Эндокринные дизрапторы в канцерогенном фоне биосферы
Автор: Белицкий Г.А., Кирсанов К.И., Лесовая Е.А., Жидкова Е.М., Хитрово И.А., Якубовская М.Г.
Журнал: Сибирский онкологический журнал @siboncoj
Рубрика: Обзоры
Статья в выпуске: 5 т.22, 2023 года.
Бесплатный доступ
Цель исследования - представить современную концепцию по влиянию эндокринных дизрапторов, вносящих существенный вклад в общий уровень загрязнения биосферы антропогенными ксенобиотиками, на процесс канцерогенеза. материал и методы. При подготовке обзора были проанализированы статьи по изучению эффектов эндокринных дизрапторов, имеющиеся в информационных базах биомедицинской литературы SciVerse Scopus, PubMed, Web of Science, РИНЦ. В обзоре процитированы 65 современных публикаций, из которых 21 работа была опубликована в течение трех последних лет, 3 статьи представляют собой официальные документы по рискам, сопряженным с использованием эндокринных дизрапторов, а 10 статей относятся к публикациям, создающим предпосылки для выделения данных соединений в отдельную функциональную группу.
Эндокринные дизрапторы, промоторы, канцерогенез, гормоны, эстрогены, андрогены, пестициды, молочная железа, эпигенетика, предстательная железа, легкие
Короткий адрес: https://sciup.org/140303537
IDR: 140303537 | УДК: 577.17:574.2:615.277.4 | DOI: 10.21294/1814-4861-2023-22-5-145-160
Текст научной статьи Эндокринные дизрапторы в канцерогенном фоне биосферы
Понятия «промоторы канцерогенеза» и «негенотоксические канцерогены» возникли более 80 лет назад после того, как выяснилось, что некоторые немутагенные соединения повышают частоту возникновения злокачественных опухолей. Под эти частично перекрывающиеся понятия подходят ксенобиотики, нарушающие гормональный гомеостаз организма и обозначаемые как эндокринные дизрапторы (ЭД). Изучение таких соединений началось с выявления неблагоприятных эффектов диэтилстильбэстрола в 1971 г. [1]. Несмотря на введение определенных регламентирующих норм, ограничивающих использование ЭД, экономический ущерб, связанный с неблагоприятными последствиями их воздействия на здоровье людей, составляет в Евросоюзе 217 млрд долларов, в США – 340 млрд долларов [2]. К ЭД относят компоненты промышленных растворителей и смазочных материалов (полихлорированные бифенилы, полибромированные дифенилы и диоксины), пластмасс (бисфенол А), пластификаторов (фталаты), некоторые пестициды (метоксихлор, хлорпирифос, дихлордифенилтрихлорэтан) и фармацевтические средства (диэтилстильбэстрол). Неблагоприятные последствия воздействий ЭД включают репродуктивную дисфункцию, врожденные дефекты, ожирение, сахарный диабет, сердечно-легочные заболевания, нейроповеденческие дисфункции и снижение когнитивных способностей. Кроме того, с экспозицией ЭД связывают рост заболеваемости злокачественными новообразованиями гормон-зависимых органов. По прогнозу Американского противоракового сообщества к 2040 г. заболеваемость раком молочной железы в мире составит
28,4 млн случаев, что примерно на 47 % превысит показатели 2020 г. Заболеваемость раком предстательной железы (РПЖ) в мире занимает 3-е место после рака молочной железы (РМЖ) и легкого и составила в 2020 г. 1 414 259 новых случаев [3, 4]. Затраты на лечение этих заболеваний в 2015 г. составили в странах Евросоюза 163 млрд евро, в США – 340 млрд долларов [5, 6].
В этиологии этих новообразований играют роль как наследуемые генетические особенности, так и факторы окружающей среды и компоненты питания. Анализ онкологической заболеваемости 44 788 пар близнецов из Швеции, Дании и Финляндии показал, что вклад генетических особенностей не является решающим. Его доля в возникновении РПЖ составила 42 %, РМЖ – 27 %, рака толстой кишки – 35 %. Остальной риск в большей степени связывали с влиянием внешних факторов и в первую очередь с растущим загрязнением биосферы канцерогенными ксенобиотиками [7, 8].
В обзоре представлены данные о наиболее распространенных ЭД и освещены основные механизмы их влияния на процессы злокачественной трансформации клеток и промоции канцерогенеза.
Эндокринные дизрапторы биосферы
Основную массу ЭД составляют антропогенные ксенобиотики, используемые в качестве растворителей, пластификаторов, антипиренов, фунгицидов, пестицидов и фармацевтических препаратов. Такими же свойствами обладают и некоторые природные соединения. Их структура и химические свойства крайне разнообразны и отличны от натуральных лигандов эндокринных рецепторов [9–11] (рис. 1).
Эндокринные дизрапторы используются в различных сферах жизнедеятельности, и к настоящему времени к ним относят более 200 химических соединений. Наиболее распространенными ЭД являются бисфенолы, фталаты, парабены, диоксины, алкилфенолы, оловосодержащие органические соединения, полихлорированные бифенилы, перфто-ралканы, бензофеноны и природные соединения, такие как фитоэстрогены генистеин и ресвератрол, микоэстроген α-зераланол (табл. 1). К возможным эндрокринным дизраптором относят и алкалоид синефин, широко используемый в качестве жиросжигающей биологически активной добавки и обладающий потенциальными антиэстрогенными свойствами (рис. 1).
Существенно, что один и тот же ЭД может обладать тропностью к нескольким ядерным рецепторам. Например, оловоорганические соединения, типа трибутилтина, широко используемые в промышленности и сельском хозяйстве, ковалентно связываются с ретиноидным X рецептором и рецепторами активации пролиферации пероксисом, а синтетический нестероидный микоэстроген α-зеараланол, используемый в качестве стимулятора роста домашнего скота, обладает таким же сродством к ядерным рецепторам эстрогенов, как и эстрадиол, и, следовательно, может конкурировать с ним или/и воспроизводить его эффект у человека, если в мясе сохраняются его остатки [9]. р-Синефрин, агонист β-адреновых рецепторов, обладает антиэстрогенным потенциалом [11], а также косвенно может влиять на функциональную активность глюкокортикоидного рецептора, о чем свидетельствует анализ молекулярных эффектов других, структурно схожих с синефрином агонистов β-адреновых рецепторов [12].
С рецептором прегнана X (PXR), который влияет на биосинтез, распределение и метаболизм стероидов, желчных кислот и ксенобиотиков, связывается большое количество структурно разнообразных соединений с широким диапазоном значений коэффициента сродства (Kd от 0,1 до 100 мМ). Его лигандами являются пестициды, фенолы, фитоэстрогены, косметические и лекарственные
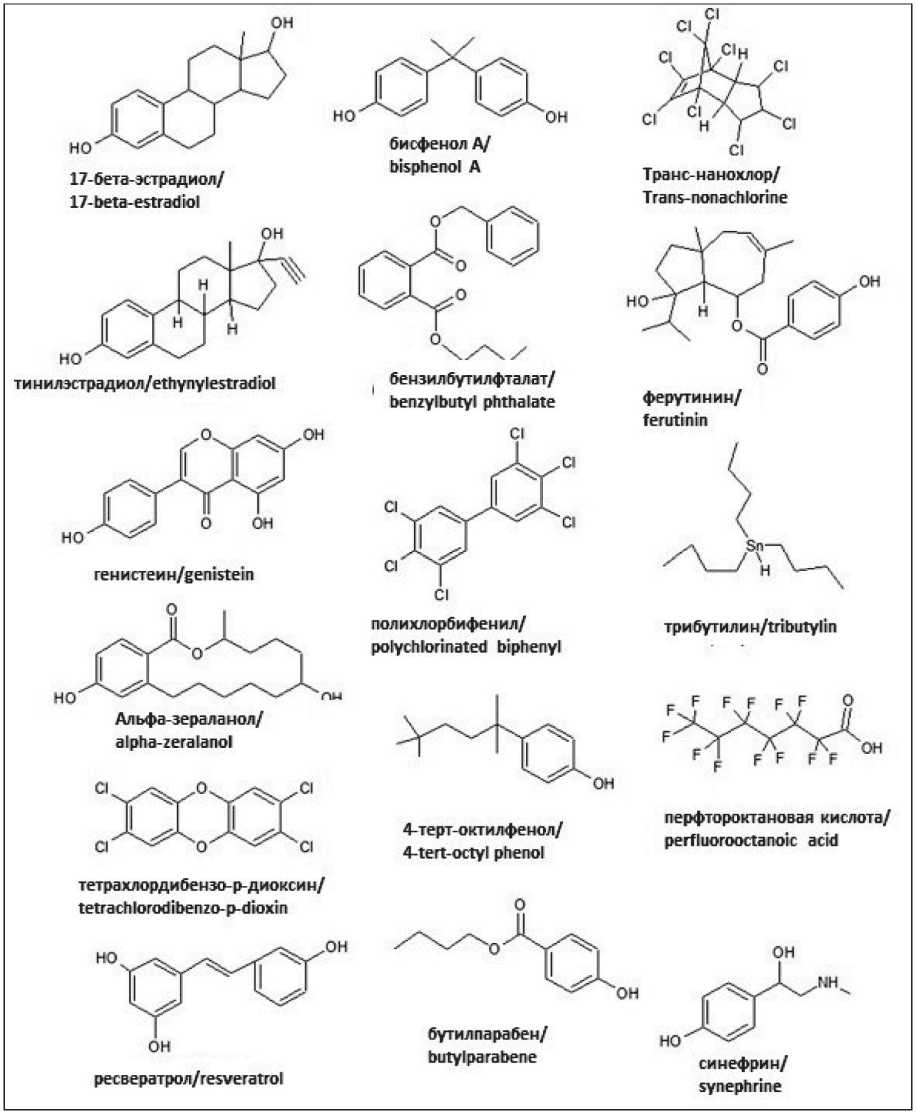
Рис. 1. Структуры эндокринных дизрапторов в сравнении с 17 β-эстрадиолом. Примечание: рисунок выполнен авторами
Fig. 1. Structures of the endocrine disruptors in comparison with 17 β-estradiol.
Note: created by the authors
Таблица 1/Table 1
Наиболее распространенные эндокринные дизрапторы, их использование и производство The most widespread endocrine disruptors, their use and manufacturing
|
Соединение/ Compound |
Группа/Group |
Использование/Use |
Объем производства/ The amount of manufacturing |
|
Бисфенол А/ Bisphenol A |
Органическое фенольное соединение/ Organic phenolic compound |
Компонент широкого спектра поликар-бонатных пластиков и эпоксидных смол, проявителя термобумаги, красок, покрытия банок для пищевых продуктов и напитков, упаковок пищевых продуктов, зубных герметиков и других стоматологических материалов/ A component of a wide range of polycarbonate plastics and epoxy resins, developer of thermal paper, paints, coating of cans for food and beverages, food packaging, dental sealants and other dental materials. |
Более 10 млн тонн ежегодно/ More than 10 million tons annually |
|
Диоктилфталат (диэ-тилгексилфтолат)/ Dioctyl phthalate (diethylhexyl phthalate) |
Пластификатор/ Plasticizer |
Компонент косметических средств (гелей, шампуней), в 2015 г. запрещен в Евросоюзе в производстве детских товаров/ A component of cosmetics (gels, shampoos), in 2015, was banned in the European Union in the production of children's goods |
До 1980 г. ежегодное производство только в Европе более 1 млн тонн, в настоящее время около 100 тонн производится ежегодно, производство и использование регламентируется/ Until 1980, the annual production in Europe alone was more than 1 million tons, currently about 100 tons are produced annually, production and use are regulated |
|
Метоксиацетат/ Methoxyacetate |
Метаболит растворителя метоксиэтанола/ Metabolite of the solvent methoxyethanol |
Метоксиэтанол используют в производстве полупроводников и красок/ Methoxyethanol is used in the production of semiconductors and dyes |
Использование метоксиэтанола в косметической продукции запрещено СанПиН 1.2.676, т. к. при его метаболизме образуется метоксиацетат (МАА)/ The use of methoxyethanol in cosmetic products is prohibited by SanPiN 1.2.676, because its metabolism produces methoxyacetate (MAA). |
|
Перхлораты/ Perchlorates |
Соли, сложные эфиры хлорной кислоты (HClO4)/ Salts, esters of perchloric acid (HClO4) |
Загрязнение хлорсодержащих отбеливателей, компонент ракетного топлива/ Contamination of chlorine-containing bleach, a component of rocket fuel |
Производится 1 млн тонн, есть способы очистки питьевой воды от контаминации этим ЭД/ 1 million tons are produced, there are ways to purify drinking water from contamination with this ED |
Окончание таблицы 1/End ofTable 1
|
Полихлорированные бифенилы/ Polychlorinated biphenyls |
Органические соединения хлора с формулой C12H10-xClx / Organic chlorine compounds with the formula C12H10-xClx |
Изготовление копировальной бумаги, в производстве диэлектриков и охлаждающих жидкостей для электрооборудования/ Production of carbon paper, in the production of dielectrics and coolants for electrical equipment |
Было произведено более 1,2 млн тонн, с 1979 г. производство запрещено, но загрязнение остается из-за стабильности соединений/ More than 1.2 million tons were produced, production has been banned since 1979, but pollution remains due to the stability of the compounds |
|
Диэтилстильбэстрол/ Diethylstilbestrol |
Лиганд ЭР/ ER ligand |
Фармацевтический препарат/ Pharmaceutical preparation |
Используют в терапии РПЖ /Prostate cancer therapy |
|
Алкалоид синефин/ Alkaloid synephrine |
Фитоэстроген/ Phytoestrogen |
Компонент биологически активных добавок/ Biologically active supplement |
Производился в качестве противо-астматического, гипотензивного и противошокового препарата/ It was produced as an anti-asthmatic, antihypertensive and anti-shock drug |
Примечание: таблица составлена авторами.
Note: created by the authors.
Дихлордифенилтри-хлорэтан (ДДТ)/ Dichlorodiphenyltrichloroethane (DDT)
Инсектицид/ Insecticide
Дихлордифенилтри-хлорэтилен/ Dichlorodiphenyltrichloroethylene
Метаболит инсектицида ДДТ/ Metabolite of the insecticide DDT
Использовали в качестве инсектицида, до 1972 г. применяли более 800 тыс. тонн в год, объем суммарного потребления в США превысил 600 тыс. тонн. Химически стабильное соединение, накапливается в окружающей среде/
They were used as an insecticide, until 1972 they used more than 800 thousand tons per year, the volume of total consumption in the USA exceeded 600 thousand tons. Chemically stable compound, accumulates in the environment
В настоящее время производится в Китае, Корейской народнодемократической республике и Индии (около 5 тыс. тонн ежегодно) как средство борьбы с вспышками малярии и лейшманелеза/ Currently produced in China, the Democratic People's Republic of Korea and India (about 5 thousand tons annually) as a means of combating outbreaks of malaria and leishmanellosis
Производится в целях контроля и исследований/
Produced for the purposes of control and research
препараты и т.д. В связи с таким структурным и функциональным разнообразием предсказать по строению молекулы наличие свойств ЭД у вновь создаваемых химических соединений затруднительно. В то же время нераспознанные ЭД уже в наномолярных концентрациях могут вызывать широкий спектр повреждений в эндокринной системе, поскольку зависимость их действия от дозы может носить нелинейный характер. Это особенно важно иметь в виду, поскольку их эффекты могут проявляться в диапазоне концентраций, соответствующих нормальному уровню эндогенных гормонов в организме человека [13, 14].
Влияние эндокринных дизрапторов на систему гормональной регуляции процессов жизнедеятельности клеток у млекопитающих
Эндокринная система наряду с нервной играет основную роль в становлении и интеграции всех тканей организма, она определяет его пренатальное и постнатальное развитие, половые различия, фертильность и многие другие свойства и функции. Дисбаланс ее гормонов влечет за собой тяжелые последствия, в том числе канцерогенез во многих органах. Эндокринные дизрапторы вызывают изменения эндокринной регуляции организма на всех его уровнях – от центральных до периферических – и в первую очередь воздействуют на гипоталамо-гипофизарную систему. Выработка гипоталамусом полипептидных гормонов гонадотропина, кортикотропина, соматотропина и тиреотропина стимулирует синтез гипофизом гормонов, регулирующих секрецию периферических эндокринных желез, в частности половых. Такая организация системы предполагает в случае повреждения одного из ее звеньев эффект домино для всех эфферентных, а затем и афферентных компонентов по механизму отрицательной обратной связи.
Помимо желез внутренней секреции, гомеостаз тканей регулирует диффузная эндокринная система, элементы которой присутствуют практически во всех тканях в виде гормонпродуцирующих клеток. Образование стероидных гормонов в периферических тканях-мишенях обозначают как интракринный синтез. Этот автономный процесс особенно выражен в клетках таких новообразований, как рак предстательной и молочной желез. В этих случаях основными стероидными предшественниками андрогенов и эстрогенов являются полифункциональный стероидный гормон надпочечников дегидроэпиандростерон (ДГЭА) и его сульфат ДГЭА-SO4 [15–19]. Рассматривая эффекты ЭД в различных органах и тканях, следует четко представлять, что они складываются из действия этих агентов на центральную нейроэндокринную и периферическую интракринную системы.
Установленные факты влияния ЭД на риск развития злокачественных новообразований
Влияния ЭД на риск развития рака молочной железы
Из широкого круга ЭД, загрязняющих биосферу, следует выделить пестициды, как наиболее распространенные и связанные с потребностями человека. Эти соединения, наряду с бисфенолом А (БФA), наиболее изучены в качестве факторов, повышающих риск РМЖ [20]. Показано, что этот риск был в 5 раз выше у женщин, которые в 1940-е гг., когда ДДТ считался безопасным пестицидом, работали с ним в возрасте до полового созревания. Впоследствии опухоли возникали у них в пременопаузальном периоде, когда болезнь протекает наиболее злокачественно. Работа с этим ЭД у взрослых женщин также увеличивала частоту РМЖ, т.е. клетки молочной железы чувствительны к действию ДДТ в любом возрасте [21, 22].
Особую чувствительность к повреждающему действию ЭД проявляют эндокринные органы плодов и новорожденных. Помимо канцерогенного действия при этом может изменяться половая диморфная организация гипоталамуса с последующим нарушением репродуктивной функции у взрослых [23–25].
Помимо пестицидов одним из наиболее активных ЭД является БФА. Пренатальная обработка крыс этим соединением вызывает изменения и в центральных эндокринных органах, в частности, в гиппокампе и гипоталамусе она изменяет экспрессию нескольких генов: ( DNMT1 , DNMT3A , DNMT3B , ESR1 , ESR2 , AVP , AR , OXT , OTR и BDNF ), причем в случае BDNF , кодирующего нейротрофин, DNMT3B , гене метилтрансфераз, и ESR1 , гене эстрогенового рецептора ERα, это происходит путем изменения метилирования ДНК в 5’-промоторных областях. Обработка новорожденных животных также значительно снижала в их гипоталамусе экспрессию эстрогеновых рецепторов ERα, ERβ и белка киссептина (Kiss1), который играет важную роль в секреции гонадотропин-рилизинг гормона в период полового созревания.
Пренатальное и раннее постнатальное воздействие БФА на крыс также вызывало у потомков ранние неопластические изменения в молочных железах и аденокарциномы в возрасте после 3 мес. При этом содержание неконъюгированного БФА в крови самок, плодов и детенышей было на том же уровне, что обнаруживается и у человека. Культивирование клеток эпителия молочной железы человека HME1 и MCF7 в течение 2 мес в присутствии наномолярных концентраций БФA, 4-трет-октилфенола и гексабромциклододекана вызвало активацию маркеров повреждения ДНК – pH2A.X, pCHK1, pCHK2, p-P53 и эпигенетические изменения в виде метилирования онкосупрессоров TIMP3, CHFR, ESR1, IGSF4, CDH13 и GSTP1. Кроме того, данные агенты индуцировали фосфорилирование киназ канцерогенного сигналинга EGFR, CREB, CTE6, C-Jun, Stat3, HSP6, HSP27, AMPKα1, FAK, P53, GSK-3α/β и P70S6 в HME1. В результате клетки приобрели способность формировать колонии в мягком агаре и прорастать в коллаген, что характерно для злокачественной трансформации [26, 27].
Влияния ЭД на риск развития рака предстательной железы
Заболеваемость раком предстательной железы также коррелирует с повышенной экспозицией к ЭД. Эпидемиологические исследования демонстрируют более высокий риск РПЖ у занятых в сельском хозяйстве и контактирующих с пестицидами, чем среди населения в целом. Особенную опасность представляют хлорорганические пестициды, такие как упоминавшийся ДДТ, лордан, диэлдрин и гептахлор, которые кумулируются в жировой ткани. В частности, есть данные о том, что в сыворотке больных РПЖ находили больше β- и γ-гексахлоргексана и ДДТ, чем у здоровых [28, 29]. В эксперименте у самцов, подвергшихся в период эмбрионального развития воздействию таких ЭД, как фунгицид винклозолин, диоксин, ДДТ, сумма углеводородов ракетного топлива или смесь пестицида с репеллентом (перметрин плюс N, N-диэтилметатолуамид), происходят наследуемые изменения эпигенома, которые и в третьем поколении приводят к аномалиям семенников, дефектам сперматогенеза, аномалиям строения предстательной железы и почек. У самок в данном эксперименте по наследству передавался поликистоз яичников [30, 31].
Помимо пестицидов в повышении заболеваемости РПЖ играет роль также и БФА. Косвенно на это указывает то, что в моче у таких больных в ряде случаев обнаруживается более высокое содержание этого дизраптора по сравнению со здоровым контролем [32–34].
Прямое и опосредованное канцерогенное действие БФА на клетки предстательной железы многогранно [29, 31]. Оно вызывает аномальные посттранскрипционные и посттрансляционные эпигенетические модификации в генах, относящихся к пропролиферативным сигнальным путям модификации хроматина, метилирования промоторов генов-супрессоров и модификации гистонов. Кроме того, он усиливает фосфорилирование AKT и ERK в стволовых клетках, экспрессирующих эстрогеновый рецептор и связанный с G-белком рецептор GPR30. БФA повышает у стареющих самцов уровень эстрадиола, стимулирующего канцерогенез в предстательной железе, а также нарушает кальциевую сигнализацию, в результате активируются миграция клеток и метастазирование.
Влияния ЭД на риск развития рака легкого
Клетки легкого обладают высокой гормонпроду-цирующей активностью, и их малигнизация также связана с гормональными факторами, в частности с эстрогенами. Риск развития рака легких у курящих женщин в 3 раза выше, чем у курящих мужчин. Особенно это касается женщин, принимающих заместительную гормональную терапию или оральные контрацептивы. При этом уровни аддуктов ДНК, так же как и мутации в супрессоре p53 и протоонкогене K-RAS, у них выше. Предполагается, что эндогенные и экзогенные половые стероиды усиливают действие канцерогенов табака. Это предположение подтверждается и тем, что у курящих мужчин, получавших эстрогены для уменьшения частоты сердечных приступов, отмечена повышенная смертность от рака легких. В связи с этим данный профилактический прием был запрещен [35, 36].
Связанность эффектов полициклических ароматических углеводородов (ПАУ) и эстрогенов объясняется тем, что оба типа соединений являются лигандами рецептора углеводородов (AhR), который регулирует изоформы CYP1A1 и CYP1B1 цитохрома P450. При этом бенз(а)пирен из табачного дыма активируется до канцерогенного диолэ-поксида, а 17β-эстрадиол – до катехолэстрогенов 2-ОН и 4-ОН. Производные 4-катехолэстрогенов (электрофильные 3,4-хиноны), как и диолэпокси-ды ПАУ, канцерогенны в связи со значительной реакционной способностью и образованием аддуктов с нуклеофильными группами ДНК [37] (рис. 2).
Таким образом, ПАУ табачного дыма в качестве ЭД могут, с одной стороны, ослаблять эффект эстрогенов, поскольку комплекс AhR-ARNT может связываться с последовательностями ДНК генов, ингибирующих ER-зависимую транскрипцию, однако, по-видимому, преобладает взаимная стимуляция метаболизма ER и ПАУ за счет повышения активности изоформ CYP1A1 и CYP1B1 и усиления взаимной метаболической активации.
Ключевые характеристики ЭД
В связи с многообразием химической структуры ЭД их выявление по структурным характеристикам весьма затруднено. Для решения этого вопроса в последние годы Совет экспертов США, Японии, Англии и Франции предложил ориентироваться на 10 ключевых характеристик (КХ) ЭД подобно тому, как это принято в оценке потенциальной канцерогенной активности химических соединений [38]. Эти характеристики соответствуют способности ЭД оказывать следующие эффекты на систему гормональной регуляции гомеостаза организма: (1) активировать или усиливать активность гормонального рецептора; (2) подавлять активность гормонального рецептора; (3) вызывать изменение уровня экспрессии гормонального рецептора; (4) нарушать передачу сигнала активированного гор- монального рецептора; (5) влиять на эпигенетическую регуляцию экспрессии генов; (6) нарушать синтез гормонов; (7) нарушать секрецию синтезированных гормонов; (8) нарушать транспортировку и распределение гормонов в организме; (9) влиять на процесс деградации и экскреции гормона; (10) влиять на дифференцировку и функционирование гормон-продуцирующих и гормон-респонсивных клеток (табл. 2).
Влияние ЭД на систему эпигенетической регуляции экспрессии генов
Одним из главных молекулярных механизмов действия ЭД на дифференцировку клеток, уровень экспрессии гормональных рецепторов и белков, участвующих в процессах транскрипции, передачи сигнала от активированного гормонального рецептора, метаболизма и транспорта гормона, является их влияние на систему эпигенетической регуляции экспрессии генов, которое происходит благодаря метилированию/деметилированию ДНК по пятому углеродному атому цитозина в динуклеотидах CpG, посттрансляционной модификации N-концевых хвостов гистоновых белков путем их фосфорилирования, метилирования, ацетилирования, сумоилирования, убиквитинирования и других модификаций, а также изменению профиля экспрессии некодирующих РНК.
Метилирование динуклеотидов CpG осуществляется ДНК-метилтрансферазами DNMT3 (DNMT3A и DNMT3B), и это состояние сохраняется при репликации ДНК с помощью ДНК-метилтрансферазы DNMT1. Деметилирование этих сайтов происходит по двум разным механизмам: пассивно во время деления клетки и активно с помощью ТЕТ белков, последовательно окисляющих 5-meC до 5-гидроксиметилцитозина
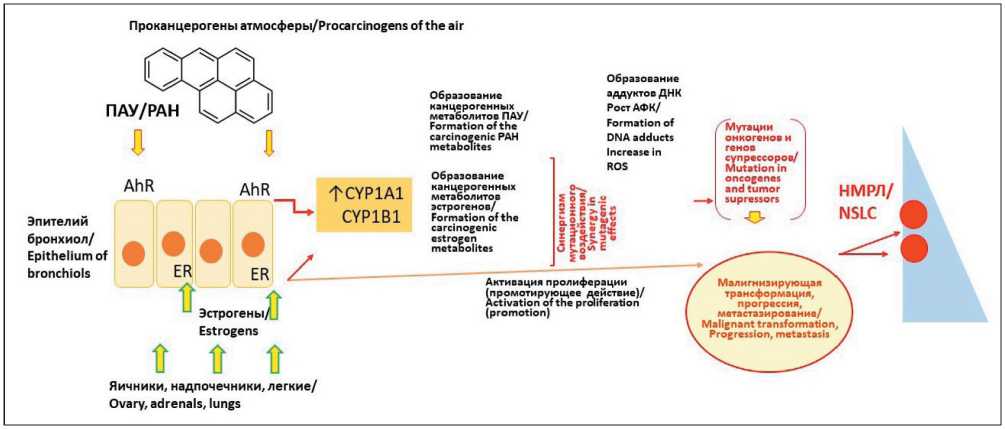
Рис. 2. Эстрогены и ПАУ табачного дыма в генезе рака легкого:
Е2 – 17β-эстрадиол; ER – рецептор эстрогена; 2-OH/4-OH E – оксидпроизводные эстрогена; PAH – полициклические ароматические углеводороды; CYP1A1/B1 – изоформы цитохрома Р450; ROS – активные формы кислорода. Примечание: рисунок выполнен авторами
Fig. 2. Estrogens and polycyclic aromatic hydrocarbons from tobacco smoke. Notes: Е2 – 17β-estradiol; ER – estrogen receptor; 2-OH/4-OH E –oxyderivatives of the estrogen; PAH – polycyclic aromatic hydrocarbons; CYP1A1/B1 – cytochrome Р450 isoforms;
ROS – reactive oxygen species. Created by the authors
и 5-карбоксицитозина, которые затем удаляются путем эксцизионной репарации с заменой на цитозин.
Другим механизмом эпигенетической регуляции экспрессии генов, как уже упоминалось, являются посттрансляционные модификации N-концевых хвостов гистоновых белков, которые играют роль «гистоновых кодов» и влияют на упаковку хроматина. Как и метилирование ДНК, модификации гистонов регулируются балансом между «пишущими» и «стирающими» системами. К первым относятся гистоновые фосфорилазы, ацетилтрансферазы и метилтрасферазы, которые модифицируют гистон, ко вторым – гистоновые фосфатазы, деацетилазы и деметилазы, которые удаляют модифицирующие радикалы.
Одной из самых ранних реакций на различные типы повреждения ДНК является фосфорилирование гистона H2АХ по серину 139 с образованием ɣ-H2AX. Введение беременным крысам метоксихлора, пестицида, предложенного взамен ДДТ, вызывало у рожденных самок поликистоз яичников, который сохранялся в ряде поколений. При этом наблюдалось избирательное гиперметилирование CpG в промоторе ERβ, при нормальном метилировании промотора ERα. Поскольку в данном эксперименте наблюдалась также повышенная экспрессия в клетках яичника Dnmt3b, предпола-
Таблица 2/Table 2
Ключевые характеристики эндокринных дизрапторов [38]Key features of endocrine disruptors [38]
Ключевая характери- Молекулярный механизм стика ЭД/ реализации эффекта/
Пример воздействия ЭД/ Example of ED effect
Key characteristic of Molecular mechanism of
ED the effect
КХ1: Активирующее или усиливающее активность действие соединения на рецепторы гормонов/ KC1: Activating or enhancing the activity
ЭД является лигандом или агонистом гормонального рецептора/
ED is a ligand or agonist of the hormone receptor
Дихлордифенилтрихлорэтан (ДДТ), связываясь с ERα и ERβ. стимулирует ER-зависимую пролиферацию, а путем взаимодействия с рецепторами, связанными G-белком, действует как положительный аллостерический модулятор фолликулостимулирующего гормона (FSHR)/
Dichlorodiphenyltrichloroethane (DDT), by binding to ERa and ERß, stimulates ER-dependent proliferation, and by interacting with G-protein-bound receptors, acts as a positive allosteric modulator of follicle-stimulating hormone (FSHR)
of the compound on hormone receptors
КХ2: Антагонизм с гормональными рецепторами/ KC2: Antagonism with hormonal receptors
ЭД является антагонистом трансмембранного или ядерного гормонального рецептора/ ED is an antagonist of the transmembrane or nuclear hormone receptor
ДДТ ингибирует связывание андрогенов с рецептором (AR) и андрогензависи-мую трансактивацию AR в клетках предстательной железы человека. Другие хлорорганические пестициды также ингибируют связывание дигидротестостерона с АР. Их действие во время внутриутробного развития может демаскулини-зировать плод мужского пола/
DDT inhibits androgen receptor binding (AR) and androgen-dependent AR transactivation in human prostate cells. Other organochlorine pesticides also inhibit the binding of dihydrotestosterone to AR. Their action during intrauterine development can demascularize the male fetus
КХ3: Нарушение экспрессии гормональных рецепторов/ KC3: Impaired expression of hormonal receptors
ЭД взаимодействует с белками, участвующими в транскрипции гена рецептора гормона и ее регуляции/
ED interacts with proteins involved in the transcription of the hormone receptor
gene and its regulation
ДДТ препятствует интернализации рецептора тиреотропного гормона (ТТГ), а пластификатор ди(2-этилгексил) фталат снижает экспрессию рецептора минералокортикоидов, которые необходимы для синтеза тестостерона в семенниках. BPA уменьшает протеасомно-опосредованную деградацию ERβ/
DDT prevents the internalization of the thyroid-stimulating hormone receptor (TSH), and the plasticizer di(2-ethylhexyl)phthalate reduces the expression of the mineralocorticoid receptor, which are necessary for the synthesis of testosterone in the testes. BPA reduces proteasome-mediated degradation of ERß
КХ4: Нарушение передачи гормонального сигнала/ KC4: Disruption of hormonal signaling
ЭД взаимодействует с белками, участвующими в транскрипции генов компонентов гормонального сигналинга/
ED interacts with proteins involved in the transcription of genes of hormonal signaling components
BPA блокирует передачу сигнала в α-клетки поджелудочной железы, секретирующие глюкагон через ингибирование ионотропного кальциевого рецептора, индуцируемого низким уровнем глюкозы. Фунгицид толилфлуанид ослабляет действие инсулина, уменьшая размер инсулинового рецептора, а широко применяемая метоксиуксусная кислота и противосудорожная вальпроевая кислота резко повышают чувствительность клеток к эстрогенам, прогестинам и другим лигандам ядерных гормональных рецепторов за счет повышения эффективности транскрипции этих рецепторов/
BPA blocks signal transmission to the alpha cells of the pancreas secreting glucagon through inhibition of the ionotropic calcium receptor induced by low glucose levels. The fungicide tolylfluanide weakens the effect of insulin, reducing the size of the insulin receptor, and the widely used methoxyacetic acid and anticonvulsant valproic acid dramatically increase the sensitivity of cells to estrogens, progestins and other ligands of nuclear hormonal receptors, by increasing the efficiency of transcription of these receptors
Продолжение таблицы 2/Table 2
КХ5: Влияние на систему эпигенетической регуляции экспрессии генов/ KC5: Influence on the system of epigenetic regulation of gene expression
ЭД влияет на уровень и профиль метилирования ДНК, модификации гистонов или экспрессию некодирующих РНК/ ED affects the level and profile of DNA methylation, histone modification, or expression of non-coding RNAs
Обработка самок крыс в процессе развития плода метоксихлором увеличивает в яичниках потомков экспрессию ДНК-метилтрансферазы DNMT3B, гиперметилирующей ДНК гена ESR2, который кодирует ERβ. Полихлордифенилы изменяют экспрессию микроРНК гипоталамуса, определяющих половой диморфизм, а BPA и фталаты изменяют экспрессию микроРНК в культурах клеток плаценты, клеток Сертоли и рака молочной железы. BPA и диэтилстильбэстрол индуцируют антисмысловую РНК (HOTAIR), стимулирующую триметилирование гистона H3K4 и увеличение пула H3K4-специфически метилтрансфераз, активирующих транскрипцию и повышающих чувствительность соответствующих генов к тестостерону/ Treatment of female rats during fetal development with methoxychlor increases the expression of DNA methyltransferase DNMT3B in the ovaries of offspring, hypermethylating DNA of the ESR2 gene that encodes ERß.Polychlorodiphenyls alter the expression of hypothalamic microRNAs that determine sexual dimorphism, and BPA and phthalates alter the expression of microRNAs in cultures of placenta cells, Sertoli cells and breast cancer. BPA and diethylstilbestrol induce antisense RNA (HOTAIR) stimulating trimethylation of histone H3K4 and an increase in the pool of H3K4-specifically methyltransferases that activate transcription and increase the sensitivity of the corresponding genes to testosterone
КХ6: Нарушение процесса синтеза гормонов/ KC6: Disruption of hormone synthesis
ЭД влияет на экспрессию и функциональную активность белков, участвующих в синтезе и метаболизме гормонов/
ED affects the expression and functional activity of proteins involved in the synthesis and metabolism of hormones
Перхлораты ингибируют синтез тиреоидного гормона, блокируя поступление йода в клетки щитовидной железы. Фталаты ингибируют синтез тестостерона в семенниках плодов крыс, что впоследствии приводит к тестостероновой недостаточности, а гербицид атразин и некоторые неоникотиноидные пестициды, действуя на новорожденных самцов мышей, стимулируют синтез эстрогенов путем активации ароматазы, превращающей тестостерон в эстроген/ Perchlorates inhibit the synthesis of thyroid hormone, blocking the flow of iodine into the cells of the thyroid gland. Phthalates inhibit the synthesis of testosterone in the testes of rat fetuses, which subsequently leads to testosterone deficiency, and the herbicide atrazine and some neonicotinoid pesticides, acting on newborn male mice, stimulate the synthesis of estrogens by activating aromatase, which converts testosterone into estrogen
КХ7: Нарушение секреции гормонов и их транспортировки/ KC7: Disruption of hormone secretion and transportation
ЭД влияет на экспрессию и функциональную активность белков, обеспечивающих секрецию гормонов из клетки/
ED affects the expression and functional activity of proteins that secrete hormones from the cell
Низкие дозы ДДТ снижают секрецию кортикостерона из надпочечников грызунов. БПА в низкой дозе ингибирует поступление кальция в β-клетки поджелудочной железы, необходимого для снижения секреции инсулина, в то время как фунгицид имидазолин изменяет поступление ионов, необходимых для активации секреции инсулина/
Low doses of DDT reduce the secretion of corticosterone from the adrenal glands of rodents. BPA in a low dose inhibits the intake of calcium into the beta cells of the pancreas, which is necessary to reduce insulin secretion, while the fungicide imidazoline alters the intake of ions necessary to activate insulin secretion
КХ8: Нарушение распределения или содержания циркулирующих гормонов/ KC8: Disruption of the distribution or content of circulating hormones
КХ9: Изменение метаболизма гормонов и их экскреции/ KC9: Changes in hormone metabolism and excretion
ЭД влияет на экспрессию и функциональную активность белков, обеспечивающих транспорт гормонов в клетке и в организме/ ED affects the expression and functional activity of proteins that provide hormone transport in the cell and in the body
ЭД влияет на экспрессию и функциональную активность белков, обеспечивающих метаболизм гормонов в разных тканях организма/
ED affects the expression and functional activity of proteins that provide hormone metabolism in different tissues of the body
Введенный внутривенно диэтилстильбэстрол (ДЭС) повышает в 7 раз концентрацию в крови глобулинов, связывающих половые гормоны, за счет чего у мужчин снижается в 6 раз общий уровень тестостерона, на 20 % свободного и эстрогена в 5 раз. ВРА и пестицид малатион по такому же механизму снижают в крови у человека и животных уровни андростендиона и тестостерона/ Intravenously administered diethylstilbestrol (DES) increases the concentration of globulins binding sex hormones in the blood by 7 times, due to which the total level of testosterone in men decreases by 6 times, by 20 % free and estrogen by 5 times. VERA and the pesticide malathion by the same mechanism reduce the levels of androstenedione and testosterone in the blood of humans and animals
Производные ПХБ ингибируют сульфотрансферазу эстрогенов, которая снижает клиренс этих гормонов из крови путем сульфатации. Таким же свойством обладает основной метаболит фунгицида гексахлорбензола и несколько хлорфенольных консервантов древесины. Многие соединения активируют глюкуронидазы, увеличивающие клиренс из крови гормонов щитовидной железы/ PCB derivatives inhibit estrogen sulfotransferase, which reduces the clearance of these hormones from the blood by sulfation. The main metabolite of the fungicide hexachlorobenzene and several chlorophenolic wood preservatives have the same property. Many compounds activate glucuronidases, which increase the clearance of thyroid hormones from the blood
Окончание таблицы 2/End of Table 2
КХ10: Изменение морфологии и функционирования гормон-продуцирующих и гормон-распознающих клеток/
KC10: Changes in the morphology and functioning of hor-mone-producing and hormone-recognizing cells
ЭД влияет на процессы дифференцировки ткани, вызывая изменение функциональной активности или гибель клеток/
ED affects the processes of tissue differentiation, causing a change in functional activity or cell death
ПХБ нарушают у новорожденных сигналинг гормона щитовидной железы, который контролирует в развивающемся мозжечке пролиферацию клеток и апоптоз. У взрослых это проявляется изменением структуры мозжечка. Обработка новорожденных ПХБ приводит также и к повреждению ядер гипоталамуса, которое резко снижает у взрослых самок число клеток, экспрессирующих ERα. Оксибензон (бензофенон-3), используемый в солнцезащитных очках в качестве фильтра УФ, введенный при беременности или в период лактации, вызывает у новорожденных длительную пролиферацию клеток молочных желез. Экстракт горького апельсина Citrus aurantum (с содержанием р-синефрина 3 %) при применении у самок крыс Wistar в течение 3 сут в дозе 50 мг/кг в сутки проявлял антиэстро-генный потенциал, о чем судили по тенденции к снижению массы матки и по статистически значимому снижению массы надпочечников [11]/
PCBs disrupt the signaling of thyroid hormone in newborns, which controls cell proliferation and apoptosis in the developing cerebellum. In adults, this is manifested by a change in the structure of the cerebellum. Treatment of newborn PCBs also leads to damage to the nuclei of the hypothalamus, which dramatically reduces the number of cells expressing ERa in adult females.Oxybenzone (benzophenone-3), used in sunglasses as a UV filter, introduced during pregnancy or lactation, causes prolonged proliferation of mammary gland cells in newborns.
Citrus aurantum bitter orange extract (with a p-synephrine content of 3 %), when used in female Wistar rats for 3 days at a dose of 50 mg/kg per day, showed antiestrogenic potential, as judged by the tendency to decrease uterine mass and by a statistically significant decrease in adrenal mass [11].
Примечание: таблица составлена авторами.
Note: created by the authors.
гается, этот эффект играет главную роль в гиперметилировании промотора [8, 39].
Такой же эффект ЭД оказывают и на клетки эмбриональной предстательной железы, что предрасполагает их к злокачественной трансформации во взрослом организме. В норме одним из факторов, влияющих на пролиферацию клеток предстательной железы, является цАМФ- фосфодиэстераза PDE4D4, которая, расщепляя цАМФ, активирует сигнальный путь цАМФ/протеинкиназа А, связанный с G-белком, регулирующим транскрипцию генов, управляющих этим процессом [40].
Устойчивая экспрессия PDE4D4 повышает пул внутриклеточного цАМФ, создавая возможность аберрантного сигналинга и неопластической трансформации секреторного эпителия предстательной железы. В активно пролиферирующих клетках предстательной железы, в том числе злокачественных, также наблюдается повышенная экспрессия этого фермента, связанная с гипометилированием CpG-островков промотора гена PDE4D4. БФА формирует этот паттерн метилирования промотора PDE4D4 в стволовых клетках эмбриональной предстательной железы, вследствие чего он сохраняется пожизненно. В нормальных условиях промотор PDE4D4 с возрастом гиперметилируется, что приводит к репрессии гена PDE4D4, тогда как у животных, кратковременно подвергшихся в неонатальном периоде воздействию бисфенола А, его промотор остается гипометилированным, что вызывает стойкую гиперэкспрессию PDE4D4. Клетки предстательной железы с такой эпигенетической модификацией проявляют повышенную чувствительность к канцерогенному действию эстрадиола, уровень которого повышается при старении. Помимо инволюции центральных эндокринных органов этому способствует и усиление в клетках стромы предстательной железы, содержащих рецепторы ERα и ERß, экспрессии ароматазы, превращающей тестостерон в эстрадиол путем его дегидрирования. Кроме того, в результате действия многих ЭД ингибируется тестикулярная 3β-Гидрокси-Δ5-стероиддегидрогеназа/Δ5,Δ4-изомераза, которая катализирует окислительное превращение Δ5-3β-гидроксистероидов в Δ4-3-оксостероиды в коре надпочечников, половых железах и различных периферических тканях и экспрессия которой необходима для синтеза тестостерона [41].
По данным экспериментов, повышение уровня эстрадиола в плазме самцов крыс, неонатально обработанных БФА в дозах, соответствующих его содержанию в биосфере, вызывает РПЖ. В норме при физиологическом уровне эстрадиола и тестостерона этого не происходит. В канцерогенном действии БФА существенно и то, что он ингибирует мРНК и белок рецептора ERβ, который, по-видимому, является антипролиферативным и проапоптотическим фактором. Кроме того, известна способность ЭД связываться с рецептором Ahr, стимулирующим экспрессию ферментов системы цитохрома Р450, которые активируют многие проканцерогенные соединения до активных электрофильных форм. Учитывая, что эти соединения, в частности ПАУ, также интенсивно загрязняют биосферу, усиление их метаболической активации может также быть значительным канцерогенным фактором [42–44].
Еще один тип эпигенетических модификаций, связанных с ЭД, состоит в их воздействии на некодирующие РНК различных классов, которые не только участвуют в регуляции домашнего хозяйства клетки, но и влияют на экспрессию многих других генов. Из них наиболее важную роль в канцерогенезе играют микроРНК (миРНК), осуществляющие транскрипционную репрессию генов путем связывания с 3’-некодирующими областями соответствующих мРНК, и длинные некодирующие РНК (днРНК), которые модулируют структуру ДНК, гистонов, координируют динамику хроматина и стабилизируют мРНК. Изменения профиля миРНК обнаруживались в клетках всех типов изученных опухолей, причем было показано, что они могут быть как супрессорами, так и онкогенами. При этом оказалось, что одна молекула способна воздействовать на несколько мишеней, в числе которых находятся такие онкогены, как RAS, MYC и EGFR, и супрессоры TP53, PTEN и BRCA1. Длинные некодирующие РНК активно экспрессируются при раке молочной железы и яичников, а их нокдаун ингибирует пролиферацию культуры опухолевых клеток этих органов.
В клетках рака предстательной железы экспрессируются две днРНК – PCGEM1 и PRNCR1, последовательно связывающиеся с рецептором андрогена и значительно усиливающие как лиганд-зависимую, так и лиганд-независимую активацию генов в опухолевых клетках и их пролиферацию.
Эндокринные дизрапторы влияют и на экспрессию миРНК. Введение мышам на протяжении жизни смеси, состоящей из трех фталатов и двух алкилфенолов в дозах, соответствующих их содержанию в биосфере, вызвало активацию двух и ингибирование восьми миРНК в клетках семенников. Это привело к изменению гормонального баланса и нарушению структуры органа. У человека ДДТ и бисфенол А изменяют экспрессию miR-21, которая играет важную роль при раке, особенно при развитии рака молочной и поджелудочной желез. Эпигенетические модификации в экспрессии миРНК могут наследоваться в ряду поколений. Пренатальное воздействие БФA в дозе 5 мг/кг увеличивает в гипоталамусе экспрессию материнской длинной некодирующей РНК Meg3, высокий уровень которой сохраняется и в 3-м поколении. Экспрессия Meg3 связана с центральным контролем преждевременного полового созревания, которое наблюдается при действии BPA как у лабораторных животных, так и у женщин, и коррелирует с повышенным риском возникновения рака молочной железы. Этот результат важен для экстраполяции действия БФА на человека, поскольку содержание ЭД в крови мышей после данной дозы было эквивалентно содержанию БФA в крови беременных женщин (0,3–18,9 нг/мл) [45, 46].
Влияние ЭД на экспрессию миРНК существенно для прогрессирования не только опухолей половой сферы. В частности, на культуре клеток рака толстой кишки человека и на его ксенотрансплантатах показано, что повышенная экспрессия miR-21 приводит к активации путей ERK, AKT и ROCK и ингибированию молекул адгезии плотных контактов JAM-A, что стимулирует пролиферацию опухолевых клеток, а в клинике является маркером плохого прогноза [47–50].
Роль эстрогенов в легочном канцерогенезе подтверждает и тот факт, что у женщин, получающих заместительную гормональную терапию или оральные контрацептивы, рак легкого возникает чаще, как и у мужчин, получающих эстрогенные препараты для снижения риска сердечнососудистых заболеваний [35, 51–55].
Эндокринные дизрапторы играют значительную роль и в прогрессировании злокачественного роста. В частности, фатальная гормон-независимая пролиферация опухолевых клеток предстательной железы может быть вызвана фоновой концентрацией БФА, несмотря на то, что его сродство к рецепторам эстрогенов и андрогенов на 3–4 порядка ниже, чем у естественных гормонов. Такой эффект связывается с его способностью активировать сигнальные пути MAPK и PI3K через трансмембранный рецептор андрогенов GPCR [56, 57].
Изменение энергетического баланса клетки
Эндокринные дизрапторы способны изменять также энергетику клетки путем воздействия на метаболизм холестерина, желчных кислот и окислительные процессы в митохондриях. В результате происходят сдвиги обмена, дающие селективное преимущество злокачественным клеткам. Например, БФА, который содержится в твердых пластиках, в том числе для медицинского использования, в детских игрушках, посуде, термобумаге и внутреннем покрытии консервных банок и др., ингибирует аэробный гликолиз, препятствуя транспорту электронов в митохондриях, что приводит к компенсаторному повышению перекисного окисления липидов. При этом падает производство АТФ и происходит накопление реактивных форм кислорода, повреждающих макромолекулы, в том числе ДНК [58]. Несколько ЭД могут суммировать свою эффективность, связываясь с одним рецептором. Например, действие смеси двух разнородных соединений – противозачаточного эстрогена 17a-этинилестрадиола и стойкого хлорорганического пестицида транс -нонахлора – на рецептор прегнана X активирует его в 100 раз сильнее, чем простая сумма их действий по отдельности. Механизм этого эффекта состоит в том, что каждый лиганд резко увеличивает аффинность другого, т. е. в данном случае происходит образование «супрамоле-кулярных лигандов» внутри лиганд-связывающего кармана ядерных рецепторов. В результате бинарная смесь вызывает выраженный биологический эффект в дозах компонентов, находящихся за пределами их видимой индивидуальной активности. и суммарное действие признанного безопасным противозачаточного препарата вместе со следовым количеством пестицида вызывает существенный сдвиг эндокринного баланса [9, 59, 60].
Нарушение эндокринной сигнализации
БФА взаимодействует и со многими эндокринными рецепторами. Он влияет на сигнальные пути половых гормонов, эпидермального фактора роста (EGFR), тироидного гормона (TR), рецептора прегнана X (PXR), рецептора, активируемого также пролифераторами пероксисом (PPAR), и рецептора ароматических углеводородов (AhR), который регулирует экспрессию ферментов с помощью сигнальных путей, сходных с сигнализацией эстрогеновых, а также стимулирует протеосомную деградацию рецепторов эстрогенов . Результатом его действия являются многие патологические процессы, в том числе стимуляция канцерогенеза в молочной и предстательной железах, яичниках, легких, печени и эндометрии [33, 61]. В культуре клеток многих органов – шейки матки (SiHa, HeLa, C33A), молочной железы (MCF7, MDA-MB-231, BT-549), предстательной железы (LNCaP), трофобластов человека (HTR-8/SVneo), мезенхимальных стволовых клеток (hUM-MSC), яичника (OVCAR-3, SkBr3), легкого (A549) и толстой кишки (SW480) – БФА стимулировал пролиферацию, миграцию и инвазивный рост путем активации сигнальных путей JAK-STAT, PI3-AKT, MAPK и других [33, 62].
Многие ЭД способны влиять на активацию сигнального пути глюкокортикоидного рецептора (GR). Описано синергическое действие дексаметазона и некоторых эфиров фталевой кислоты на активацию GR, их способность усиливать ядерную транслокацию рецептора и экспрессию глюкокортикоид-зависимых генов по механизму трансактивации. BPA может взаимодействовать и с глюкокортикоидным рецептором, но с гораздо меньшей аффинностью, чем кортизол или дексаметазон. В связи с этим он не считается полным агонистом этого рецептора [63, 64]. Влияние на GR предполагают для алкалоида синефрина на основании анализа свойств других, структурно схожих с синефрином агонистов β-адреновых рецепторов [12]. Аналогичные данные получены для 3,4,4'-трихлоркарбанилида, изопропил-N-фенилкарбамата и 2-[4-хлорфенил]-бензтиазола [65]. Поскольку индукция трансактивации в химиотерапии опухоли ассоциирована с увеличением пролиферативной активности и развитием побочных эффектов, можно предположить, что ЭД некоторых классов способны усиливать нежелательные эффекты глюкокортикоидов, в частности, приводить к прогрессированию опухоли и развитию метастазирования. Другие ЭД также действуют в качестве антагонистов GR, в частности, некоторые эфиры фталевой кислоты, неорганические соединения мышьяка и др. [66, 67]. Для БФА также показано, что одним из его механизмов малигнизации клеток является действие на ERK1/2/ERRγ. Связывание БФА с ERRγ приводит к его ядерной транслокации и активации факторов транскрипции, таких как AP1, которые активируют пролиферацию. Активация ERK1/2 может происходить и через GPER/EGFR, который активирует транскрипцию и активацию онкогенов, таких как c-fos и ингибитор апоптоза BCL2.
Проблемы контроля загрязнений биосферы ЭД
Несмотря на большой прогресс в понимании механизмов действия ЭД, попытки законодательно ограничить загрязнение биосферы этими соединениями, предпринимаемые в развитых странах, пока направлены на отдельные виды продукции и на выявление специфических типов гормональной активности. В США нормативные акты предписывают тестирование косметических изделий, пестицидов и загрязнителей питьевой воды только на эстрогенную активность, тогда как Европейское агентство по химическим веществам и Европейское управление по безопасности пищевых продуктов рекомендуют выявлять не только эстрогенные, но и андрогенные и тиреотропные свойства соединений [68, 69]. Оценка глюкокортикоид-подобной активности на данный момент не регламентирована в нормативных документах по профилактике злокачественных новообразований и заболеваний другого генеза.
Углубленное изучение вопроса показало, что методический арсенал для выявления ЭД нуждается в усовершенствовании, поскольку используемые тесты не охватывают все типы соединений и механизмы их действия, а также не обладают достаточной чувствительностью, поскольку разрабатывались для изучения баланса эндогенных гормонов. Так, тест на эстрогенную активность по изменению массы матки подходит для изучения колебаний эстрогенного баланса в организме, но применительно к ЭД этот метод эффективен только в случае сверхвысоких концентраций. В частности, описана тенденция к антиэстрогенному эффекту у алкалоида синефрина при применении у самок крыс Wistar в течение 3 сут, однако для регистрации статистически значимых изменений необходимы проведение более длительных исследований и использование более высоких доз синефрина. К тому же воздействия ЭД на регулирующие и интегрирующие звенья эстроген-эффекторных сигнальных путей могут вызывать изменения в гормонозависимых органах и без влияния на массу матки. В связи с этим предполагается включить в нормативные требования США и ЕС широкий круг современных методик для изучения влияния ЭД на экспрессию ядерных и неядерных рецепторов, синтез и транспорт гормонов, а также сигнальные пути и эпигенетические эффекты [11, 70, 71].
Поскольку одним из основных механизмов действия ЭД является их эпигенетическая активность, существенным вкладом в их распознавание может стать разработанная в отделе химического канцеро- генеза ФГБУ «НМИЦ онкологии им. Н.Н. Блохина» тест-система выявления эпигенетически активных промоторов канцерогенеза, состоящая из популяции генетически модифицированных клеток, имеющих в своем геноме эпигенетически репрессированный репортерный ген флуорофора, уровень экспрессии которого определяется экспозицией к эпигенетически активным соединениям [72].
Список литературы Эндокринные дизрапторы в канцерогенном фоне биосферы
- Herbst A.L., Ulfelder H., Poskanzer D.C. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. N Engl J Med. 1971; 284(15): 878–81. doi: 10.1056/NEJM197104222841604.
- Attina T.M., Hauser R., Sathyanarayana S., Hunt P.A., Bourguignon J.P., Myers J.P., DiGangi J., Zoeller R.T., Trasande L. Exposure to endocrine-disrupting chemicals in the USA: a population-based disease burden and cost analysis. Lancet Diabetes Endocrinol. 2016; 4(12): 996–1003. doi: 10.1016/S2213-8587(16)30275-3.
- Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022; 72(1): 7–33. doi: 10.3322/caac.21708.
- Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021; 71(3): 209–49. doi: 10.3322/caac.21660.
- Bellanger M., Demeneix B., Grandjean P., Zoeller R.T., Trasande L. Neurobehavioral deficits, diseases, and associated costs of exposure to endocrine-disrupting chemicals in the European Union. J Clin Endocrinol Metab. 2015; 100(4): 1256–66. doi: 10.1210/jc.2014-4323.
- Hauser R., Skakkebaek N.E., Hass U., Toppari J., Juul A., Andersson A.M., Kortenkamp A., Heindel J.J., Trasande L. Male reproductive disorders, diseases, and costs of exposure to endocrine-disrupting chemicals in the European Union. J Clin Endocrinol Metab. 2015; 100(4): 1267–77. doi: 10.1210/jc.2014-4325.
- Lichtenstein P., Holm N.V., Verkasalo P.K., Iliadou A., Kaprio J., Koskenvuo M., Pukkala E., Skytthe A., Hemminki K. Environmental and heritable factors in the causation of cancer--analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000; 343(2): 78–85. doi: 10.1056/NEJM200007133430201.
- Koual M., Tomkiewicz C., Cano-Sancho G., Antignac J.P., Bats A.S., Coumoul X. Environmental chemicals, breast cancer progression and drug resistance. Environ Health. 2020; 19(1): 117. doi: 10.1186/s12940-020-00670-2.
- Balaguer P., Delfosse V., Grimaldi M., Bourguet W. Structural and Functional Evidences for the Interactions between Nuclear Hormone Receptors and Endocrine Disruptors at Low Doses. C. R. Biol. 2017; 340(9–10): 414–20, doi:10.1016/j.crvi.2017.08.002.
- Gore A.C., Chappell V.A., Fenton S.E., Flaws J.A., Nadal A., Prins G.S., Toppari J., Zoeller R.T. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev. 2015; 36(6): 1–150. doi: 10.1210/er.2015-1010.
- Arbo M.D., Franco M.T., Larentis E.R., Garcia S.C., Sebben V.C., Leal M.B., Dallegrave E., Limberger R.P. Screening for in vivo (anti)estrogenic activity of ephedrine and p-synephrine and their natural sources Ephedra sinica Stapf. (Ephedraceae) and Citrus aurantium L. (Rutaceae) in rats. Arch Toxicol. 2009; 83(1): 95–9. doi: 10.1007/s00204-008-0324-8.
- Korn S.H., Wouters E.F., Wesseling G., Arends J.W., Thunnissen F.B. Interaction between glucocorticoids and beta2-agonists: alpha and beta glucocorticoid-receptor mRNA expression in human bronchial epithelial cells. Biochem Pharmacol. 1998; 56(12): 1561–9. doi: 10.1016/s0006-2952(98)00179-8.
- Conolly R.B., Lutz W.K. Nonmonotonic dose-response relationships: mechanistic basis, kinetic modeling, and implications for risk assessment. Toxicol Sci. 2004; 77(1): 151–7. doi: 10.1093/toxsci/kfh007. Erratum in: Toxicol Sci. 2004; 77(2): following table of contents.
- Graceli J.B., Sena G.C., Lopes P.F., Zamprogno G.C., da Costa M.B., Godoi A.F., Dos Santos D.M., de Marchi M.R., Dos Santos Fernandez M.A. Organotins: a review of their reproductive toxicity, biochemistry, and environmental fate. Reprod Toxicol. 2013; 36: 40–52. doi: 10.1016/j. reprotox.2012.11.008.
- Oyola M.G., Handa R.J. Hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes: sex differences in regulation of stress responsivity. Stress. 2017; 20(5): 476–94. doi: 10.1080/10253890.2017.1369523.
- Iaglov V.V. Aktualnye problemy biologii diffuznoĭ éndokrinnoĭ sistemy [Current problems of the biology of the diffuse endocrine system]. Arkh Anat Gistol Embriol. 1989; 96(1): 14–29.
- Simpson E., Rubin G., Clyne C., Robertson K., O’Donnell L., Jones M., Davis S. The role of local estrogen biosynthesis in males and females. Trends Endocrinol Metab. 2000; 11(5): 184–8. doi: 10.1016/s1043-2760(00)00254-x.
- McNamara K.M., Sasano H. The intracrinology of breast cancer. J Steroid Biochem Mol Biol. 2015; 145: 172–8. doi: 10.1016/j.jsbmb.2014.04.004.
- Penning T.M., Detlefsen A.J. Intracrinology-revisited and prostate cancer. J Steroid Biochem Mol Biol. 2020; 196. doi: 10.1016/j.jsbmb.2019.105499.
- Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68(6): 394–424. doi: 10.3322/caac.21492. Erratum in: CA Cancer J Clin. 2020; 70(4): 313.
- Cohn B.A., Wolff M.S., Cirillo P.M., Sholtz R.I. DDT and breast cancer in young women: new data on the significance of age at exposure. Environ Health Perspect. 2007; 115(10): 1406–14. doi: 10.1289/ehp.10260.
- Cohn B.A., Cirillo P.M., Terry M.B. DDT and Breast Cancer: Prospective Study of Induction Time and Susceptibility Windows. J Natl Cancer Inst. 2019; 111(8): 803–10. doi: 10.1093/jnci/djy198.
- Cheong A., Johnson S.A., Howald E.C., Ellersieck M.R., Camacho L., Lewis S.M., Vanlandingham M.M., Ying J., Ho S.M., Rosenfeld C.S. Gene expression and DNA methylation changes in the hypothalamus and hippocampus of adult rats developmentally exposed to bisphenol A or ethinyl estradiol: a CLARITY-BPA consortium study. Epigenetics. 2018; 13(7): 704–20. doi: 10.1080/15592294.2018.1497388.
- Cao J., Mickens J.A., McCaffrey K.A., Leyrer S.M., Patisaul H.B. Neonatal Bisphenol A exposure alters sexually dimorphic gene expression in the postnatal rat hypothalamus. Neurotoxicology. 2012; 33(1): 23–36. doi: 10.1016/j.neuro.2011.11.002.
- Eckstrum K.S., Edwards W., Banerjee A., Wang W., Flaws J.A., Katzenellenbogen J.A., Kim S.H., Raetzman L.T. Effects of Exposure to the Endocrine-Disrupting Chemical Bisphenol A During Critical Windows of Murine Pituitary Development. Endocrinology. 2018; 159(1): 119–31. doi: 10.1210/en.2017-00565.
- Nair V.A., Valo S., Peltomäki P., Bajbouj K., Abdel-Rahman W.M. Oncogenic Potential of Bisphenol A and Common Environmental Contaminants in Human Mammary Epithelial Cells. Int J Mol Sci. 2020; 21(10): 3735. doi: 10.3390/ijms21103735.
- Acevedo N., Davis B., Schaeberle C.M., Sonnenschein C., Soto A.M. Perinatally administered bisphenol a as a potential mammary gland carcinogen in rats. Environ Health Perspect. 2013; 121(9): 1040–6. doi: 10.1289/ehp.1306734.
- Cockburn M., Mills P., Zhang X., Zadnick J., Goldberg D., Ritz B. Prostate cancer and ambient pesticide exposure in agriculturally intensive areas in California. Am J Epidemiol. 2011; 173(11): 1280–8. doi: 10.1093/aje/kwr003.
- Bleak T.C., Calaf G.M. Breast and prostate glands affected by environmental substances (Review). Oncol Rep. 2021; 45(4): 20. doi: 10.3892/or.2021.7971.
- Kandaraki E., Chatzigeorgiou A., Livadas S., Palioura E., Economou F., Koutsilieris M., Palimeri S., Panidis D., Diamanti-Kandarakis E. Endocrine disruptors and polycystic ovary syndrome (PCOS): elevated serum levels of bisphenol A in women with PCOS. J Clin Endocrinol Metab. 2011; 96(3): 480–4. doi: 10.1210/jc.2010-1658.
- Manikkam M., Tracey R., Guerrero-Bosagna C., Skinner M.K. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLoS One. 2013; 8(1). doi: 10.1371/journal.pone.0055387.
- Ho S.M., Tang W.Y., Belmonte de Frausto J., Prins G.S. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 2006; 66(11): 5624–32. doi: 10.1158/0008-5472.CAN-06-0516.
- Khan N.G., Correia J., Adiga D., Rai P.S., Dsouza H.S., Chakrabarty S., Kabekkodu S.P. A comprehensive review on the carcinogenic potential of bisphenol A: clues and evidence. Environ Sci Pollut Res Int. 2021; 28(16): 19643–63. doi: 10.1007/s11356-021-13071-w.
- Prins G.S., Ye S.H., Birch L., Zhang X., Cheong A., Lin H., Calderon- Gierszal E., Groen J., Hu W.Y., Ho S.M., van Breemen R.B. Prostate Cancer Risk and DNA Methylation Signatures in Aging Rats following Developmental BPA Exposure: A Dose-Response Analysis. Environ Health Perspect. 2017; 125(7). doi: 10.1289/EHP1050.
- Stapelfeld C., Dammann C., Maser E. Sex-specificity in lung cancer risk. Int J Cancer. 2020; 146(9): 2376–82. doi: 10.1002/ijc.32716.
- The Coronary Drug Project. Findings leading to discontinuation of the 2.5-mg day estrogen group. The coronary Drug Project Research Group. JAMA. 1973; 226(6): 652–7.
- Słowikowski B.K., Jankowski M., Jagodziński P.P. The smoking estrogens – a potential synergy between estradiol and benzo(a)pyrene. Biomed Pharmacother. 2021; 139. doi: 10.1016/j.biopha.2021.111658.
- La Merrill M.A., Vandenberg L.N., Smith M.T., Goodson W., Browne P., Patisaul H.B., Guyton K.Z., Kortenkamp A., Cogliano V.J., Woodruff T.J., Rieswijk L., Sone H., Korach K.S., Gore A.C., Zeise L., Zoeller R.T. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat Rev Endocrinol. 2020; 16(1): 45–57. doi: 10.1038/s41574-019-0273-8.
- Zama A.M., Uzumcu M. Fetal and neonatal exposure to the endocrine disruptor methoxychlor causes epigenetic alterations in adult ovarian genes. Endocrinology. 2009; 150(10): 4681–91. doi: 10.1210/en.2009-0499.
- Fimia G.M., Sassone-Corsi P. Cyclic AMP signalling. J Cell Sci. 2001; 114(Pt 11): 1971–2. doi: 10.1242/jcs.114.11.1971.
- Ye L., Guo J., Ge R.S. Environmental Pollutants and Hydroxysteroid Dehydrogenases. In Vitamins & Hormones. Elsevier. 2014; 94: 349–90.https://doi.org/10.1016/B978-0-12-800095-3.00013-4.
- Amir S., Shah S.T.A., Mamoulakis C., Docea A.O., Kalantzi O.I., Zachariou A., Calina D., Carvalho F., Sofikitis N., Makrigiannakis A., Tsatsakis A. Endocrine Disruptors Acting on Estrogen and Androgen Pathways Cause Reproductive Disorders through Multiple Mechanisms: A Review. Int J Environ Res Public Health. 2021; 18(4): 1464. doi: 10.3390/ijerph18041464.
- Xin F., Jiang L., Liu X., Geng C., Wang W., Zhong L., Yang G., Chen M. Bisphenol A induces oxidative stress-associated DNA damage in INS-1 cells. Mutat Res Genet Toxicol Environ Mutagen. 2014; 769: 29–33. doi: 10.1016/j.mrgentox.2014.04.019.
- Tarnow P., Tralau T., Luch A. Chemical activation of estrogen and aryl hydrocarbon receptor signaling pathways and their interaction in toxicology and metabolism. Expert Opin Drug Metab Toxicol. 2019; 15(3): 219–29. doi: 10.1080/17425255.2019.1569627.
- Drobná Z., Henriksen A.D., Wolstenholme J.T., Montiel C., Lambeth P.S., Shang S., Harris E.P., Zhou C., Flaws J.A., Adli M., Rissman E.F. Transgenerational Effects of Bisphenol A on Gene Expression and DNA Methylation of Imprinted Genes in Brain. Endocrinology. 2018; 159(1): 132–44. doi: 10.1210/en.2017-00730.
- Lucaccioni L., Trevisani V., Marrozzini L., Bertoncelli N., Predieri B., Lugli L., Berardi A., Iughetti L. Endocrine-Disrupting Chemicals and Their Effects during Female Puberty: A Review of Current Evidence. Int J Mol Sci. 2020; 21(6): 2078. doi: 10.3390/ijms21062078.
- Lampis A., Hahne J.C., Gasparini P., Cascione L., Hedayat S., Vlachogiannis G., Murgia C., Fontana E., Edwards J., Horgan P.G., Terracciano L., Sansom O.J., Martins C.D., Kramer-Marek G., Croce C.M., Braconi C., Fassan M., Valeri N. MIR21-induced loss of junctional adhesion molecule A promotes activation of oncogenic pathways, progression and metastasis in colorectal cancer. Cell Death Differ. 2021; 28(10): 2970–82. doi: 10.1038/s41418-021-00820-0.
- Knoll M., Lodish H.F., Sun L. Long non-coding RNAs as regulators of the endocrine system. Nat Rev Endocrinol. 2015; 11(3): 151–60. doi: 10.1038/nrendo.2014.229.
- Pardini B., Calin G.A. MicroRNAs and Long Non-Coding RNAs and Their Hormone-Like Activities in Cancer. Cancers (Basel). 2019; 11(3): 378. doi: 10.3390/cancers11030378.
- Derghal A., Djelloul M., Trouslard J., Mounien L. An Emerging Role of micro-RNA in the Effect of the Endocrine Disruptors. Front Neurosci. 2016; 10: 318. doi: 10.3389/fnins.2016.00318.
- Schveigert D., Krasauskas A., Didziapetriene J., Kalibatiene D., Cicenas S. Smoking, hormonal factors and molecular markers in female lung cancer. Neoplasma. 2016; 63(4): 504–9. doi: 10.4149/neo_2016_402.
- Meireles S.I., Esteves G.H., Hirata R. Jr., Peri S., Devarajan K., Slifker M., Mosier S.L., Peng J., Vadhanam M.V., Hurst H.E., Neves E.J., Reis L.F., Gairola C.G., Gupta R.C., Clapper M.L. Early changes in gene expression induced by tobacco smoke: Evidence for the importance of estrogen within lung tissue. Cancer Prev Res (Phila). 2010; 3(6): 707–17. doi: 10.1158/1940-6207.CAPR-09-0162.
- Meza R., Meernik C., Jeon J., Cote M.L. Lung cancer incidence trends by gender, race and histology in the United States, 1973–2010. PLoS One. 2015; 10(3). doi: 10.1371/journal.pone.0121323.
- Lortet-Tieulent J., Soerjomataram I., Ferlay J., Rutherford M., Weiderpass E., Bray F. International trends in lung cancer incidence by histological subtype: adenocarcinoma stabilizing in men but still increasing in women. Lung Cancer. 2014; 84(1): 13–22. doi: 10.1016/j.lungcan.2014.01.009.
- Smida T., Bruno T.C., Stabile L.P. Influence of Estrogen on the NSCLC Microenvironment: A Comprehensive Picture and Clinical Implications. Front Oncol. 2020; 10: 137. doi: 10.3389/fonc.2020.00137.
- Hirao-Suzuki M. Estrogen Receptor β as a Possible Double-Edged Sword Molecule in Breast Cancer: A Mechanism of Alteration of Its Role by Exposure to Endocrine-Disrupting Chemicals. Biol Pharm Bull. 2021; 44(11): 1594–7. doi: 10.1248/bpb.b21-00468.
- Zhang C., Schilirò T., Gea M., Bianchi S., Spinello A., Magistrato A., Gilardi G., Di Nardo G. Molecular Basis for Endocrine Disruption by Pesticides Targeting Aromatase and Estrogen Receptor. Int J Environ Res Public Health. 2020; 17(16): 5664. doi: 10.3390/ijerph17165664.
- Küblbeck J., Vuorio T., Niskanen J., Fortino V., Braeuning A., Abass K., Rautio A., Hakkola J., Honkakoski P., Levonen A.L. The EDCMET Project: Metabolic Effects of Endocrine Disruptors. Int J Mol Sci. 2020; 21(8): 3021. doi: 10.3390/ijms21083021.
- Delfosse V., Dendele B., Huet T., Grimaldi M., Boulahtouf A., Gerbal-Chaloin S., Beucher B., Roecklin D., Muller C., Rahmani R., Cavaillès V., Daujat-Chavanieu M., Vivat V., Pascussi J.M., Balaguer P., Bourguet W. Synergistic activation of human pregnane X receptor by binary cocktails of pharmaceutical and environmental compounds. Nat Commun. 2015; 6: 8089. doi: 10.1038/ncomms9089.
- Kassotis C.D., Stapleton H.M. Endocrine-Mediated Mechanisms of Metabolic Disruption and New Approaches to Examine the Public Health Threat. Front Endocrinol (Lausanne). 2019; 10: 39. doi: 10.3389/fendo.2019.00039.
- Safe S., Wormke M. Inhibitory aryl hydrocarbon receptor-estrogen receptor alpha cross-talk and mechanisms of action. Chem Res Toxicol. 2003; 16(7): 807–16. doi: 10.1021/tx034036r.
- Nomiri S., Hoshyar R., Ambrosino C., Tyler C.R., Mansouri B. A mini review of bisphenol A (BPA) effects on cancer-related cellular signaling pathways. Environ Sci Pollut Res Int. 2019; 26(9): 8459–67. doi: 10.1007/s11356-019-04228-9.
- Leng Y., Ren L., Niu S., Zhang T., Zhang J. In vitro and in silico investigations of endocrine disruption induced by metabolites of plasticizers through glucocorticoid receptor. Food Chem Toxicol. 2021; 155. doi: 10.1016/j.fct.2021.112413.
- Atlas E., Pope L., Wade M.G., Kawata A., Boudreau A., Boucher J.G. Bisphenol A increases aP2 expression in 3T3L1 by enhancing the transcriptional activity of nuclear receptors at the promoter. Adipocyte. 2014; 3(3): 170–9. doi: 10.4161/adip.28436.
- de la Rosa R., Vazquez S., Tachachartvanich P., Daniels S.I., Sillé F., Smith M.T. Cell-Based Bioassay to Screen Environmental Chemicals and Human Serum for Total Glucocorticogenic Activity. EnvironToxicol Chem. 2021; 40(1): 177–86. doi: 10.1002/etc.4903.
- Meakin C.J., Szilagyi J.T., Avula V., Fry R.C. Inorganic arsenic and its methylated metabolites as endocrine disruptors in the placenta: Mechanisms underpinning glucocorticoid receptor (GR) pathway perturbations. Toxicol Appl Pharmacol. 2020. doi: 10.1016/j.taap.2020.115305.
- Leng Y., Sun Y., Huang W., Lv C., Cui J., Li T., Wang Y. Identification of dicyclohexyl phthalate as a glucocorticoid receptor antagonist by molecular docking and multiple in vitro methods. Mol Biol Rep. 2021; 48(4): 3145–54. doi: 10.1007/s11033-021-06303-2.
- European Parliament. Directorate General for Internal Policies of the Union. Endocrine Disruptors: From Scientific Evidence to Human Health Protection Policy. Publications Office: LU, 2019.
- Kassotis C.D., Vandenberg L.N., Demeneix B.A., Porta M., Slama R., Trasande L. Endocrine-disrupting chemicals: economic, regulatory, and policy implications. Lancet Diabetes Endocrinol. 2020; 8(8): 719–30. doi: 10.1016/S2213-8587(20)30128-5.
- Hormonally Active Agents in the Environment. Committee on Hormonally Active Agents in the Environment. National Research Council, 1999.
- European Chemical Agency (ECHA) and European Food Safety Authority (EFSA) with the technical support of the Joint Research Centre (JRC); Andersson N., Arena M., Auteri D., Barmaz S., Grignard E., Kienzler A., Lepper P., Lostia A.M., Munn S., Parra Morte J.M., Pellizzato F., Tarazona J., Terron A., Van der Linden S. Guidance for the identification of endocrine disruptors in the context of Regulations (EU) No 528/2012 and (EC) No 1107/2009. EFSA J. 2018; 16(6). doi: 10.2903/j.efsa.2018.5311.
- Максимова В.П., Бугаева П.Е., Жидкова Е.М., Усалка О.Г.,Лесовая Е.А., Белицкий Г.А., Якубовская М.Г., Кирсанов К.И. Современные подходы к выявлению и изучению эпигенетически активных ксенобиотиков. Успехи молекулярной онкологии. 2019; 6(3): 8–27. [Maksimova V.P., Bugaeva P.E., Zhidkova E.M., Usalka O.G., Lesovaya E.A., Belitsky G.A., Yakubovskaya M.G., Kirsanov K.I. Modern approaches for the screening of epigenetically active xenobiotics. Advances in Molecular Oncology. 2019; 6(3): 8–27. (in Russian)]. doi: 10.17650/2313-805X-2019-6-3-8-27.


