J Obstet Gynecol. 2015;54 (1):43-47. doi:10.1016/j. tjog. 2014.11.005
- Mitchell SK, Carson LF, Judson P, Downs LS. Efficacy and tolerability of lower-dose topotecan in recurrent ovarian cancer: a retrospective case review. Int J Gynecol Cancer. 2005;15 (5):793-798. doi:10.1111/j. 1525-1438.2005.00138. x
- Piura B, Rabinovich A. Topotecan in heavily pretreated patients with recurrent ovarian, peritoneal, and fallopian tube carcinoma. Int J Gynecol Cancer. 2005;15 (4):612-617. doi:10.1111/j. 1525-1438.2005.00116. x
- O'Malley DM, Azodi M, Makkenchery A, et al. Weekly topotecan in heavily pretreated patients with recurrent epithelial ovarian carcinoma. Gynecol Oncol. 2005;98 (2):242-248. doi:10.1016/j. ygyno. 2005.04.032
- Anand A, Chan SY. The use of topotecan for relapsed ovarian cancer in accordance with the National Institute for Clinical Excellence guidance 2001: the Nottingham experience. Clin Oncol. 2004;16 (8):543-548. doi:10.1016/j. clon. 2004.07.003
- Bruchim I, Ben-Harim Z, Piura E, Haran G, Fishman A. Analysis of two topotecan treatment schedules in patients with recurrent ovarian cancer. J Chemother. 2016;28 (2):129-134. doi:10.1080/1120009X. 2015.1115195
- Le T, Hopkins L, Baines KA, Rambout L, Fung-Kee-Fung M. Prospective evaluation of weekly topotecan in recurrent platinum-resistant epithelial ovarian cancer. Int J Gynecol Cancer. 2008;18 (3):428-431. doi:10.1111/j. 1525-1438.2007.01041.
- Karabulut B, Sezgin C, Terek MC, et al. Topotecan in Platinum-Resistant Epithelial Ovarian Cancer. Chemotherapy. 2005;51 (6):347-351. doi:10.1159/000088959
- Rose PG, Gordon NH, Fusco N, et al. A Phase II and Pharmacokinetic Study of Weekly 72-h Topotecan Infusion in Patients with Platinum-Resistant and Paclitaxel-Resistant Ovarian Carcinoma. Gynecol Oncol. 2000;78 (2):228-234. doi:10.1006/gyno. 2000.5844
- Safra T, Menczer J, Bernstein R, et al. Efficacy and toxicity of weekly topotecan in recurrent epithelial ovarian and primary peritoneal cancer. Gynecol Oncol. 2007;105 (1):205-210. doi:10.1016/j. ygyno. 2006.11.017
- Markman M, Kennedy A, Webster K, Kulp B, Peterson G, Belinson J. Phase 2 Evaluation of Topotecan Administered on a 3-Day Schedule in the Treatment of Platinum- and Paclitaxel-Refractory Ovarian Cancer. Gynecol Oncol. 2000;79 (1):116-119. doi:10.1006/gyno. 2000.5902
- Poveda A, del Campo J, Ray-Coquard I, et al. Phase II randomized study of PM01183 versus topotecan in patients with platinum-resistant/ refractory advanced ovarian cancer. Ann Oncol. 2017;28 (6):1280-1287. doi:10.1093/annonc/ mdx111 | 1281
- Conteduca V, Gurioli G, Rossi L, et al. Oxaliplatin plus leucovorin and 5-fluorouracil (FOLFOX-4) as a salvage chemotherapy in heavily-pretreated platinum-resistant ovarian cancer. BMC Cancer. 2018;18 (1). doi:10.1186/s12885-018-5180-1
- Montazeri A, Culine S, Laguerre B, et al. Individual Adaptive Dosing of Topotecan in Ovarian Cancer.:7.
- Rodriguez M, Rose PG. Improved Therapeutic Index of Lower Dose Topotecan Chemotherapy in Recurrent Ovarian Cancer. Gynecol Oncol. 2001;83 (2):257-262. doi:10.1006/gyno. 2001.6365
- Gronlund B, Hansen HH, H0gdall C, Engelholm SA. Efficacy of low-dose topotecan in second-line treatment for patients with epithelial ovarian carcinoma: Low-Dose Topotecan and Ovarian Cancer. Cancer. 2002;95 (8):1656-1662. doi:10.1002/cncr. 10838
- Largillier R, Valenza B, Ferrero JM, et al. Haematological Evaluation of Weekly Therapy with Topotecan for the Treatment of Recurrent Ovarian Cancer Resistant to Platinum-Based Therapy. Oncology. 2007;73 (3-4):177-184. doi:10.1159/000127384
- Chekerov R, Hilpert F, Mahner S, et al. Sorafenib plus topotecan versus placebo plus topotecan for platinum-resistant ovarian cancer (TRIAS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2018;19 (9):1247-1258. doi:10.1016/S1470-2045 (18) 30372-3
- Meier W, du Bois A, Reuss A, et al. Topotecan versus treosulfan, an alkylating agent, in patients with epithelial ovarian cancer and relapse within 12 months following 1st-line platinum/paclitaxel chemotherapy. A prospectively randomized phase III trial by the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group (AGO-OVAR). Gynecol Oncol. 2009;114 (2):199-205. doi:10.1016/j. ygyno. 2009.04.026
- Calcagno M, Bellati F, Palaia I, et al. Three-Day Topotecan Schedule in Heavily Pretreated Recurrent Ovarian Cancer Patients. Int J Gynecol Cancer. 2009;19 (3):455-459. doi:10.1111/IGC. 0b013e3181a1a7d2
- Clarke-Pearson DL, Van Le L, Iveson T, et al. Oral Topotecan as Single-Agent Second-Line Chemotherapy in Patients With Advanced Ovarian Cancer. J Clin Oncol. 2001;19 (19):3967-3975. doi:10.1200/Jœ. 2001.19.19.3967
- Abushahin F, Singh DK, Lurain JR, Grendys EC, Rademaker AW, Schink JC. Weekly topotecan for recurrent platinum resistant ovarian cancer. Gynecol Oncol. 2008;108 (1):53-57. doi:10.1016/j. ygyno. 2007.08.062
- Safra T, Berman T, Yachnin A, et al. Weekly Topotecan for Recurrent Ovarian, Fallopian Tube and Primary Peritoneal Carcinoma: Tolerability and Efficacy Study—The Israeli Experience. Int J Gynecol Cancer. 2013;23 (3):475-480. doi:10.1097/lGC. 0b013e3182866944
- Sehouli J, Stengel D, Harter P, et al. Topotecan Weekly Versus Conventional 5-Day Schedule in Patients With Platinum-Resistant Ovarian Cancer: A Randomized Multicenter Phase II Trial of the North-Eastern German Society of Gynecological Oncology Ovarian Cancer Study Group. J Clin Oncol. 2011;29 (2):242-248. doi:10.1200/JCO. 2009.27.8911
- Kita T, Kikuchi Y, Takano M, et al. The effect of single weekly paclitaxel in heavily pretreated patients with recurrent or persistent advanced ovarian cancer. Gynecol Oncol. 2004;92 (3):813-818. doi:10.1016/j. ygyno. 2003.12.002
- Zanotti KM, Belinson JL, Kennedy AW, Webster KD, Markman M. Treatment of Relapsed Carcinoma of the Ovary with Single-Agent Paclitaxel Following Exposure to Paclitaxel and Platinum Employed as Initial Therapy. Gynecol Oncol. 2000;79 (2):211-215. doi:10.1006/gyno. 2000.5958
- Niwa Y, Nakanishi T, Kuzuya K, Nawa A, Mizutani S. Salvage treatment with docetaxel for recurrent epithelial ovarian cancer. Int J Clin Oncol. 2003;8 (6):343-347. doi:10.1007/s10147-003-350-8
- Ghamande S, Lele S, Marchetti D, Baker T, Odunsi K. Weekly paclitaxel in patients with recurrent or persistent advanced ovarian cancer. Int J Gynecol Cancer. 2003;13 (2):142-147. doi:10.1046/j. 1525-1438.2003.13045. x
- Linch M, Stavridi F, Hook J, Barbachano Y, Gore M, Kaye SB. Experience in a UK cancer centre of weekly paclitaxel in the treatment of relapsed ovarian and primary peritoneal cancer. Gynecol Oncol. 2008;109 (1):27-32. doi:10.1016/j. ygyno. 2008.01.007
- Berkenblit A, Seiden MV, Matulonis UA, et al. A phase II trial of weekly docetaxel in patients with platinum-resistant epithelial ovarian, primary peritoneal serous cancer, or fallopian tube cancer. Gynecol Oncol. 2004;95 (3):624-631. doi:10.1016/j. ygyno. 2004.08.028
- Markman M, Zanotti K, Webster K, Peterson G, Kulp B, Belinson J. Phase 2 trial of single agent docetaxel in platinum and paclitaxel-refractory ovarian cancer, fallopian tube cancer, and primary carcinoma of the peritoneum. Gynecol Oncol. 2003;91 (3):573-576. doi:10.1016/j. ygyno. 2003.08.001
- Le T, Hopkins L, Baines KA, Rambout L, Al Hayki M, Kee Fung MF. Prospective evaluations of continuous weekly paclitaxel regimen in recurrent platinum-resistant epithelial ovarian cancer. Gynecol Oncol. 2006;102 (1):49-53. doi:10.1016/j. ygyno. 2005.11.025
- Katsumata N, Tsunematsu R, Tanaka K, et al. A phase II trial of docetaxel in platinum pre-treated patients with advanced epithelial ovarian cancer: A Japanese Cooperative Study. Ann Oncol. 2000;11 (12):1531-1536. doi:10.1023/A:1008337103708
- McNeish IA, Ledermann JA, Webber L, et al. A randomised, placebo-controlled trial of weekly paclitaxel and sara-catinib (AZD0530) in platinum-resistant ovarian, fallopian tube or primary peritoneal cancer"1". Ann Oncol. 2014;25 (10):1988-1995. doi:10.1093/annonc/mdu363
- Pignata S, Lorusso D, Scambia G, et al. Pazopanib plus weekly paclitaxel versus weekly paclitaxel alone for platinum-resistant or platinum-refractory advanced ovarian cancer (MITO 11): a randomised, open-label, phase 2 trial. Lancet Oncol. 2015;16 (5):561-568. doi:10.1016/S1470-2045 (15) 70115-4
- Coleman RL, Brady WE, McMeekin DS, et al. A phase II evaluation of nanoparticle, albumin-bound (nab) paclitaxel in the treatment of recurrent or persistent platinum-resistant ovarian, fallopian tube, or primary peritoneal cancer: A Gynecologic Oncology Group Study. Gynecol Oncol. 2011;122 (1):111-115. doi:10.1016/j. ygyno. 2011.03.036
- Markman M, Blessing J, Rubin SC, Connor J, Hanjani P, Waggoner S. Phase II trial of weekly paclitaxel (80 mg/m2) in platinum and paclitaxel-resistant ovarian and primary peritoneal cancers: A Gynecologic Oncology Group study. Gynecol Oncol. 2006;101 (3):436-440. doi:10.1016/j. ygyno. 2005.10.036
- Coleman RL, Moon J, Sood AK, et al. Randomised phase II study of docetaxel plus vandetanib versus docetaxel followed by vandetanib in patients with persistent or recurrent epithelial ovarian, fallopian tube or primary peritoneal carcinoma: SWOG S0904. Eur J Cancer. 2014;50 (9):1638-1648. doi:10.1016/j. ejca. 2014.03.005
- Lortholary A, Largillier R, Weber B, et al. Weekly paclitaxel as a single agent or in combination with carboplatin or weekly topotecan in patients with resistant ovarian cancer: the CARTAXHY randomized phase II trial from Groupe d'Investigateurs Nationaux pour l'Etude des Cancers Ovariens (GINECO). Ann Oncol. 2012;23 (2):346-352. doi:10.1093/ annonc/mdr149
- Rose PG, Blessing JA, Ball HG, et al. A phase II study of docetaxel in paclitaxel-resistant ovarian and peritoneal carcinoma: a gynecologic oncology group study. Gynecol Oncol. 2003;88 (2):130-135. doi:10.1016/S0090-8258 (02) 00091-4
- Liu JF, Ray-Coquard I, Selle F, et al. Randomized Phase II Trial of Seribantumab in Combination With Paclitaxel in Patients With Advanced Platinum-Resistant or -Refractory Ovarian Cancer. J Clin Oncol. 2016;34 (36):4345-4353. doi:10.1200/JCO. 2016.67.1891
- Omura GA, Brady MF, Look KY, et al. Phase III Trial of Paclitaxel at Two Dose Levels, the Higher Dose Accompanied by Filgrastim at Two Dose Levels in Platinum-Pretreated Epithelial Ovarian Cancer: An Intergroup Study. J Clin Oncol. 2003;21 (15):2843-2848. doi:10.1200/JCO. 2003.10.082
- Rumyantsev A, Tyulyandina A, Fedyanin M, et al. Efficacy of platinum-based chemotherapy in platinum-resistant ovarian cancer: A systematic review with pooled analysis of outcomes. J Clin Oncol. 2020;38 (15_suppl): e18076-e18076. doi:10.1200/Jœ. 2020.38.15_suppl. e18076
- Rose PG, Tian C, Bookman MA. Assessment of tumor response as a surrogate endpoint of survival in recurrent/ platinum-resistant ovarian carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol. 2010;117 (2):324-329. doi:10.1016/j. ygyno. 2010.01.040
- Siddiqui MK, Tyczynski J, Pahwa A, Fernandes AW. Objective response rate is a possible surrogate endpoint for survival in patients with advanced, recurrent ovarian cancer. Gynecol Oncol. 2017;146 (1):44-51. doi:10.1016/j. ygyno. 2017.03.515
- Blackledge G, Lawton F, Redman C, Kelly K. Response of patients in phase II studies of chemotherapy in ovarian cancer: implications for patient treatment and the design of phase II trials. Br J Cancer. 1989;59 (4):650-653. doi:10.1038/ bjc. 1989.132
- Alsop K, Fereday S, Meldrum C, et al. BRCA Mutation Frequency and Patterns of Treatment Response in BRCA Mutation-Positive Women With Ovarian Cancer: A Report From the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012;30 (21):2654-2663. doi:10.1200/JCO. 2011.39.8545
- Hanker LC, Loibl S, Burchardi N, et al. The impact of second to sixth line therapy on survival of relapsed ovarian cancer after primary taxane/platinum-based therapy. Ann Oncol. 2012;23(10):2605-2612. doi:10.1093/annonc/mds203


