Эволюция системной лекарственной терапии диссеминированного рака эндометрия. Обзор литературы
Автор: Даренская А. Д., Румянцев А. А., Гуторов С. Л., Тюляндина А. С.
Журнал: Злокачественные опухоли @malignanttumors
Рубрика: Обзоры и аналитика
Статья в выпуске: 2 т.13, 2023 года.
Бесплатный доступ
«Золотым стандартом» I линии лекарственной терапии диссеминированного рака эндометрия (РЭ) является комбинация «ТС» (Паклитаксел + Карбоплатин). Гормонотерапия (ГТ) в качестве терапии I линии рекомендована ограниченной группе пациенток. До недавнего времени прогноз больных диссеминированным РЭ, несмотря на проводимые стандартные методы лечения (химиотерапия (ХТ) и ГТ), оставался неутешительным. Ни один из цитостатиков, имеющихся в арсенале онкологов-химиотерапевтов, не обеспечивал долговременного контроля болезни и длительной выживаемости пациенток, получивших стандартную платиносодержащую терапию. Очевидно, что неудовлетворительные результаты лечения больных диссеминированным РЭ требовали изменения подходов к терапии и указывали на необходимость разработки более эффективных режимов лечения. Углубленное понимание механизмов канцерогенеза, появление новой молекулярной классификации РЭ и разделение лечебных подходов в зависимости от биологического потенциала опухоли привели к значительному прорыву в лечении диссеминированного РЭ. Одним из наиболее значительных прорывов следует считать открытие роли микро-сателлитной нестабильности (microsatellite instability, MSI) и нарушений в системе репарации неспаренных оснований ДНК (mismatch repair system, MMR) как предиктора высокой эффективности иммунотерапии - нового направления системной лекарственной терапии диссеминированного РЭ. Данная обзорная статья посвящена эволюции системной лекарственной терапии диссеминированного РЭ. В статье мы подробно обсуждаем результаты основных международных исследований по вопросам ГТ, ХТ I и II линий, таргетной терапии, иммунотерапии, а также иммунотаргетной терапии диссеминированного РЭ. Подробно рассматриваем такие биологические маркеры, как MSI, PD-L1, их влияние на эффективность лечения; разбираем механизм, лежащий в основе синергетического эффекта комбинации пембролизумаб + ленватиниб.
Рак эндометрия, микросателлитная нестабильность, msi, mss, пембролизумаб, ленватиниб, иммунотерапия, химиотерапия, гормонотерапия, таргетная терапия, mmr, pd-1, pd-l1
Короткий адрес: https://sciup.org/140300130
IDR: 140300130 | DOI: 10.18027/2224-5057-2023-13-2-6
Текст обзорной статьи Эволюция системной лекарственной терапии диссеминированного рака эндометрия. Обзор литературы
Рак эндометрия (РЭ) занимает одну из лидирующих позиций по распространенности злокачественных новообразований (ЗНО) как в мире, так и в России. Так, в структуре заболеваемости ЗНО женского населения России в 2021 году РЭ — на 3 месте (8,1 % всех ЗНО) после рака молочной железы (22,1 %) и ЗНО кожи (кроме меланомы, 13,4%). Абсолютное число впервые в жизни установленных диагнозов ЗНО тела матки в России в 2021 году составило 25482 случая заболевания. В 14,8% случаев РЭ был выявлен на III–IV стадиях опухолевого процесса [1,2], т. е. на тех стадиях, когда вопрос о проведении системной лекарственной терапии не вызывает сомнений.
ГОРМОНОТЕРАПИЯ
В течение нескольких десятилетий в лечении диссеминированного РЭ успешно используется гормонотерапия (ГТ). В качестве терапии I линии диссеминированного РЭ
ГТ может быть рекомендована ограниченной группе пациенток: при положительном гормональном статусе опухоли (рецепторы эстрогенов (РЭ +), рецепторы прогестерона (РП +)), степени дифференцировки G1-G2, при вялотекущем прогрессировании заболевания (ПЗ), отсутствии поражения висцеральных органов и симптомов болезни. У данной когорты больных РЭ ГТ может контролировать болезнь довольно долго.
ГТ прогестинами основывается на их физиологическом ингибировании эндометриальной стимуляции, индуцированной эстрогенами. Роль прогестинов в лечении диссеминированного РЭ впервые была продемонстрирована R. M. Kelley и W. H. Baker в 1961 году (n = 21). Частота объективного ответа (ЧОО) составила 29% [3].
Изучение трех наиболее часто используемых прогестинов в лечении диссеминированного РЭ (гидроксипрогестерона капроната, медроксипрогестерона ацетата (МПА) и мегестрола ацетата (МГА)) не выявило существенных различий в их эффективности [3–5].
Таблица 1. Эффективность ГТ при диссеминированном РЭ.
|
Препарат |
Режим лечения |
Число пациентов, n |
ЧОО, % |
Медиана ВБП, мес. |
Медиана ОВ, мес. |
|
МПА [4] (исследование III фазы) |
МПА 200 мг/сутки, per os |
145 |
25 |
3,2 |
11,1 |
|
МПА 1000 мг/сутки, per os |
154 |
15 |
2,5 |
7,0 |
|
|
МПА или тамоксифен [16] |
МПА 1 г в неделю, внутримышечно |
48 |
56,2 |
– |
– |
|
Тамоксифен 20 мг х 2 раза в сутки, per os |
45 |
53,4 |
– |
– |
|
|
Тамоксифен [17] |
Тамоксифен 20 мг х 2 раза в сутки, per os |
68 |
10 |
1,9 |
8,8 |
|
Арзоксифен [18] |
Арзоксифен 20 мг/сутки, per os |
29 |
31 |
3,7 |
– |
|
МПА+тамоксифен [11] |
Тамоксифен 40 мг, per os, – ежедневно, в сочетании с альтернирующим еженедельным приемом МПА 200 мг, per os, – ежедневно |
58 |
33 |
3 |
13 |
|
МГА или МГА+тамоксифен [5] |
МГА |
20 |
20 |
– |
12 |
|
МГА+тамоксифен |
42 |
19 |
– |
8,6 |
|
|
МГА, чередующийся с тамоксифеном [10] |
MГA 80 мг х 2 раза/сутки, – каждые 3 недели, чередующийся с тамоксифеном 20 мг х 2 раза в сутки, per os, – каждые 3 недели |
56 |
27 |
2,7 |
14,0 |
|
Анастрозол [12] |
Анастрозол 1 мг/сутки, per os, – в течение ≥28 дней |
23 |
9 |
1 |
6 |
|
Летрозол [13] |
Летрозол 2,5 мг/сутки, – непрерывно |
28 |
9,4 |
– |
– |
|
Фулвестрант [19] |
Фулвестрант 250 мг, внутримышечно, – каждые 4 недели |
22ER- |
0 |
2 |
3 |
|
31ER+ |
16 |
10 |
26 |
||
|
Фулвестрант [20] |
Фулвестрант 250 мг, внутримышечно, – каждые 4 недели |
35 |
11,4 |
2,3 |
13,2 |
|
Гозерелина ацетат [14] |
Гозерелина ацетат 3,6 мг, подкожно, – ежемесячно |
40 |
12 |
1,9 |
7,3 |
|
Трипторелин (агонист ГнРГ замедленного высвобождения) [15] |
Трипторелин 3,75 мг, – каждые 4 недели |
23 |
8,7 |
– |
7,2 |
* ГТ - гормонотерапия; РЭ - рак эндометрия; МПА - медроксипрогестерона ацетат; МГА - мегестрола ацетат; ГнРГ - гонадотропин рилизинг-гормон;
ЧОО – частота объективного ответа; ВБП – выживаемость без прогрессирования; ОВ – общая выживаемость; мес. – месяцы.
Эскалация дозы прогестинов не способствовала улучшению результатов прогестинотерапии [4].
Гипотеза о возможности антиэстрогенов увеличивать экспрессию РП [6,7] легла в основу назначения комбинации тамоксифена с прогестинами с целью достижения пролонгированных ремиссий. Однако большинство клинических исследований не выявили преимуществ комбинации тамоксифена с прогестинами в постоянном или последовательном режимах по сравнению с прогестинотерапией в монорежиме [5,8–11].
Ингибиторы ароматазы также не продемонстрировали значимых результатов в лечении распространенного РЭ [12,13].
При РЭ широко изучались агонисты гонадотропин рилизинг-гормона (ГнРГ) [14,15]. Результаты основных исследований по эффективности ГТ при диссеминированном РЭ представлены в табл. 1.
ХИМИОТЕРАПИЯ И ТАРГЕТНАЯ ТЕРАПИЯ
Показаниями к назначению химиотерапии (ХТ) в качестве I линии являются: рецидив или исходно диссеминированная форма РЭ, быстрый рост опухоли, низкая степень дифференцировки, поражение висцеральных органов.
Эффективность I линии ХТ при распространенном или рецидивирующем РЭ изучалась в ряде рандомизированных клинических исследований III фазы.
В рандомизированное исследование III фазы, проведенное Гинекологической исследовательской группой (GOG), — GOG-48 включали пациенток распространенным или рецидивирующим РЭ, которым ранее проводились хирургическое лечение, лучевая терапия (ЛТ) и ГТ; ни одна из них не получала предшествующую терапию цитостатиками. Пациентки были рандомизированы в 2 группы: группу монотерапии доксорубицином 60 мг/м2 либо в группу комбинации «АС» (доксорубицин 60 мг/м2 + циклофосфамид 500 мг/м2, каждые 3 недели), суммарно — 8 курсов терапии [21]. Из 132 пациенток, получивших доксорубицин в монорежиме, полных регрессий (ПР) было зарегистрировано 7 (5%), частичных регрессий (ЧР) — 22 (17%), стабилизация болезни (СБ) отмечена в 73 (55%) случаях. Из 144 пациенток, получивших комбинацию «АС», ПР наблюдалась в 18 (13%) случаях, ЧР — в 25 (17%), СБ — в 75 (52%) случаях. Медиана выживаемости без прогрессирования (ВБП) достигла 3,2 месяцев (мес.) и 3,9 мес., медиана общей выживаемости (ОВ) — 6,7 мес. и 7,3 мес. в группах доксорубицина и комбинации «АС» соответственно (р = 0,048). Таким образом, комбинация «АС» продемонстрировала некоторое преимущество перед монотерапией доксорубицином в лечении пациенток РЭ.
В исследование III фазы GOG-107 включали пациенток рецидивирующим или распространенным РЭ III–IV стадий, которые были рандомизированы в 2 группы: группу монотерапии доксорубицином 60 мг/м 2 либо в группу комбинации «АР» (доксорубицин 60 мг / м 2 + цисплатин 50 мг/м 2 , каждые 3 недели), до ПЗ, неприемлемой токсичности или кумулятивной дозы доксорубицина — 500 мг/м 2 [22]. Среди 150 пациенток, получивших доксорубицин в монорежиме, зарегистрировано 12 (8%) ПР и 26 (17%) ЧР, в то время как среди пациенток, получавших комбинацию «АР» (n = 131), ПР отмечена у 25 больных (19 %), ЧР — у 30 (23%) (р = 0,004). Медиана ВБП в 2 группах составила 3,8 мес. и 5,7 мес. соответственно (отношение рисков (HR) — 0,736; 95% доверительный интервал (ДИ) 0,577–0,939; р = 0,014), медиана ОВ — 9,2 мес. и 9,0 мес. соответственно (HR — 0,928; 95%ДИ 0,727– 1,185). Наиболее частыми нежелательными явлениями (НЯ) были: лейкопения III–IV степени (40% — в группе монотерапии доксорубицином против 62% — в группе комбинации «АР»), тромбоцитопения (2% против 14% соответственно), анемия (4% против 22%) и тошнота/рвота (3 % против 13 % соответственно). Таким образом, было показано, что добавление цисплатина к доксорубицину при распространенном РЭ увеличивает ЧОО и улучшает ВБП, но не оказывает статистически значимого влияния на ОВ, при этом сопровождается более высокой частотой побочных эффектов.
Эти результаты послужили основой для последующих рандомизированных исследований III фазы у пациенток диссеминированным и местнораспространенным РЭ. Так, целью исследования III фазы GOG 177 [23] было определить, улучшает ли ОВ добавление паклитаксела к доксорубицину и цисплатину у женщин распространенным/ рецидивирующим РЭ (n = 263). Вторичными конечными точками являлись ВБП, ЧОО и безопасность. Пациентки получали комбинацию «АР» (доксорубицин 60 мг /м2 + цисплатин 50 мг/м2) или трехкомпонентный режим «ТАР» (доксорубицин 45 мг/м2 + цисплатин 50 мг/м2, день 1, затем паклитаксел 160 мг/м2, день 2, с поддержкой филграстимом). Начальная доза доксорубицина в группе АР была снижена до 45 мг/м2 у пациентов с ЛТ на органы малого таза (ОМТ) в анамнезе и у пациентов старше 65 лет. Оба режима повторялись каждые 3 недели, суммарно — до 7 курсов. ЧОО (57% против 34%; р < 0,01), медиана ВБП (8,3 мес. против 5,3 мес.; р < 0,01) и медиана ОВ (15,3 мес. против 12,3 мес.; р = 0,037) были достоверно выше в группе пациентов, получающих режим «ТАР». Профиль токсичности был приемлемым. Фебрильная нейтропения отмечена только у 2% пациентов, получавших «АР», и у 3 % пациентов — получавших «ТАР». Однако в группе с Паклитакселом значительно чаще, чем в группе «АР», лечение осложнялось периферической сенсорной полинейропатией (ПСН) II–III степени (ПСН II степени —у 27% и 4%, ПСН III степени — у 12% и 1% больных соответственно). Таким образом, было продемонстрировано, что режим «TAP» значительно улучшает ЧОО, ВБП и OВ по сравнению с «AP», однако высокая токсичность лечения зачастую не позволяла широко применять данный режим.
В настоящее время «золотым стандартом» I линии ХТ распространенного РЭ или его рецидивов является комбинация «ТС» (паклитаксел + карбоплатин). Ее эффективность была продемонстрирована в рандомизированном исследовании III фазы NRG Oncology GOG-209, посвященном сравнению схемы «TAP» (доксорубицин 45 мг/м 2 + цисплатин 50 мг/м 2 , день 1 + паклитаксел 160 мг/м 2 , день 2) и комбинации «ТС» (паклитаксел 175 мг/ м 2 + карбоплатин AUC6, день 1) в качестве первоначальной терапии распространенного РЭ [24,25]. Статистический дизайн исследования был спланирован таким образом, чтобы доказать равную эффективность изучаемых комбинаций препаратов (non-inferiority). Суммарно в исследование была включена 1381 пациентка; сформированные группы сбалансированы по основным демографическим характеристикам. По результатам исследования медиана ВБП составила 13 мес. и 14 мес. в группах схемы «TAP» и комбинации «ТС» (HR — 1,032; 90% ДИ 0,93–1,15), медиана ОВ — 41 мес. и 37 мес. соответственно (HR — 1,002; 90% ДИ 0,895–1,121). ЧОО в обеих группах — 52%. При этом режим ХТ «TAP» характеризовался неблагоприятным профилем безопасности вследствие повышения частоты развития ряда НЯ III–IV степени, включая астению, тошноту и рвоту, диарею, нефротоксичность и др. Таким образом, достаточно высокая эффективность и удовлетворительная переносимость комбинации «ТС» закрепила данный режим ХТ в качестве режима I линии системной терапии диссеминированного РЭ [26,27].
Было проведено несколько исследований II фазы по изучению эффективности комбинации бевацизумаба, паклитаксела и карбоплатина по сравнению с комбинацией «ТС» в I линии лекарственной терапии диссеминированного РЭ. В исследовании MITO END-2 (n = 108) медиана ВБП в группе комбинации бевацизумаба, паклитаксела и карбоплатина и группе «ТС» составила 13 мес. (95%ДИ 9,2–16,8) и 8,7 мес. (95%ДИ 6,3–11,2) соответственно (p = 0,036) [28]. При этом в группе бевацизумаба, паклитаксела и карбоплатина достоверно чаще регистрировалась токсичность > III степени со стороны сердечно-сосудистой системы.
В рандомизированное исследование II фазы GOG-86P (n = 329) включали пациенток распространенным и рецидивирующим РЭ, ранее не получавших системной ХТ. Была проведена сравнительная оценка эффективности стандартной ХТ «ТС» и 3 режимов лечения (комбинация бевацизумаба, паклитаксела и карбоплатина с последующей поддерживающей терапией бевацизумабом, темсиролимус, паклитаксел и карбоплатин с последующей поддерживающей терапией темсиролимусом и комбинация бевацизумаба, карбоплатина и иксабепилона с последующей поддерживающей терапией бевацизума-бом) [29]. На ASCO-2015 были представлены результаты этого исследования. ЧОО составляла 60%, 55% и 53% в 3 подгруппах соответственно. Преимуществ в медиане ВБП не было продемонстрировано ни в одной изучаемой комбинации по сравнению с группой контроля: HR — 0,81, 92%ДИ 0,63–1,02; HR — 1,22, 92%ДИ 0,96–1,55 и HR — 0,87, 92%ДИ 0,68–1,11 соответственно. Однако было достигнуто достоверное увеличение медианы ОВ в группе комбинации бевацизумаба, паклитаксела и карбоплатина по сравнению с группой контроля — 34,0 мес. и 22,7 мес. соответственно (р < 0,039). Токсичность > III степени в группе комбинации бевацизумаба, паклитаксела и карбоплатина составила 93,7%. Таким образом, добавление к стандартной ХТ бева-цизумаба позволяет улучшить отдаленные результаты лечения, однако его применение должно сопровождаться тщательным мониторированием побочных эффектов.
Традиционно РЭ разделяли на два подтипа в зависимости от патоморфологического варианта опухоли:
-
• РЭ I патогенетического типа (эндометриоидный) встречается чаще (примерно у 80% больных РЭ), развивается в более молодом возрасте, на фоне длительной гипер-эстрогении и гиперплазии эндометрия. У больных РЭ I патогенетического типа часто наблюдаются ожирение, сахарный диабет и гипертоническая болезнь, возможны эстроген-секретирующие опухоли яичников или синдром склерокистозных яичников. Опухоли I патогенетического варианта, как правило, высокодифференцированные, имеют более благоприятный прогноз.
-
• РЭ II патогенетического типа (неэндометриоидный) (серозный) (выявляется в 20% случаев) обычно низкодифференцированный, имеет менее благоприятный
прогноз, возникает в старшем возрасте, в отсутствие гиперэстрогении, на фоне атрофии эндометрия [26]. Установлено, что при серозном РЭ часто выявляется гиперэкспрессия HER-2/neu — белка-рецептора эпидермального фактора роста человека 2-го типа, который присутствует в тканях и в норме, участвуя в регуляции деления и дифференцировки клеток. Гиперэкспрессия этого рецептора на поверхности опухолевых клеток ассоциирована с более агрессивным течением заболевания, повышенным метастатическим потенциалом опухоли и неблагоприятным прогнозом.
В многоцентровом рандомизированном исследовании II фазы [30] оценивали эффективность применения трастузумаба в комбинации с ХТ при серозном РЭ. Трастузумаб — рекомбинантное гуманизированное моноклональное антитело (МКА), которое селективно взаимодействует с внеклеточным доменом HER-2/neu на поверхности злокачественных клеток и тормозит их пролиферацию. В исследование включали пациенток распространенным (III–IV стадии) и рецидивирующим серозным РЭ с гиперэкспрессией HER-2/neu. Все больные (n = 61) были рандомизированы в 2 группы: «ТС» (контрольная группа) и «ТС» (6 курсов) в комбинации с внутривенным капельным (в/в кап.) введением трастузумаба (экспериментальная группа), до прогрессирования или непереносимой токсичности. Первичная конечная точка — ВБП. Среди всех участниц медиана ВБП составила 8,0 мес. (контрольная группа) по сравнению с 12,6 мес. (экспериментальная группа) (р = 0,005; HR — 0,44; 90%ДИ 0,26–0,76). Медиана
Таблица 2. Эффективность ХТ II линии при диссеминированном РЭ. Исследования II фазы.
|
Препарат |
Режим лечения |
Число пациентов, n |
ЧОО, % |
Медиана ВБП, мес. |
Медиана ОВ, мес. |
|
Паклитаксел [31] |
Паклитаксел 200 мг/м 2 , в/в кап., каждые 21 день (175 мг/м 2 -у пациенток, ранее получавших ЛТ на ОМТ) |
44 |
27,3 |
— |
10,3 |
|
Паклитаксел [32] |
Паклитаксел 80 мг/м 2 , в/в кап., 1-часовая инфузия, каждые 7 дней |
15 |
26,7 |
— |
— |
|
Доцетаксел [33] |
Доцетаксел 36 мг/м 2 , в/в кап., 1-часовая инфузия, дни 1, 8 и 15, каждые 28 дней |
26 |
7,7 |
2,0 |
6,4 |
|
Гемцитабин [34] |
Гемцитабин 800 мг/м², в/в кап., 30-минутная инфузия, дни 1 и 8, каждые 21 день |
23 |
4,0 |
1,7 |
— |
|
Оксалиплатин [35] |
Оксалиплатин 130 мг/м 2 , в/в кап., 2-х-часовая инфузия, каждые 21 день |
52 |
13,5 |
— |
— |
|
Доксорубицин [36] |
Доксорубицин 60 мг/м 2 , в/в кап., день 1, каждые 3 недели |
17 |
0 |
2,1 |
5,8 |
|
Доксорубицин [37] |
Доксорубицин 60 мг/м 2 , в/в кап., день 1, каждые 3 недели |
33 |
12,1 |
4,4 |
8,1 |
|
ПЛД [38] |
ПЛД 50 мг/м 2 , в/в кап., 1-часовая инфузия, каждые 4 недели |
42 |
9,5 |
— |
8,2 |
|
Ифосфамид [39] |
Ифосфамид, 1,2 г/м 2 , в/в кап., ежедневно, в течение 5 дней, каждые 4 недели + месна 300 мг/м 2 , в/в, каждые 4 часа, в течение 5 дней |
40 |
15 |
— |
— |
|
Этопозид [40] |
Этопозид 50–60 мг/м 2 /день (30 мг/м 2 /день — с предшествующей ЛТ), per os, в течение 21 дня |
22 |
0 |
— |
— |
|
Топотекан [41] |
Топотекан 0,5–1,5 мг/м 2 , в/в кап., — ежедневно, в течение 5 дней, каждые 3 недели |
22 |
9 |
— |
— |
|
Пеметрексед [42] |
Пеметрексед 900 мг/м 2 , в/в кап., 10-минутная инфузия, каждые 21 день |
25 |
4 |
2,7 |
9,4 |
|
Иксабепилон [43] |
Иксабепилон 40 мг/м 2 , 3-х-часовая инфузия, каждые 3 недели |
50 |
12 |
2,9 |
— |
* ХТ — химиотерапия; РЭ — рак эндометрия; ЧОО — частота объективного ответа; ВБП — выживаемость без прогрессирования; ОВ — общая выживаемость; мес. — месяцы; в / в кап. — внутривенно капельно; ЛТ — лучевая терапия; ОМТ — органы малого таза; ПЛД — пегилированный липосомальный доксорубицин.
ВБП достигла 9,3 мес. (контрольная группа) по сравнению с 17,9 мес. (экспериментальная группа) — среди 41 пациентки с заболеванием стадии III или IV, получавших I линию терапии (р = 0,013; HR — 0,40; 90%ДИ 0,20–0,80), и 6,0 мес. (контрольная группа) и 9,2 мес. (экспериментальная группа) соответственно — среди 17 пациенток с рецидивом заболевания (р = 0,003; ОР 0,14; 90 %ДИ 0,04–0,53). Токсичность между группами не различалась. Таким образом, было показано, что добавление трастузумаба к «ТС» характеризуется благоприятным профилем безопасности и способствует увеличению медианы ВБП. При этом наибольший эффект отмечен при использовании трастузумаба в I линии лечения.
В отличие от ХТ I линии, II линия ХТ диссеминированного РЭ демонстрирует более худшие результаты: ЧОО намного ниже. Поскольку результаты применения ХТ II линии при РЭ неудовлетворительные, рандомизированных исследований, посвященных этой теме, мало и большинство данных по эффективности химиопрепаратов во II линии терапии диссеминированного РЭ получены в ходе нерандомизированных исследований II фазы (табл. 2).
Как видно из табл. 2, наилучший результат был получен при использовании паклитаксела в монорежиме (3-х-не-дельный режим), где ЧОО составила 27,3% [31].
Еженедельное введение паклитаксела продемонстрировало значимую эффективность у пациенток метастати-
Таблица 3. Эффективность таргетной терапии при диссеминированном РЭ.
|
Препарат |
Режим лечения |
Число пациентов, n |
ЧОО, % |
Медиана ВБП, мес. |
Медиана ОВ, мес. |
|
Афлиберцепт [48] |
Афлиберцепт 4 мг/кг, в/в кап., каждые 14 дней |
44 |
7 |
2,9 |
14,6 |
|
Далантерцепт [49] |
Далантерцепт 1,2 мг/кг, подкожно, каждые 3 недели |
28 |
0 |
2,1 |
14,5 |
|
Бевацизумаб [50] |
Бевацизумаб 15 мг/кг, в/в кап., каждые 3 недели |
52 |
13,5 |
4,2 |
10,5 |
|
Сунитиниб [51] |
Сунитиниб 50 мг/сутки, на протяжении 4 недель, 2 недели — перерыв |
33 |
18,1 |
3 |
19,4 |
|
Сорафениб [52] |
Сорафениб 400 мг х 2 раза в сутки, per os, цикл — каждые 28 дней |
40 |
5 |
— |
11,4 |
|
Бриваниб [53] |
Бриваниб 800 мг, per os, ежедневно |
43 |
18,6 |
3,3 |
10,7 |
|
Нинтеданиб [54] |
Нинтеданиб 200 мг х 2 раза в сутки |
32 |
9,4 |
— |
— |
|
Цедираниб [55] |
Цедираниб 30 мг/сутки, per os, ежедневно, цикл — каждые 28 дней |
48 |
12,5 |
3,65 |
12,5 |
|
Требаниб [56] |
Требаниб 15 мг/кг, в/в кап., еженедельно |
32 |
3,1 |
1,97 |
6,6 |
|
Гефитиниб [57] |
Гефитиниб 500 мг/сутки, per os, ежедневно |
26 |
3,8 |
— |
— |
|
Эрлотиниб [58] |
Эрлотиниб 150 мг/сутки |
32 |
12,5 |
— |
— |
|
Цетуксимаб [59] |
Цетуксимаб 400 мг/м 2 , в/в кап., (начальная доза), далее — 250 мг/м 2 , еженедельно, цикл — каждые 4 недели |
20 |
5 |
— |
— |
|
Трастузумаб [60] |
Трастузумаб 4 мг/кг, в/в кап., в течение 1 недели, далее — по 2 мг/кг, еженедельно |
33 |
0 |
— |
— |
|
Лапатиниб [61] |
Лапатиниб 1500 мг/сутки, per os, ежедневно |
30 |
3,3 |
— |
— |
|
Эверолимус [62] |
Эверолимус 10 мг, per os, ежедневно, цикл — каждые 28 дней |
28 |
0 |
— |
— |
|
Эверолимус [63] |
Эверолимус 10 мг, per os, ежедневно |
44 |
5 |
2,8 |
8,1 |
|
Темсиролимус [64] |
Темсиролимус 25 мг, в/в кап., еженедельно, цикл — каждые 4 недели |
29, не получавших х/т |
14 |
— |
— |
|
25, получавших х/т |
4 |
— |
— |
||
|
Темсиролимус или тем-сиролимус + МГА, чередующийся с тамоксифеном [65] |
Темсиролимус 25 мг, в/в кап., еженедельно |
50 |
22 |
— |
— |
|
Комбинация темсиролимуса еженедельно + МГА 80 мг х 2 раза в сутки, в течение 3 недель, с чередованием тамоксифена 20 мг х 2 раза в сутки, в течение 3 недель |
21 |
14 |
— |
— |
|
|
Ридафоролимус [66] |
Ридафоролимус 12,5 мг, в/в кап., в течение 5 дней, каждые 2 недели; цикл — каждые 4 недели. |
45 |
11 |
— |
— |
|
Ридафоролимус или прогестины/ХТ [67] |
Ридафоролимус, per os |
64 |
0 |
3,6 |
— |
|
Прогестины или ХТ |
66 |
4 |
1,9 |
— |
|
|
Эверолимус + летрозол [47] |
Эверолимус 10 мг/сутки, per os + летрозол 2,5 мг в сутки, per os, цикл — каждые 4 недели |
35 |
32 |
— |
— |
|
Темсиролимус + бева-цизумаб [68] |
Темсиролимус 25 мг, в/в кап., еженедельно + бевацизумаб 10 мг/кг, в/в кап., каждые 2 недели |
49 |
24,5 |
5,6 |
16,9 |
* РЭ — рак эндометрия; ЧОО — частота объективного ответа; ВБП — выживаемость без прогрессирования; ОВ — общая выживаемость; мес. — месяцы; в / в кап. — внутривенно капельно.
ческим или рецидивирующим РЭ, предлеченных комбинацией паклитаксела и препаратов платины [44].
Такие препараты, как ифосфамид, оксалиплатин, пегилированный липосомальный доксорубицин (ПЛД), топотекан, доцетаксел в монорежиме обладают меньшей противоопухолевой активностью, нежели паклитаксел [33,35,38,39,41]. При использовании этопозида ЧОО равнялась 0 [40].
Отдельного внимания заслуживают случаи длительной ремиссии опухолевого процесса после ранее проведенной платиносодержащей ХТ — так называемые «платиночувствительные» рецидивы заболевания. Прогностическая значимость бесплатинового интервала (PFI) после I линии ХТ распространенного РЭ изучалась во многих исследованиях [45,46]. В обзоре GOG данных 5 рандомизированных исследований III фазы по системной ХТ РЭ (n = 586) у пациенток с PFI > 6 мес. отмечено снижение относительного риска смерти на 30% по сравнению с подгруппой больных с PFI < 6 мес. (HR—0,70; 95%ДИ 0,59–0,84, p < 0,0001) [45].
В многоцентровом ретроспективном исследовании SGSG-012/GOTIC-004/Intergroup [46] (n=262) проводили оценку эффективности повторного назначения платиносодержащей ХТ при рецидивах РЭ. У больных РЭ с PFI < 6 мес., 6–11 мес., 12–23 мес. и > 24 мес. ЧОО составила 25%, 38%, 61 % и 65% соответственно. При проведении платиносодержащей ХТ II линии у пациенток с PFI < 6 мес., 6–11 мес., 12–23 мес. и > 24 мес., при медиане длительности наблюдения 16,9 мес., медиана ВБП составила 3,2 мес. (95%ДИ 2,3–4,3), 6,0 мес. (95%ДИ 4,4– 7,3), 7,8 мес. (95 %ДИ 5,8–10,6) и 13,4 мес. (95%ДИ 10,2– 20,0) соответственно (р < 0,0001); медиана ОВ — 11,3 мес. (95%ДИ 7,9–17,5), 14,8 мес. (95% ДИ 11,5–19,5), 27,8 мес. (95%ДИ 16,6–36,1) и 43,0 мес. (95 %ДИ 27,4–74,7) соответственно (р < 0,0001). Таким образом, «платиночувствительный» рецидив РЭ является фактором благоприятного прогноза в отношении непосредственной эффективности ХТ II линии, а также выживаемости пациенток.
Проведен ряд исследований [47–68] по изучению роли таргетной терапии в лечении диссеминированного РЭ, результаты которых, в большинстве случаев, не продемонстрировали значительных успехов: ЧОО составила 0–24,5%, медиана ВБП не превышала 5–6 мес. (табл. 3). Как видно из табл. 3, комбинация эверолимуса и летрозола является наиболее эффективной, ЧОО — 32% [47].
ИММУНОТЕРАПИЯ
Несомненно, что столь неудовлетворительные результаты лечения пациенток диссеминированным РЭ требовали изменения подходов к терапии, разработки более эффективных режимов лечения.
Принимая во внимание геномные, транскриптомные и протеомные особенности опухолевых клеток, специалисты The Cancer Genome Atlas Research Network (TCGA) разработали новую молекулярную классификацию РЭ, основанную на молекулярном профиле опухоли, в которой выделяют 4 молекулярно-генетических подтипа: POLE-ультрамутированный (POLEmut), MSI-гипермутированный (MSI-h), с низким числом копий генов (не имеющий специфического молекулярного профиля, NSMP) и высоким числом копий генов (серозо-подобный, самый неблагоприятный подтип, несущий мутации ТР53, p53mut) [69–73].
Попытки персонификации лекарственной терапии показали, что наибольшее значение для терапии распространенного РЭ имеет микросателлитная нестабильность (microsatellite instability, MSI). Наличие высокой микросателлитной нестабильности (high level microsatellite instability, MSI-h) указывает на дефекты в системе репарации неспаренных оснований ДНК (deficient mismatch repair system, dMMR). Система репарации неспаренных нуклеотидов ДНК (mismatch repair system, MMR) является одной из составляющих сложного механизма поддержания стабильности генома клетки. Основная функция данной системы — устранение ошибок репликации ДНК, возникающих при делении клеток, когда во время построения новой нити происходит ошибочная вставка некомплементарного нуклеотида, в результате чего возникает несоответствие. За работу системы MMR отвечают 6 генов: MLH1, MSH2, MSH6, PMS2, MSH3 и MLH3. Наличие герминальных мутаций в этих генах приводит к развитию синдрома Линча (5% случаев РЭ). Чаще встречается другой, ненаследственный, механизм формирования dMMR, в подавляющем большинстве случаев, заключающийся в гиперметилировании участка CpG в промотере MLH1 в самой опухоли, и, как следствие, — инактивация MLH1 [74;75]. В результате dMMR появляется большое число мутаций со сдвигом рамки считывания, что приводит к формированию стоп-кодонов и синтезу нефункциональных белков. Микросателлиты представляют собой короткие последовательности в ДНК из 1–5 оснований, повторяющиеся до нескольких десятков раз. Микросателлиты встречаются и в норме, однако при dMMR их число увеличивается, что и может быть выявлено. Понятия dMMR и MSI описывают один и тот же процесс.
Результаты проведенных исследований продемонстрировали, что РЭ является одним из лидеров по частоте встречаемости MSI-h/dMMR. В мета-анализе 26 исследований, опубликованном M. Lorenzi et al. в 2020 году (n = 1302), частота встречаемости MSI-h при РЭ составила 25%, причем была обнаружена схожая частота данной генетической альтерации при ранних и поздних стадиях заболевания [76].
В результате дефектной системы репликации ДНК, появления POLE-инактивирующих мутаций и dMMR (MLH1, MSH2, MSH6, PMS2), происходит значительное увеличение мутационной нагрузки в опухоли (TMB-high), что коррелирует с высоким уровнем неоантигенов и опухоль-инфильтрирующих лимфоцитов CD3 + и CD8 + (TIL). Это создает определенное микроокружение опухоли, которое является благоприятным для иммунологического ответа.
Таким образом, молекулярная характеристика РЭ имеет большое значение для обоснования использования ингибиторов контрольных точек иммунитета в лечении данной нозологии. Одним из таких препаратов является пембролизумаб — гуманизированное МКА, которое селективно блокирует взаимодействие между рецептором PD-1 (Programmed cell death-1, рецептор сигнального пути программируемой клеточной гибели-1) на Т-лимфоцитах и его лигандами PD-L1 и PD-L2 (programmed cell death-1 /-2 pathway ligand, лиганд рецептора сигнального пути программируемой клеточной гибели-1/-2) на опухолевой клетке (рис. 1). PD-1-это рецептор иммунной контрольной точки, ограничивающий активность Т-лимфоцитов в периферических тканях. Опухолевые клетки могут использовать метаболический путь с участием PD-1 для ингибирования активного Т-клеточного иммунологического надзора. В результате ингибирования связывания рецептора PD-1 с его лигандами пембролизумаб реактивирует опухоль-специфичные цитотоксические Т-лимфоциты в микроокружении опухоли и таким образом реактивирует противоопухолевый иммунитет.
Проведенные исследования в области иммунотерапии злокачественных опухолей позволили выявить группу больных с MSI-h, высокочувствительных к терапии пем-бролизумабом в монорежиме [77–81], а также к терапии некоторыми другими антагонистами PD-L1/PD-1 [82,83].
В многоцентровом нерандомизированном открытом мультикогортном исследовании II фазы KEYNOTE-158 (NCT02628067) приняли участие предлеченные больные (n = 21), получившие одну и более стандартные линии ХТ, имеющие различные MSI-h/dMMR-распространен-ные солидные опухоли, включая РЭ (n = 4) [84]. Все больные получали пембролизумаб 200 мг, в/в кап., каждые 3 недели, до ПЗ или непереносимой токсичности. Первичная конечная точка — ЧОО, оцениваемая по критериям RECIST (версия 1.1). Вторичные конечные точки включали: длительность ответа, ВБП, ОВ и безопасность. При медиане длительности наблюдения 4,5 мес. ЧОО составила 42,9% (95%ДИ 21,8–66,0).
На основании первых обнадеживающих результатов данного исследования, несмотря на II фазу исследования и небольшое число больных, в 2017 году пембролизумаб в монорежиме был одобрен FDA (Американским управлением по надзору за качеством пищевых продуктов и медикаментов) для пациентов с MSI-h солидными опухолями, у которых зарегистрировано прогрессирование после предшествующей системной терапии и не имеющих альтернативных вариантов противоопухолевого лечения.
В 2022 году были представлены обновленные результаты исследования II фазы KEYNOTE-158 (NCT02628067) [85]. Следует напомнить, что в когорту D вошли пациентки РЭ независимо от статуса MSI-h/dMMR, в когорту K — больные, имеющие любую солидную опухоль MSI-h/dMMR, кроме колоректального рака. Все участники получали пембролизумаб 200 мг, в/в кап., каждые 3 недели, в течение 35 циклов. По состоянию на 5 октября 2020 года, 18 (20%) из 90 пролеченных больных РЭ завершили 35 циклов пем-бролизумаба и 52 (58%) пациента прекратили лечение. При медиане длительности наблюдения 42,6 мес. ЧОО составила 48% (95%ДИ 37–60), а медиана длительности ответа не была достигнута (2,9–49,7 мес. +). Медиана ВБП составила 13,1 мес. (95%ДИ 4,3–34,4), медиана ОВ не была достигнута (27,2 мес. +). Среди всех пролеченных больных у 76% было ≥ 1 НЯ, связанное с лечением (III–IV степени — 12%). Летальных исходов, связанных с лечением, не было. Иммуноопосредованные НЯ или инфузионные реакции возникали у 28 % больных (III–IV степени — у 7%; летальных исходов не было). На сегодняшний день данная терапевтическая опция прочно закреплена в российских и зарубежных клинических рекомендациях по лечению РЭ [26,27].
При диссеминированном РЭ были изучены и другие моноклональные ингибиторы иммунных контрольных точек, включая ниволумаб (полностью человеческое IgG4-анти-PD-1 МКА), 240 мг, в/в кап., каждые 2 недели, авелумаб (анти-PD-L1), 10 мг/кг, в/в кап., каждые 2 недели, дурвалумаб (анти-PD-L1), 1500 мг, в/в кап., каждые 4 недели и достарлимаб (анти-PD-1 МКА), 500 мг, в/в кап., каждые 3 недели, 4 введения, затем — 1000 мг, в/в кап., каждые 6 недель. ЧОО при использовании данных препаратов у пациенток с MSI-h-опухолями — 23%, 26,7%, 47% и 42,3% соответственно [83,86–88].
В когорте пациенток диссеминированным РЭ с микро-сателлитно-стабильными опухолями (microsatellite-stable, MSS), без дефекта в системе репарации неспаренных оснований ДНК (proficient mismatch repair system, рMMR), эффективность монотерапии ингибиторами контрольных точек намного более скромная. Например, для дурвалу-маба ЧОО составляет лишь 3% [83,89].
В 2017 году были опубликованы результаты мультикогорт-ного исследования Ib фазы KEYNOTE-028 (NCT02054806) [90], в которое включали больных распространенными PD-L1-позитивными солидными опухолями. В одну из когорт входили пациентки местно-распространенным или метастатическим PD-L1-позитивным РЭ (n = 24) с ПЗ на фоне стандартной терапии. Дополнительный анализ 19 образцов опухолевой ткани РЭ не выявил MSI-h в 94,7% случаев. Пятнадцать (62,5%) из этих 24 участниц получили, по крайней мере, две предшествующие линии терапии по поводу диссеминированной болезни. Пациентки получали пем-бролизумаб в монорежиме, в дозе 10 мг/кг, каждые 2 недели, на срок до 24 месяцев или до прогрессирования и/или непереносимой токсичности. Первичная конечная точка оценки эффективности — ЧОО по RECIST (версия 1.1). ЧР была зарегистрирована лишь у 13% (95%ДИ 2,8–33,6) больных (n = 3), еще у 13 % достигнута СБ. У 13 (54,2 %) участниц наблюдались связанные с лечением НЯ: утомляемость (20,8%), зуд (16,7%), лихорадка (12,5 %) и снижение аппетита (12,5%). Связанные с лечением НЯ III степени зарегистрированы у 4 пациенток, ни у одной не было НЯ IV степени, и ни одна не прекратила лечение из-за НЯ.
Современные возможности применения иммунотерапии (в частности пембролизумаба) в монорежиме имеют свои ограничения из-за первичной резистентности, что часто связывают с иммуносупрессивными характеристиками микроокружения опухоли. Это характеризуется большей инфильтрацией иммуносупрессивных клеток, снижающих активность эффекторных иммунных клеток: цитоксических Т-лимфоцитов и Т-хелперов [91].
ИММУНОТАРГЕТНАЯ ТЕРАПИЯ
Ленватиниб является пероральным мультитаргетным ингибитором рецепторов тирозинкиназ, избирательно подавляющим киназную активность рецепторов фактора роста эндотелия сосудов 1–3 типов — VEGFR1 (FLT1), VEGFR2 (KDR) и VEGFR3 (FLT4). Также ленватиниб оказывает ингибирующее воздействие на другие рецепторы тирозин-киназ, задействованные в проангиогенных и онкогенных механизмах, включая рецепторы фактора роста фибробластов 1–4 типов (FGFR1–4), альфа-рецептор тромбоцитарного фактора роста (PDGFRa), а также рецепторы тирозинкиназ KIT и RET.
В исследовании I фазы [92] ленватиниб в монорежиме показал многообещающую противоопухолевую актив- ность у больных с запущенными солидными опухолями, в том числе у 4 пациенток РЭ.
В международном открытом несравнительном многоцентровом исследовании II фазы [93,94] оценивали эффективность ленватиниба в монорежиме во II линии терапии у пациенток рецидивирующим нерезектабельным РЭ после ХТ I линии на основе препаратов платины. Первичной конечной точкой была ЧОО. Вторичные конечные точки включали: медиану ВБП, медиану ОВ и контроль над болезнью. Участницы (n = 133) получали ленватиниб в монорежиме, 24 мг/сутки, per os, каждые 28 дней. ЧОО составила 14,3% (95%ДИ 8,8–21,4). Длительная СБ (≥ 23 недель) наблюдалась в 23,3% случаев. Таким образом, контроль над болезнью зарегистрирован в 37,6% случаев (95%ДИ 29,3–46,4). Медиана ВБП составила 5,6 мес. (95%ДИ 3,7– 6,3), а медиана ОВ — 10,6 мес. (95%ДИ 8,9–14,9). Наиболее частыми НЯ (любой степени), связанными с лечением, были: утомляемость/астения (48%), артериальная гипертензия (49%), тошнота/рвота (32%), снижение аппетита (32 %) и диарея (31%).
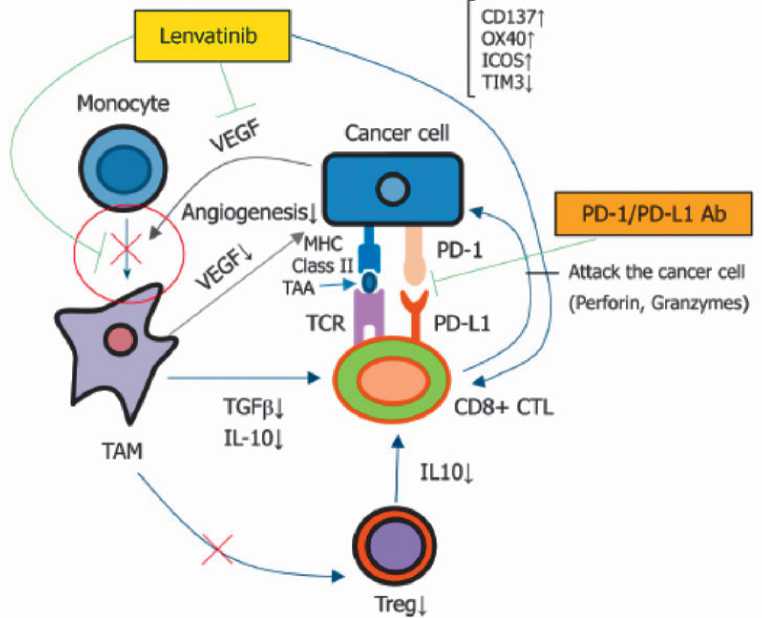
Рисунок 1. Механизм, лежащий в основе синергетического эффекта ленватиниба и пемболизумаба [97].
VEGF (Vascular endothelial growth factor) — фактор роста эндотелия сосудов;
ТАМ (Tumor-associated macrophage) — опухолеассоциированный макрофаг;
Treg (Regulatory T-cell) — регуляторная Т-клетка;
IL (Interleukin) — интерлейкин;
MHC (Major histocompatibility complex) — главный комплекс гистосовместимости;
TAA (Tumor-associated antigen) — опухолеассоциированный антиген;
TCR (T-cell receptor) — Т-клеточный рецептор;
PD-1 (Programmed cell death-1) — рецептор сигнального пути программируемой клеточной гибели-1;
PD-L1 (Programmed cell death-1 pathway ligand) — лиганд рецептора сигнального пути программируемой клеточной гибели-1.
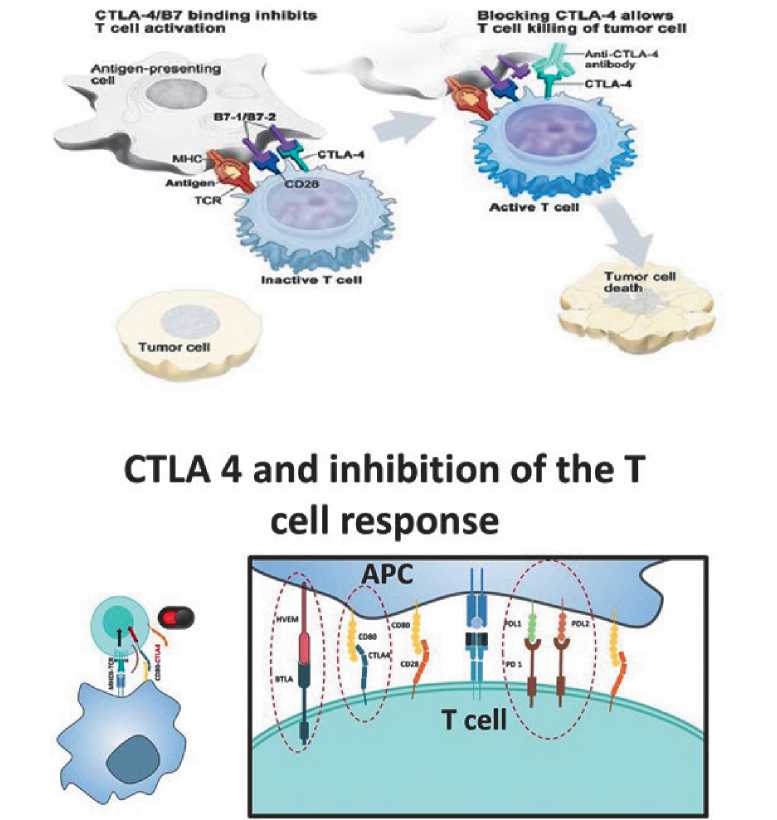
Рисунок 2. Роль CTLA4 и PD-1 в подавлении гиперактивации иммунитета.
CTLA-4 (Cytotoxic T-lymphocyte antigen-4) — цитотоксический Т-лимфо-цитарный антиген-4;
T-cell — Т-клетка;
APC (Antigen-presenting cell) — антигенпрезентирующая клетка;
MHC (Major histocompatibility complex) — главный комплекс гистосовместимости;
TCR (T-cell receptor) — Т-клеточный рецептор;
Tumor cell — опухолевая клетка;
PD-1 (Programmed cell death-1) — рецептор сигнального пути программируемой клеточной гибели-1;
PD-L1 (Programmed cell death-1 pathway ligand) — лиганд рецептора сигнального пути программируемой клеточной гибели-1.
В доклинических исследованиях ленватиниб уменьшал популяцию макрофагов, связанных с опухолью, и увеличивал количество клеток CD8 + , вызывая иммунную активацию. Таким образом, комбинация анти-VEGF препарата лен-ватиниба и анти-PD-1 препарата пембролизумаба обладает противоопухолевым эффектом [95]. В экспериментальных исследованиях на моделях ксенографтных мышей комбинация ленватиниба и МКА к PD-1/PD-L1 продемонстрировала более высокую противоопухолевую активность в сравнении с монотерапией антиPD-1/PD-L1 [96].
Механизм, лежащий в основе синергетического эффекта данной комбинации препаратов, представлен на рис. 1. Рассмотрим его более подробно.
Известно, что клетки эндометриальной карциномы и микроокружение опухоли модулируют иммунный ответ. Клетки эндометриальной карциномы обладают способностью активировать путь PD-1 [98–100]. PD-1 впервые был описан в 1992 году как предполагаемый медиатор апоптоза, хотя последние данные свидетельствуют о его роли в подавлении гиперактивации иммунитета, как это делает, например, CTLA-4 [101].
Белки контрольных точек, такие как B7–1 / B7–2 на антиген-презентирующих клетках (antigen-presenting cell, АРС) и CTLA-4 на Т-клетках, помогают контролировать иммунные реакции организма. Когда T-клеточный рецептор (T-cell receptor, TCR) связывается с антигеном и бел- ками главного комплекса гистосовместимости (major histocompatibility complex, MHC) на APC, а CD28 связывается с B7–1/B7–2 на APC, Т-клетка может быть активирована. Однако связывание B7–1 /B7–2 с CTLA-4 удерживает Т-клетки в неактивном состоянии, поэтому они не способны уничтожать опухолевые клетки в организме. Блокирование связывания B7–1/B7–2 с CTLA-4 с помощью ингибитора иммунных контрольных точек (антитело против CTLA-4) позволяет Т-клеткам быть активными и уничтожать опухолевые клетки (рис. 2).
Являясь трансмембранным гликопротеином I типа в суперсемействе иммуноглобулинов, PD-1 обладает аминокислотной последовательностью, идентичной таковой у CTLA-4 и CD28 на 20% и 15% соответственно [102]. Человеческий PD-1 экспрессируется на Т-лимфоцитах после стимуляции TCR и связывает гомологи B7 PD-L1 (также известный как B7 H1) и PD-L2 (также известный как B7-DC, Dendritic cell), которые постоянно присутствуют на поверхности APC и в негематопоэтических тканях могут быть индуцированы провоспалительными цитокинами [103–105] (рис. 2).
Ленватиниб подавляет ангиогенез и рост опухоли посредством ингибирования VEGFR1-R3 и FGFR1-R4. Лен-ватиниб также ингибирует VEGF-опосредованное опухолесупрессивное микроокружение — иммуносупрессивные клетки (ТАМ (Tumor-associated macrophage) — опухолеассоциированный макрофаг, Treg (Regulatory T cell) — регуляторная Т-клетка, MDSC (Myeloid-derived suppressor cell) — супрессорная клетка миелоидного происхождения) и опухолесупрессивные цитокины (IL10 или TGF-β). Кроме того, ленватиниб подавляет TIM 3 (ко-ингибирующий ингибитор контрольной точки) и увеличивает содержание ко-стимулирующих молекул: CD137, OX40 и ICOS. Пембро-лизумаб, в свою очередь, восстанавливает истощенную активность Т-клеток, что, в конечном итоге, приводит к гибели опухолевой клетки. Таким образом, достигается синергетический эффект (рис. 1).
Впервые эффективность и безопасность комбинации ленватиниба и пембролизумаба была продемонстрирована в многоцентровом нерандомизированном несравнительном открытом исследовании Ib/II фазы Study 111/KEYNOTE-146 [106], к участию в котором допускались пациентки распространенным РЭ, с ПЗ после предшествующей системной противоопухолевой терапии. Пациенток включали вне зависимости от MSI-статуса, PD-L1-статуса и гистологического подтипа опухоли (n = 108). Примерно половине больных до участия в исследовании провели ≥ 2 линий противоопухолевой терапии. У 11 (10,1%) пациенток был выявлен MSI-h. Все участницы исследования получали ленватиниб 20 мг/сутки, per os, ежедневно и пем-бролизумаб 200 мг, в/в кап., день 1, цикл — каждые 3 недели. Первичная конечная точка — ЧОО через 24 недели наблюдения. Вторичные конечные точки эффективности включали длительность ответа, ВБП, ОВ. Оценка ответа опухоли на лечение проводилась в соответствии с критериями иммуноассоциированного RECIST (immune-related RECIST, irRECIST). ЧОО через 24 недели среди всех включенных в исследование пациенток составила 38,0% (95%ДИ 28,8– 47,8), в подгруппе MSI-h/dMMR (n = 11) — 63,6% (95%ДИ 30,8–89,1), в подгруппе MSS (n = 94) — 36,2% (95%ДИ 26,5– 46,7). Медианы длительности ответа, ВБП и ОВ 108 больных составили 21,2 мес., 7,4 мес. (95%ДИ 5,3–8,7) и 16,7 мес. соответственно. Наиболее распространенными НЯ любой степени были артериальная гипертензия, диарея, снижение аппетита, слабость и гипотиреоз. НЯ III–IV степени, связанные с лечением, наблюдались у 83 (66,9%) пациенток. Все побочные эффекты — управляемые.
Результаты этого исследования стали регистрационными и послужили основанием для ускоренного одобрения комбинации ленватиниба и пембролизумаба для клинического применения во многих странах мира, в том числе России (FDA (Food and Drug Administration) — Управлением по санитарному надзору за качеством пищевых продуктов и медикаментов США, Австралийской администрацией лекарственных средств (Australian Therapeutic Goods Administration), Министерством здравоохранения Канады (Health Canada)) для лечения пациенток распространенным РЭ в случае отсутствия MSI-h или dMMR при ПЗ после предшествующей системной терапии и отсутствии показаний для хирургического лечения или ЛТ [107,108].
Эффективность комбинации ленватиниба и пембролизу-маба была подтверждена в мультицентровом рандомизированном исследовании III фазы Study 309/KEYNOTE-775 [109], в которое включали пациенток с наличием морфологически верифицированного диссеминированного РЭ, ранее получивших ≥ 1 линии системной терапии на основе препаратов платины и имеющих образец опухоли для определения статуса MMR. В общей сложности 827 участниц (697 из них — с MSS/pMMR и 130-c MSI-h/dMMR) были рандомизированы в 2 группы в соотношении 1:1 — в группу комбинации ленватиниба 20 мг/сутки, per os, ежедневно, непрерывно длительно, и пембролизумаба 200 мг, в/в кап., день 1 (максимально — 35 циклов), цикл — каждые 3 недели (n = 411) либо в группу ХТ по выбору лечащего врача (доксорубицин 60 мг/м 2 , в/в кап., день 1, цикл — каждые 3 недели (максимальная кумулятивная доза — 500 мг/м 2 ) или паклитаксел 80 мг/м 2 , в/в кап., еженедельно, 3 недели лечения, 1 неделя перерыв) ( n = 416). Больные были стратифицированы по статусу MMR (MSI-h/dMMR против MSS/pMMR), затем пациентки pMMR были стратифицированы по общему статусу по шкале ECOG (0 против 1), географическому региону и предшествующему облучению ОМТ. Первичными конечными точками были ВБП и ОВ. Медиана ВБП оказалась значительно выше при применении комбинации ленватиниба с пембролизумабом, нежели при использовании стандартной ХТ (в популяции MSS/pMMR: 6,6 мес. против 3,8 мес.; HR — 0,60; 95%ДИ 0,50–0,72; р < 0,001; все участницы: 7,2 мес. против 3,8 мес.; HR — 0,56; 95%ДИ 0,47– 0,66; р < 0,001). Медиана ОВ также была достоверно выше при применении комбинации ленватиниба с пембролизу-мабом, нежели при использовании ХТ (популяция pMMR: 17,4 мес. против 12,0 мес.; HR — 0,68; 95%ДИ 0,56–0,84; р < 0,001; общая популяция: 18,3 мес. против 11,4 мес.;
Таблица 4. Эффективность комбинации ленватиниба с пембролизумабом при диссеминированном РЭ. Результаты подгруппового анализа в рамках исследования Study 309/KEYNOTE-775 [110].
|
Показатель |
Группа |
Гистологический тип опухоли (РЭ) |
||||||
|
Эндометриоидный |
Светлоклеточный |
Серозный |
||||||
|
Вся когорта |
Медиана ВБП |
Комбинация ленватиниба с пембролизумабом |
7,6 мес. |
HR-0,52; 95%ДИ 0,41–0,65 |
3,9 мес. |
HR-0,47; 95%ДИ 0,24–0,92 |
5,7 мес. |
HR-0,53; 95%ДИ 0,38–0,72 |
|
ХТ по выбору лечащего врача |
3,9 мес. |
2,0 мес. |
3,6 мес. |
|||||
|
Медиана ОВ |
Комбинация ленватиниба с пембролизумабом |
Не достигнута |
HR-0,65; 95%ДИ 0,49–0,84 |
19,9 мес. |
HR-0,33; 95%ДИ 0,15–0,74 |
12,0 мес. |
HR-0,68; 95%ДИ 0,48–0,94 |
|
|
ХТ по выбору лечащего врача |
13,4 мес. |
8,7 мес. |
9,3 мес. |
|||||
|
Когорта pMMR |
Медиана ВБП |
Комбинация ленватиниба с пембролизумабом |
7,6 мес. |
HR-0,59; 95%ДИ 0,46–0,76 |
3,9 мес. |
HR-0,49; 95%ДИ 0,25–0,97 |
5,7 мес. |
HR-0,54; 95%ДИ 0,39–0,75 |
|
ХТ по выбору лечащего врача |
5,0 мес. |
2,0 мес. |
3,6 мес. |
|||||
|
Медиана ОВ |
Комбинация ленватиниба с пембролизумабом |
20,0 мес. |
HR-0,78; 95%ДИ 0,57–1,05 |
19,9 мес. |
HR-0,34; 95%ДИ 0,15–0,78 |
12,0 мес. |
HR-0,68; 95%ДИ 0,49–0,96 |
|
|
ХТ по выбору лечащего врача |
15,2 мес. |
8,7 мес. |
10,0 мес. |
|||||
* РЭ — рак эндометрия; pMMR (proficient mismatch repair system) — без дефекта в системе репарации неспаренных оснований ДНК; ВБП — выживаемость без прогрессирования; ОВ — общая выживаемость; ХТ — химиотерапия; мес. — месяцы; 95 %ДИ — 95 % доверительный интервал.
HR — 0,62; 95%ДИ 0,51–0,75; р < 0,001). Таким образом, на фоне применения комбинации пембролизумаба с лен-ватинибом было зафиксировано снижение относительного риска прогрессирования на 40% и снижение относительного риска смерти пациенток на 38% по сравнению со стандартной ХТ. Применение комбинации ленватиниба с пембролизумабом сопровождалось повышением риска развития НЯ. НЯ III–IV степени наблюдались у 88,9% больных, получавших комбинацию ленватиниба с пемброли-зумабом, и у 72,7% — получавших ХТ; у 33 % и 8,0 % пациенток соответственно терапия была досрочно прервана вследствие непереносимой токсичности. Среди НЯ III–IV степени в группе комбинации наиболее часто отмечались артериальная гипертензия (37,9% против 2,3% в контрольной группе), снижение массы тела (10,3% против 0,3%), снижение аппетита (7,9% против 0,5%), а также диарея (7,6% против 2,1 % в группах комбинации и ХТ соответственно). Это указывает на необходимость тщательного мониторинга состояния больных на фоне проводимой терапии ленватинибом c пембролизумабом.
Результаты исследования KEYNOTE-775 позволяют рассматривать комбинацию ленватиниба с пембролизума-бом в качестве нового стандарта терапии для пациенток распространенным РЭ, получивших ≥ 1 линии системной терапии [26,27].
На конгрессе Европейского общества медицинской онкологии (ESMO) в 2021 году Colombo N. были представлены данные подгруппового анализа в рамках исследования Study 309/KEYNOTE-775 [110]. Авторами проанализированы отдаленные результаты лечения пациенток в зависимости от ряда факторов, в том числе гистотипа опухоли, статуса MMR в опухоли, предшествующей терапии. Было выявлено, что применение комбинации ленватиниба с пемброли- зумабом позволяет значительно улучшить отдаленные результаты лечения (ВБП и ОВ) во всех подгруппах больных, независимо от гистологического подтипа опухоли, предшествующей (неоадъювантной/адъювантной) терапии, PFI, а также статуса MMR в опухоли (табл. 4). Дополнительно было выявлено, что наибольшая эффективность комбинации ленватиниба с пембролизумабом отмечалась у больных, которые до включения в исследование получили только 1 линию системной терапии на основе препаратов платины (HR — 0,54; 95%ДИ 0,44–0,67) по сравнению с пациентками, получившими большее количество линий предшествующей системной терапии (HR — 0,75; 95%ДИ 0,52–1,09).
Таким образом, после проведения подгруппового анализа в рамках исследования Study 309/KEYNOTE-775 было отмечено, что комбинация ленватиниба с пемролизумабом обладает высокой эффективностью при MSS РЭ, особенно в подгруппах пациенток с трудно поддающимися лечению гистологическими подтипами опухолей — светлоклеточной и серозной аденокарциномой [111,112]. Раннее начало терапии комбинацией позволяет рассчитывать на ее наибольшую эффективность. При этом пациентки с прогрессированием РЭ после ранее проведенной адъювантной ХТ могут быть кандидатами для назначения комбинации ленватиниба с пембролизумабом и в качестве I линии системной терапии распространенного опухолевого процесса.
ЗАКЛЮЧЕНИЕ
Пациентки с прогрессированием РЭ представляют собой гетерогенную группу, у которых на тактику лечения и прогноз влияет множество факторов, в том числе гистологический подтип опухоли, объем оперативного вмешательства, предыдущее адъювантное лечение, длительность интервала с момента завершения адъювантной терапии, а также размер рецидивной опухоли и ее локализация. Именно поэтому при выборе тактики лечения пациенток распространенным и рецидивирующим РЭ необходим мультидисциплинарный подход с участием хирурга, радиотерапевта и химиотерапевта. При отсутствии показаний к хирургическому лечению (при невозможности выполнить полную циторедуктивную операцию) и ЛТ (облучение проводилось на этапе первичного лечения), назначается системная лекарственная терапия.
ГТ имеет ограниченные возможности применения: эндометриоидная карцинома высокой степени дифференцировки, с экспрессией рецепторов стероидных гормонов (эстрогенов и прогестерона). «Золотым стандартом» I линии ХТ распространенного РЭ или его рецидивов является комбинация «ТС». При серозном РЭ с наличием позитивного статуса HER-2 / neu следует использовать комбинацию паклитаксела, карбоплатина и трастузумаба.
В случае прогрессирования на I линии ХТ цитостатическая терапия является малоэффективной опцией, за исключением «платиночувствительных» рецидивов, когда при длительном PFI возможно повторное применение комбинации «ТС».
Для планирования дальнейшей системной терапии всем пациенткам диссеминированным РЭ целесообразно проводить молекулярно-генетическое тестирование на MSI-h/dMMR. При выявлении в опухоли MSI-h/dMMR (25% случаев) и отсутствии необходимости в ЛТ и хирургическом лечении наиболее эффективным вариантом II линии противоопухолевой терапии будет иммунотерапия пембролизумабом в монорежиме. Большая часть опухолей (около 75%) — микросателлитно-стабильные опухоли, не имеющие нарушений в системе репарации ДНК (MSS/рMMR). Поэтому патогенетически обоснованным для данной категории пациенток в случае ПЗ после предшествующей системной терапии будет назначение комбинации ингибитора контрольных точек иммунитета пембролизумаба и мультитаргетного ингибитора тирозин-киназ ленватиниба.
PD-L1-тестирование при РЭ в настоящее время не обладает доказанным клиническим значением. Диагностика POLE-мутированного варианта на сегодняшний день проводится, в основном, в научно-исследовательских целях.
Таким образом, возможности современной терапии диссеминированного РЭ позволяют значимо улучшить результаты лечения и обеспечить длительную выживаемость данной категории пациенток, даже при резистентности и рефрактер-ности к стандартной противоопухолевой терапии.
Остается открытым вопрос о преимуществе комбинированной терапии ленватинибом с пембролизумабом для пациенток с MSI-h-статусом опухоли. Кроме того, до конца еще не определена оптимальная последовательность терапии для этой категории больных. Имеются данные, которые демонстрируют, что применение ленватиниба в комбинации с ингибиторами контрольных точек иммунного ответа позволяет восстановить чувствительность опухолевых клеток к иммунотерапии после ранее отмеченного прогрессирования [113]. Возможно, при лечении пациенток MSI-h/dMMR-позитивным РЭ наиболее рациональной станет стратегия применения пембролизумаба в монотерапии с переходом на комбинацию ленватиниба с пембролизумабом в случае отсутствия эффекта или прогрессирования опухолевого процесса. Дальнейшие исследования в этой области помогут ответить на поставленные вопросы и позволят достичь новых высот в лечении данной патологии.
Список литературы Эволюция системной лекарственной терапии диссеминированного рака эндометрия. Обзор литературы
- Состояние онкологической помощи населению России в 2021 году. Под ред. А.Д. Каприна, В.В. Старинского, A.О. Шахзадовой - М.: МНИОИ им. П.А. Герцена - филиал ФГБУ «НМИЦ радиологии» Минздрава России, 2022 .- илл .- 239 с.
- Злокачественные новообразования в России в 2021 году (заболеваемость и смертность). Под ред. А. Д. Каприна, B.В. Старинского, А.О. Шахзадовой — М.: МНИОИ им. П.А. Герцена - филиал ФГБУ «НМИЦ радиологии» Минздрава России, - 2022 .- илл .- 252 с.
- Kelley R. M., Baker W. H. Progestational agents in the treatment of carcinoma of the endometrium// N. Engl.J. Med .- 1961 — Vol. 264 .- 216-222. Doi: 10.1056/NEJM196102022640503.
- Thigpen J.T., Brady M.F., Alvarez R.D. et al. Oral medroxyprogesterone acetate in the treatment of advanced or recurrent endometrial carcinoma: A dose-response study by the Gynecologic Oncology Group. J Clin Oncol 1999 ; 17 (6): 1736-1744. Doi: 10.1200/jc0. 1999.17.6.1736.
- Pandya K.J., Yeap B.Y., Weiner L. M. et al. Megestrol and tamoxifen in patients with advanced endometrial cancer: An Eastern Cooperative Oncology Group Study (E4882). Am J Clin Oncol 2001 ; 24 (1): 43-46. Doi: 10.1097/00000421200102000-00007.
- Carlson J.A., Allegra J. C., Day T. G. I. et al. Tamoxifen and endometrial carcinoma: alterations in estrogen and progesterone receptors in untreated patients and combination hormonal therapy in advanced neoplasia// Amer.J. Obstet. Gynecol .- 1984 ; 149 (2) .- 149-153. Doi: 10.1016/0002-9378(84)90187-x.
- Vishnevsky A. S., Tsyrlina E.V., Sofroniy D. F. et al. Criteria of endometrial carcinoma sensitivity to hormone therapy: pathogenetic type of the disease and the tumor reaction to tamoxifen// Europ. J. Gynaecol. Oncol .- 1993 ; 14 (2) .- 139-143.
- Kline R. C., Freedman R. S., Jones L. A. et al. Treatment of recurrent or metastatic poorly differentiated adenocarcinoma of the endometrium with tamoxifen and medroxyprogesterone acetate// Cancer. Treat. Rep .- 1987 ; 71 (3) .- P. 327-328.
- Pandya K.J., Yeap B.Y., Davis T. E. Phase II study of megestrol and megestrol+ tamoxifen in advanved endometrial carcinoma: an Eastern Cooperative Oncology Group Study. Abstract// Proc. Amer.Ass. Cancer. Res .- 1989 .- Vol. 30 .- 1037 p.
- Fiorica J.V., Brunetto V.L., Hanjani P. et al. Phase II trial of alternating courses of megestrol acetate and tamoxifen in advanced endometrial carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol 2004 ; 92 (1): 10-14. Doi: 10.1016/j.ygyno.2003.11.008.
- Whitney C.W., Brunetto V.L., Zaino R.J. et al. Phase II study of medroxyprogesterone acetate plus tamoxifen in advanced endometrial carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol 2004 ; 92 (1): 4-9. Doi: 10.1016/j. ygyno.2003.09.018.
- Rose P. G., Brunetto V. L., VanLe L. et al. A phase II trial of anastrozole in advanced recurrent or persistent endometrial carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol 2000 ; 78 (2): 212-216. Doi: 10.1006/gyno.2000.5865.
- Ma B.B., Oza A., Eisenhauer E. et al. The activity of letrozole in patients with advanced or recurrent endometrial cancer and correlation with biological markers a study of the National Cancer Institute of Canada Clinical Trials Group. Int J Gynecol Cancer 2004 ; 14 (4): 650-658. Doi: 10.1111/j.1048-891X.2004.14419.x.
- Asbury R. F., Brunetto V. L., Lee R. B. et al. Goserelin acetate as treatment for recurrent endometrial carcinoma: A Gynecologic Oncology Group study. Am J Clin Oncol 2002 ; 25 (6): 557-560. Doi: 10.1097/00000421-200212000-00004.
- Lhomm'e C., Vennin P., Callet N. et al. A multicenter phase II study with triptorelin (sustained-release LHRH agonist) in advanced or recurrent endometrial carcinoma: A French Anticancer Federation Study. Gynecol Oncol 1999 ; 75 (2): 187-193. Doi: 10.1006/gyno.1999.5538.
- Rendina G.M., Donadio C., Fabri M. et al. Tamoxifen and medroxyprogesterone therapy for advanced endometrial carcinoma. Eur J Obstet Gynecol Reprod Biol, 1984 ; 17 (4): 285-91. Doi: 10.1016/0028-2243(84)90071-6.
- Thigpen T., Brady M. F., Homesley H. D. et al. Tamoxifen in the treatment of advanced or recurrent endometrial carcinoma: A Gynecologic Oncology Group study. J Clin Oncol 2001 ; 19 (2): 364-367. Doi: 10.1200/JCO.2001.19.2.364.
- McMeekin D. S., Gordon A., Fowler J. et al. A phase II trial of arzoxifene, a selective estrogen response modulator, in patients with recurrent or advanced endometrial cancer. Gynecol Oncol 2003 ; 90 (1): 64-69. Doi: 10.1016/s0090-8258(03)00203-8.
- Covens A.L., Filiaci V., Gersell D. et al. Phase II study of fulvestrant in recurrent / metastatic endometrial carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol 2011 ; 120 (2): 185-188. Doi: 10.1016/j.ygyno.2010.10.015.
- Emons G., Gunthert A., Thiel F.C. et al. Phase II study of fulvestrant 250 mg/ month in patients with recurrent or metastatic endometrial cancer: A study of the Arbeitsgemeinschaft Gynakologische Onkologie. Gynecol Oncol 2013 ; 129 (3): 495-499. Doi: 10.1016/j.ygyno.2013.02.039.
- Thigpen J. T., Blessing J. A., DiSaia P. J. et al. A randomized comparison of doxorubicin alone versus doxorubicin plus cyclophosphamide in the management of advanced or recurrent endometrial carcinoma: A Gynecologic Oncology Group study. J Clin Oncol, 1994. 12 (7): 1408-1414. Doi: 10.1200/JCO.1994.12.7.1408.
- Thigpen J. T., Brady M. F., Homesley H. D. et al. Phase III trial of doxorubicin with or without cisplatin in advanced endometrial carcinoma: a gynecologic oncology group study. J Clin Oncol 2004 ; 22 (19): 3902-3908. Doi: 10.1200/ JCO.2004.02.088.
- Fleming G.F., Brunetto V.L., Cella D. et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: a gynecologic oncology group study. J Clin Oncol 2004 ; 22 (11): 2159-2166. Doi: 10.1200/JCO.2004.07.184.
- Miller D. S., Filiaci V.L., Mannel R. S. et al. Carboplatin and paclitaxel for advanced endometrial cancer: final overall survival and adverse event analysis of a phase III trial (NRG Oncology / GOG0209). J Clin Oncol 2020 ; 38 (33): 3841-3850. Doi: 10.1200/jc0.20.01076.
- Miller D., Filiaci V., Fleming G. et al. Late-breaking abstract 1: Randomized phase III noninferiority trial of first line chemotherapy for metastatic or recurrent endometrial carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol 2012 ; 125 (3): Р. 771. Doi: 10.1016/j.ygyno.2012.03.034.
- Нечушкина В. М., Коломиец Л. А., Кравец О. А., Морхов К. Ю., Новикова Е. Г., Новикова О. В., Тюляндина А. С. и др. Практические рекомендации по лекарственному лечению рака тела матки и сарком матки. Злокачественные опухоли: Практические рекомендации RUSSCO #3s2, 2022 (том 12). 260-275. Doi: 10.18027/2224-5057-2022-12-3s2-260-275.
- National Comprehensive Cancer Network: Clinical Practice Guidelines in Oncology (NCCN Guidelines®) — Uterine Neoplasms. Version 1.2023 — December 22, 2022. Available at: https://www.nccn.org/professionals/physician_gls/ pdf/uterine.pdf.
- Lorusso D., Ferrandina G., Colombo N. et al. Randomized phase II trial of carboplatin-paclitaxel (CP) compared to carboplatin-paclitaxel-bevacizumab (CP-B) in advanced (stage III-IV) or recurrent endometrial cancer: The MITO END-2 trial. J Clin Oncol. 2015 May ; 33 (15 suppl): 5502. Doi: 10.1200/jco.2015.33.15_suppl. 5502.
- Aghajanian C., Filiaci V.L., Dizon D. S. et al. A randomized phase II study of paclitaxel / carboplatin/ bevacizumab, paclitaxel / carboplatin / temsirolimus and ixabepilone/ carboplatin / bevacizumab as initial therapy for measurable stage III or IVA, stage IVB or recurrent endometrial cancer, GOG-86P. J Clin Oncol 2015 ; 33 (suppl): Abstract 5500.
- Fader A.N., Roque D.M., Siegel E. et al. Randomized phase II trial of carboplatin-paclitaxel versus carboplatin-pa-clitaxel-trastuzumab in uterine serous carcinomas that overexpress human epidermal growth factor receptor 2 / neu. J Clin Oncol 2018 ; 36 (20): 2044-51. Doi: 10.1200/jœ.2017.76.5966.
- Lincoln S., Blessing J.A., Lee R.B. et al. Activity of paclitaxel as second-line chemotherapy in endometrial carcinoma: a gynecologic oncology group study. Gynecol Oncol. 2003 ; 88 (3): 277-281. Doi: 10.1016/S0090-8258(02)00068-9.
- Homesley H.D., Meltzer N. P., Nieves L. et al. A phase II trial of weekly 1-hour paclitaxel as second- line therapy for endometrial and cervical cancer// Int.J. Clin. Oncol .- 2008 .- 13 (1) .- P. 62-65. Doi: 10.1007/s10147-007-0731-5.
- Garcia A.A., Blessing J.A., Nolte S. et al. A phase II evaluation of weekly docetaxel in the treatment of recurrent or persistent endometrial carcinoma: A study by the Gynecologic Oncology Group. Gynecol Oncol. 2008 ; 111 (1): 22-26. Doi: 10.1016/j.ygyno.2008.06.013.
- Tait D. L., Blessing J. A., Hoffman J. S. et al. A phase II study of gemcitabine (gemzar, LY188011) in the treatment of recurrent or persistent endometrial carcinoma: A gynecologic oncology group study.// Gynecol. Oncol .- 2011 .- 121 (1) .- P. 118-121. Doi: 10.1016/j.ygyno.2010.11.027.
- Fracasso P. M., Blessing J.A., Molpus K. L. et al. Phase II study of oxaliplatin as second-line chemotherapy in endometrial carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol. 2006 ; 103 (2): 523-526. https://doi.org/10.1016/j. ygyno.2006.03.043.
- Makker V., Hensley M.L., Zhou Q. et al. Treatment of Advanced or Recurrent Endometrial Carcinoma with Doxorubicin in Patients Progressing After Paclitaxel / Carboplatin: Memorial Sloan-Kettering Cancer Center Experience From 1995 to 2009. Int J Gynecol Cancer. 2013 ; 23 (5): 929-934. https://doi.org/10.1097/IGC.0b013e3182915c20.
- Moreira E., Paulino E., Ingles Garces Ä. H. et al. Efficacy of doxorubicin after progression on carboplatin and paclitaxel in advanced or recurrent endometrial cancer: a retrospective analysis of patients treated at the Brazilian National Cancer Institute (INCA). Med Oncol 2018 ; 35 (3): 20. Doi: 10.1007/s12032-018-1086-7.
- Muggia F. M., Blessing J.A., Sorosky J. et al. Phase II Trial of the Pegylated Liposomal Doxorubicin in Previously Treated Metastatic Endometrial Cancer: A Gynecologic Oncology Group Study// J Clin Oncol. 2002 ; 20 (9): 2360-2364. Doi: 10.1200/jCO.2002.08.171.
- Sutton G.P., Blessing J.A., Homesley H.D. et al. Phase II study of ifosfamide and mesna in refractory ade-nocarcinoma of the endometrium. A Gynecologic Oncology Group study. Cancer 1994 ; 73 (5): 1453-1455. Doi: 10.1002/1097-0142(19940301)73:5<1453::aid-cncr2820730521-3.0.co;2-x.
- Rose P. G., Blessing J.A., Lewandowski G. S. et al. A phase II trial of prolonged oral etoposide (VP-16) as second-line therapy for advanced and recurrent endometrial carcinoma: A Gynecologic Oncology Group study// Gynecol. Oncol .- 1996 .- 63 (1) .- P. 101-104. Doi: 10.1006/gyno.1996.0286.
- Miller D. S., Blessing J.A., Lentz S. S. et al. A Phase II Trial of Topotecan in Patients with Advanced, Persistent, or Recurrent Endometrial Carcinoma: A Gynecologic Oncology Group Study// Gynecol Oncol. 2002 ; 87 (3): 247-251. https://doi.org/10.1006/gyno.2002.6804.
- Miller D. S., Blessing J. A., Drake R. D. et al. A phase II evaluation of pemetrexed (Alimta, LY231514, IND #40061) in the treatment of recurrent or persistent endometrial carcinoma: a phase II study of the Gynecologic Oncology. Gy-necol Oncol. 2009 ; 115 (3): 443-6. Doi: 10.1016/j.ygyno.2009.09.004.
- Dizon D.S., Blessing J.A., McMeekin D. S. et al. Phase II trial of ixabepilone as second-line treatment in advanced endometrial cancer: Gynecologic Oncology Group trial 129-P// J. Clin. Oncol .- 2009 .- 27 (19) .- P. 3104-3108. Doi: 10.1200/JC0.2008.20.6995.
- Markman M., Fowler J. Activity of weekly paclitaxel in patients with advanced endometrial cancer previously treated with both a platinum agent and paclitaxel. Gynecol Oncol 2004 ; 92 (1): 180-2. Doi: 10.1016/j.ygyno.2003.10.019.
- Moore K.N., Tian C., McMeekin S. et al. Does the progression-free interval after primary chemotherapy predict survival after salvage chemotherapy in advanced and recurrent endometrial cancer? Cancer 2010 ; 116 (23): 5407-14. Doi: 10.1002/cncr.25480.
- Nagao S., Nishio S., Michimae H. et al. Applicability of the concept of «platinum sensitivity» to recurrent endometrial cancer: The SGSG-012/ G0TIC-004/ Intergroup study. Gynecol Oncol. 2013 Dec ; 131 (3): 567-73. Doi: 10.1016/j. ygyno.2013.09.021.
- Slomovitz B. M., Jiang Y., Yates M. S. et al. Phase II study of everolimus and letrozole in patients with recurrent endometrial carcinoma. J Clin Oncol 2015 ; 33 (8): 930-936. Doi: 10.1200/jœ.2014.58.3401.
- Coleman R.L., Sill M.W., Lankes H.A. et al. A phase II evaluation of aflibercept in the treatment of recurrent or persistent endometrial cancer: A Gynecologic Oncology Group study. Gynecol Oncol 2012 ; 127 (3): 538-543. Doi: 10.1016/j.ygyno.2012.08.020.
- Makker V., Filiaci V. L., Chen L. M. et al. Phase II evaluation of dalantercept, a soluble recombinant activin receptor-like kinase 1 (ALK1) receptor fusion protein, for the treatment of recurrent or persistent endometrial cancer: an NRG Oncology/ Gynecologic Oncology Group Study 0229N. Gynecol Oncol. 2015 ; 138 (1): 24-9. Doi: 10.1016/j.ygyno.2015.04.006.
- Aghajanian C., Sill M.W., Darcy K.M. et al. Phase II trial of bevacizumab in recurrent or persistent endometrial cancer: A Gynecologic Oncology Group study. J Clin Oncol 2011 ; 29 (16): 2259-2265. Doi: 10.1200/jœ.2010.32.6397.
- Castonguay V., Lheureux S., Welch S. et al. A phase II trial of sunitinib in women with metastatic or recurrent endometrial carcinoma: A study of the Princess Margaret, Chicago and California Consortia. Gynecol Oncol 2014 ; 134 (2): 274-280. Doi: 10.1016/j.ygyno.2014.05.016.
- Nimeiri H. S., Oza A. M., Morgan R.J. et al. A phase II study of sorafenib in advanced uterine carcinoma/ carcinosarcoma: A trial of the Chicago, PMH, and California Phase II Consortia. Gynecol Oncol 2010 ; 117 (1): 37-40. Doi: 10.1016/j.ygyno.2010.01.013.
- Powell M.A., Sill M. W., Goodfellow P.J. et al. A phase II trial of brivanib in recurrent or persistent endometrial cancer: An NRGOncology/ Gynecologic Oncology Group Study. Gynecol Oncol 2014 ; 135 (1): 38-43. Doi: 10.1016/j. ygyno.2014.07.083.
- Dizon D. S, Sill M.W., Schilder J. M. et al. A phase II evaluation of nintedanib (BIBF-1120) in the treatment of recurrent or persistent endometrial cancer: An NRG Oncology / Gynecologic Oncology Group Study. Gynecol Oncol 2014 ; 135 (3): 441-445. Doi: 10.1016/j.ygyno.2014.10.001.
- Bender D., Sill M. W., Lankes H. A. et al. A phase II evaluation of cediranib in the treatment of recurrent or persistent endometrial cancer: An NRG Oncology / Gynecologic Oncology Group study. Gynecol Oncol 2015 ; 138 (3): 507-512. Doi: 10.1016/j.ygyno.2015.07.018.
- Moore K.N., Sill M.W., Tenney M.E. et al. A phase II trial of trebananib (AMG 386 ; IND#111071), a selective an-giopoietin 1/ 2 neutralizing peptibody, in patients with persistent / recurrent carcinoma of the endometrium: An NRG / Gynecologic Oncology Group trial. Gynecol Oncol 2015 ; 138 (3): 513-518. Doi: 10.1016/j.ygyno.2015.07.006.
- Leslie K.K., Sill M.W., Fischer E. et al. A phase II evaluation of gefitinib in the treatment of persistent or recurrent endometrial cancer: A Gynecologic Oncology Group study. Gynecol Oncol 2013 ; 129 (3): 486-494. Doi: 10.1016/j. ygyno.2013.02.019.
- Oza A. M., Eisenhauer E.A., Elit L. et al. Phase II study of erlotinib in recurrent or metastatic endometrial cancer: NCIC IND-148. J Clin Oncol 2008 ; 26 (26): 4319-4325. Doi: 10.1200/jœ.2007.15.8808.
- Slomovitz B. M., Chelariu-Raicu A., Schmeler K. M. et al. Phase 2 study of cetuximab (Erbitux) in patients with progressive or recurrent endometrial cancer. Int J Gynecol Cancer. 2020 ; 30 (11): 1733-1737. Doi: 10.1136/ijgc-2020-001859.
- Fleming G.F., Sill M.W., Darcy K.M. et al. Phase II trial of trastuzumab in women with advanced or recurrent, HER2-positive endometrial carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol 2010 ; 116 (1): 15-20. Doi: 10.1016/j.ygyno.2009.09.025.
- Leslie K. K., Sill M.W., Lankes H.A. et al. Lapatinib and potential prognostic value of EGFR mutations in a Gynecologic Oncology Group phase II trial of persistent or recurrent endometrial cancer. Gynecol Oncol 2012 ; 127 (2): 345-350. Doi: 10.1016/j.ygyno.2012.07.127.
- Slomovitz B. M., Lu K. H., Johnston T. et al. A phase 2 study of the oral mammalian target of rapamycin inhibitor, everolimus, in patients with recurrent endometrial carcinoma. Cancer 2010 ; 116 (23): 5415-5419. Doi: 10.1002/cncr.25515.
- Ray-Coquard I., Favier L., Weber B. et al. Everolimus as second- or third-line treatment of advanced endometrial cancer: ENDORAD, a phase II trial of GINECO. Br J Cancer 2013 ; 108 (9): 1771-1777. Doi: 10.1038/bjc.2013.183.
- Oza A. M., Elit L., Tsao M. S. et al. Phase II study of temsirolimus in women with recurrent or metastatic endometrial cancer: A trial of the NCIC Clinical Trials Group. J Clin Oncol 2011 ; 29 (24): 3278-3285. Doi: 10.1200/jc0.2010.34.1578.
- Fleming G. F., Filiaci V. L., Marzullo B. et al. Temsirolimus with or without megestrol acetate and tamoxifen for endometrial cancer: A Gynecologic Oncology Group study. Gynecol Oncol 2014 ; 132 (3): 585-592. Doi: 10.1016/j.ygyno.2014.01.015.
- Colombo N., McMeekin D. S., Schwartz P. E. et al. Ridaforolimus as a single agent in advanced endometrial cancer: Results of a single-arm, phase 2 trial. Br J Cancer 2013 ; 108 (5): 1021-1026. Doi: 10.1038/bjc.2013.59.
- Oza A. M., Pignata S., Poveda A. et al. Randomized phase II trial of ridaforolimus in advanced endometrial carcinoma. J Clin Oncol 2015 ; 33 (31): 3576-3582. Doi: 10.1200/jœ.2014.58.8871.
- Alvarez E.A., Brady W. E., Walker J. L. et al. Phase II trial of combination bevacizumab and temsirolimus in the treatment of recurrent or persistent endometrial carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol 2013 ; 129 (1): 22-27. Doi: 10.1016/j.ygyno.2012.12.022.
- Cancer Genome Atlas Research Network, Kandoth C., Schultz N., Cherniack A.D., Akbani R., Liu Y., Shen H., Robertson A. G., Pashtan I., Shen R., Benz C. C., Yau C., Laird P.W., Ding L., Zhang W., Mills G. B., Kucherlapati R., Mardis E.R., Levine D.A. Integrated genomic characterization of endometrial carcinoma. Nature. 2013 May ; 497 (7447): 67-73. Doi: 10.1038/nature12113.
- Talhouk A., McConechy M. K., Leung S. et al. A clinically applicable molecular-based classification for endometrial cancers// Br J Cancer. 2015 ; 113 (2): 299-310. Doi: 10.1038/bjc.2015.190.
- Talhouk A., McConechy M. K., Leung S. et al. Confirmation of ProMisE: a simple, genomics-based clinical classifier for endometrial cancer // Cancer .- 2017 .- №123 .- P. 802-813.
- Talhouk A., Hoang L.N., McConechy M. K. et al. Molecular classification of endometrial carcinoma on diagnostic specimens is highly concordant with final hysterectomy: earlier prognostic information to guide treatment // Gynecol. Oncol .- 2016 .- №143 (1) .- P. 46-53. Doi: 10.1016/j.ygyno.2016.07.090.
- Kandoth C., Schultz N., Cherniack A.D. et al. Integrated genomic characterization of endometrial carcinoma// Nature .- 2013 .- 497 (7447) .- P. 67-73. Doi: 10.1038/nature12113.
- Bansal N., Yendluri V., Wenham R. M. The molecular biology of endometrial cancers and the implications for pathogenesis, classification, and targeted therapies// Cancer Control .- 2009 .- №16 (1) .- P. 8-13. Doi: 10.1177/107327480901600102.
- Chernukha G. E., Dumanovskaya M. R., Burmenskaya O. V. et al. Expression of apoptosis-regulatory genes in different endometrial hyperplasia types and endometrioid carcinoma// Obstetrics and Gynecology .- 2013 .- № 1 .- P. 63-69.
- Lorenzi M., Amonkar M., Zhang J. et al. Epidemiology of Microsatellite Instability High (MSI-H) and Deficient Mismatch Repair (dMMR) in Solid Tumors: A Structured Literature Review. J Oncol. 2020 ; 1-17. https:// doi.org/10.1155/2020/1807929.
- Marabelle A., Fakih M., Lopez J. et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol 2020 ; 21 (10): 1353-65. Doi: 10.1016/S1470-2045(20)30445-9.
- Le D. T., Uram J. N., Wang H. et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015 ; 372 (26): 2509-20. Doi: 10.1056/NEJMoa1500596.
- Marabelle A., Le D. T., Ascierto P.A. et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability / mismatch repair-deficient cancer: Results from the phase II KEYNOTE-158 study. J Clin Oncol 2020 ; 38 (1): 1-10. https://doi.org/10.1200/jCO.19.02105.
- Keytruda (pembrolizumab) [package insert]. Whitehouse Station, NJ, Merck Sharp & Dohme, 2018.
- Arora E., Masab M., Mittar P. et al. Role of immune checkpoint inhibitors in advanced or recurrent endometrial cancer. Cureus 2018 ; 10: e2521.
- Oaknin A., Tinker A.V., Gilbert L. et al. Clinical activity and safety of the anti-PD- 1 monoclonal antibody dostar-limab for patients with recurrent or advanced dMMR endometrial cancer. Future Oncol. 2021 ; 17 (29): 3781-3785. https://doi.org/10.2217/fon-2021-0598.
- Antill Y., Kok P. S., Stockler M. R. et al. Updated results of activity of durvalumab in advanced endometrial cancer (AEC) according to mismatch repair (MMR) status: The phase II PHAEDRA trial (ANZGOG1601). Ann Oncol 2019 ; 30 (Suppl 9): P. ix192. https://doi.org/10.1093/annonc/mdz446.011.
- Diaz L.A., Marabelle A., Delord J.P. et al. Pembrolizumab therapy for microsatellite instability high (MSI-H) colorectal cancer (CRC) and non-CRC. J Clin Oncology. 2017 ; 35 (15): 3071. Doi: 10.1200/jœ.2017.35.15_suppl.3071.
- O'Malley D. M., Bariani G. M., Cassier P.A. et al. Pembrolizumab in patients with microsatellite instability-high advanced endometrial cancer: results from the KEYNOTE-158 study. J Clin Oncol 2022 ; 40 (7): 752-61. Doi: 10.1200/ jCO.21.01874.
- Hasegawa K., Tamura K., Katsumata N. et al. Efficacy and safety of nivolumab (Nivo) in patients (pts) with advanced or recurrent uterine cervical or corpus cancers. J Clin Oncol 2018 ; 36 (Suppl 15): 5594. Doi: 10.1200/jco.2018.36.15_ suppl.5594.
- Konstantinopoulos P. A., Luo W., Liu J. F. et al. Phase II study of avelumab in patients with mismatch repair deficient and mismatch repair proficient recurrent / persistent endometrial cancer. J Clin Oncol 2019 ; 37 (30): 2786-94. Doi: 10.1200/ JCO. 19.01021.
- Oaknin A., Tinker A.V., Gilbert L. et al. Clinical activity and safety of the antiprogrammed death 1 monoclonal antibody dostarlimab for patients with recurrent or advanced mismatch repair-deficient endometrial cancer: a non-randomized phase 1 clinical trial. JAMA Oncol 2020 ; 6 (11): 1766-1772. Doi: 10.1001/jamaoncol.2020.4515.
- Antill Y. C., Kok P .- S., Robledo K. et al. Activity of durvalumab in advanced endometrial cancer (AEC) according to mismatch repair (MMR) status: The phase II PHAEDRA trial (ANZGOG1601). J Clin Oncol 2019 ; 37 (15 Sup-pl): abstr 5501. Doi: 10.1200/jœ.2019.37.15_suppl.5501.
- Ott P. A., Bang Y.J., Berton-Rigaud D. et al. Safety and Antitumor Activity of Pembrolizumab in Advanced Programmed Death Ligand 1-Positive Endometrial Cancer: Results From the KEYNOTE-028 Study J Clin Oncol. 2017 Aug 1 ; 35 (22): 2535-2541. Doi: 10.1200/jœ.2017.72.5952.
- Binnewies M., Roberts E.W., Kersten K. et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med 2018 ; 24 (5): 541-50. Doi: 10.1038/s41591-018-0014-x.
- Hong D. S., Kurzrock R., Wheler J.J. et al. Phase I dose-escalation study of the multikinase inhibitor lenvatinib in patients with advanced solid tumors and in an expanded cohort of patients with melanoma. Clin Cancer Res 2015 ; 21: 4801-10. Doi: 10.1158/1078-0432.CCR-14-3063.
- Vergote I., Powell M.A., Teneriello M. G. et al. Second-line lenvatinib in patients with recurrent endometrial cancer. Gynecol Oncol. 2020 ; 156 (3): 575-582. Doi: 10.1016/j.ygyno.2019.12.039.
- Vergote I., Teneriello M., Powell M. A. et al. A phase II trial of lenvatinib in patients with advanced or recurrent endometrial cancer: Angiopoietin-2 as a predictive marker for clinical outcomes. J Clin Oncol 2013 ; 31 (15 Suppl): abstr 5520.
- Mittica G., Ghisoni E., Giannone G., et al. Checkpoint inhibitors in endometrial cancer: preclinical rationale and clinical activity// Oncotarget .- 2017 .- 8 (52) .- P. 90532-90544. Doi: 10.18632/ oncotarget. 20042.
- Lenvima (lenvatinib) [package insert]. Woodcliff Lake, Nj, Eisai, 2019.
- Kudo M. Targeted and immune therapies for hepatocellular carcinoma: Predictions for 2019 and betond. World J Gastroenterol 2019 ; 25 (7): 789-807. https://dx.doi.org/10.3748/wjg.v25.i7.789.
- Vanderstraeten A., Tuyaerts S., Amant F. The immune system in the normal endometrium and implications for endometrial cancer development// J. Reprod. Immunol .- 2015 .- 109 .- P. 7-16. Doi: 10.1016/j.jri.2014.12.006.
- Herzog T.J., Arguello D., Reddy S.K., et al. PD-1, PD-L1 expression in 1599 gynecological cancers: implications for immunotherapy// Gynecol. Oncol .- 2015 .- 137 (suppl 1) .- P. 204-205.
- Brahmer J. R., Tykodi S. S., Chow L. Q. M. et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer // N. Engl.J. Med .- 2012 .- 366 (26) .- P. 2455-2465. Doi: 10.1056/NEjMoa1200694.
- Ishida Y., Agata Y., Shibahara K. et al. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death // EMBO J .- 1992 .- 11 (11) .- P. 3887-3895. Doi: 10.1002/j.1460-2075.1992. tb05481.x.
- Carreno B. M., Collins M. The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune responses // Annu. Rev. Immunol .- 2002 .- 20 .- P. 29-53. Doi: 10.1146/annurev.immunol.20.091101.091806.
- Latchman Y., Wood C. R., Chernova T. et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation// Nat. Immunol .- 2001 .- 2 (3) .- P. 261-268. Doi: 10.1038/85330.
- Francisco L.M., Sage P. T., Sharpe A.H. The PD-1 pathway in tolerance and autoimmunity// Immunol. Rev .- 2010 .- 236 .- P. 219-242. Doi: 10.1111/j.1600-065X.2010.00923.x.
- Freeman G.J., Long A.J., Iwai Y. et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation// J. Exp. Med .- 2000 .- 192 (7) .- P. 1027-1034. Doi: 10.1084/ jem.192.7.1027.
- Makker V., Taylor M. H., Aghajanian C. et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol 2020 ; 38 (26): 2981-2992. Doi: 10.1200/jœ.19.02627.
- Arora S., Balasubramaniam S., Zhang W. et al. FDA Approval Summary: Pembrolizumab plus Lenvatinib for Endometrial Carcinoma, a Collaborative International Review under Project Orbis. Clin Cancer Res. 2020 ; 26 (19): 5062-5067. Doi: 10.1158/1078-0432.CCR-19-3979.
- U. S. Food and Drug Administration. Simultaneous review decisions for pembrolizumab plus lenvatinib in Australia, Canada and US. https://www.fda.gov/drugs/resources-information-approved-drugs/simultaneous-review-decisions-pem-brolizumabplus-lenvatinib-australia-canada-and-us. Accessed March 18, 2020.
- Makker V., Colombo N., Herraez A. C., et al. A multicenter, open-label, randomized, phase III study to compare the efficacy and safety of lenvatinib in combination with pembrolizumab versus treatment of physician's choice in patients with advanced endometrial cancer. Gynecol Oncol. 2021 ; 162 (suppl 1): S4 https://doi.org/10.1016/S0090-8258(21)00657-0.
- Colombo N., Lorusso D., Casado A., et al. Outcomes by histology and prior therapy with lenvatinib plus pembroli-zumab vs treatment of physician's choice in patients with advanced endometrial cancer (Study 309 / KEYNOTE-775). Presented at: European Society for Medical Oncology (ESMO) Congress 2021 ; September 16-21, 2021. Abstract 726MO.
- Hasegawa K., Nagao S., Yasuda M. et al. Gynecologic Cancer InterGroup (GCIG) Consensus Review for Clear Cell Carcinoma of the Uterine Corpus and Cervix. Int J Gynecol Cancer. 2014 ; 24 (3 Suppl.): S90 — S95. https:// doi.org/10.1097/IGC. 0000000000000297.
- Sagae S., Susumu N., Viswanathan A. N. et al. Gynecologic Cancer InterGroup (GCIG) Consensus Review for Uterine Serous Carcinoma. Int J Gynecol Cancer. 2014 ; 24 (3 Suppl.): S83 — S89. https://doi.org/10.1097/IGC. 0000000000000264.
- Lee C .- H., Shah A.Y., Hsieh J.J., Rao A., Pinto A., Bilen M.A. et al. Phase II trial of lenvatinib (LEN) plus pem-brolizumab (PEMBRO) for disease progression after PD-1/ PD-L1 immune checkpoint inhibitor (ICI) in metastatic clear cell renal cell carcinoma (mccRCC). J Clin Oncol. 2020 ; 38 (15 Suppl.): abstr 5008. https://doi.org/10.1200/JCO. 2020.38.15_suppl. 5008.


