Клатрохелатные комплексы железа (II) — высокоэффективные электрокатализаторы получения водорода при низких значениях pH
Автор: Аль-обади Ф.Т.А., Долганов А.В., Заводаева А.С., Иванцова П.М., Моисеева Д.Н., Нугаева Э.Ф., Солдатова В.И., Тюрин В.Ю., Юрова В.Ю.
Журнал: Огарёв-online @ogarev-online
Статья в выпуске: 19 т.4, 2016 года.
Бесплатный доступ
Темплатной конденсацией алифатических и ароматических α-диоксимов, а также дихлороглиоксима с 4-пиридинилборной кислотой на матрице - ионе железа (II) - синтезированы новые 4-пиридилборатные макробициклические комплексы железа (II), являющиеся эффективными электрокатализаторами реакции 2H+/H2 в водных растворах при pH 1 - 4. Получены трис-диоксиматные клатрохелаты железа (II) с различными электронодонорными и электроноакцепторными реберными заместителями, в том числе с терминальными реакционноспособными группами для иммобилизации на поверхности рабочего электрода, изучена их активность в реакции электрокаталитического получения молекулярного водорода и предложен механизм этой реакции.
Короткий адрес: https://sciup.org/147249192
IDR: 147249192 | УДК: 547.829
Текст научной статьи Клатрохелатные комплексы железа (II) — высокоэффективные электрокатализаторы получения водорода при низких значениях pH
Introduction. Sustainable and clean energy resources are strongly needed to deal with global energy and environmental issues [1]. Extensive efforts have been earlier made to develop the artificial analogs of natural hydrogenases for the hydrogen evolution reaction (HER) from acidic solutions, as the molecular hydrogen has merited increasing attention as clean, inexhaustible, efficient and cost-attractive energy carrier for the future [2]. Therefore, a search for the inexpensive non-precious metal complexes that are able to catalyze the reduction of H+ ions at low or even without overpotential similar to the natural [FeFe] and [FeNi] hydrogenazes seems to be a real challenge for the synthetic chemists [3]. Among many coordination compounds, only limited number of metal complexes may efficiently catalyze the 2H+/H2 process due to many requirements on an electrocatalyst for this reaction [4]. For this purpose, we suggest to use iron (II) clathrochelate complexes with functionalizing pyridinyl substituents, which emerged as effective homogeneous electrocatalysts for hydrogen evolution at low pH; we expected the protonation of these complexes to increase their solubility in water due to the formation of salts (Scheme 1).
2+
N
Scheme 1.
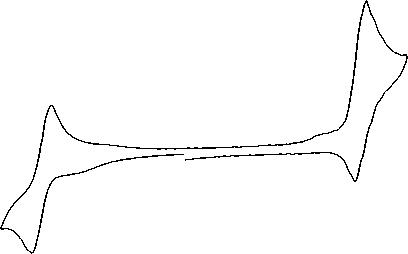
Results and discussion. Electrochemical characteristics of these iron(II) clathrochelates are summarized in Table 1. All peaks observed in their cyclic voltammograms are characteristic of diffusion-controlled current processes. In the cathodic range of these cyclic voltammograms, only one one-electron quasi-reversible wave is observed, which was assigned to redox-processes Fe2+/+, a typical CV curve is shown in Figure 1.
I, µA
– 6
–12
– 2000 1500 –1000 –500 0 500 1000 2000
E , мВ
Fig.1. Cyclic voltammogram for 0,5 mM acetonitrile solutions of FeNx 3 (B4-Py) 2 on a glassy carbon electrode (200 mV/s, GC).
Table 1
Oxidation and reduction potentials (mV) and characteristics of electrochemical processes for the iron(II) clathrochelates
|
Complexes |
Oxidation, Еox |
Reduction, Ered |
kobs |
||||
|
Еa |
Е c |
ΔE |
E c |
E a |
ΔE |
||
|
FeNx 3 (B-4-Py) 2 |
1370 |
1280 |
90 |
– 1080 |
–870 |
210 |
953 |
|
FeDm 3 (B-4-Py) 2 |
1450 |
1360 |
90 |
– 1030 |
– 900 |
130 |
874 |
|
FeBd 3 (B-4-Py) 2 |
1480 |
1400 |
80 |
–1050 |
– 800 |
250 |
658 |
|
Fe(Cl 2 Gm) 3 (B-4-Py) 2 |
1910 |
1800 |
110 |
– 450 |
– 380 |
70 |
1900 |
The anodic ranges of these CVs contain quasi-reversible oneelectron waves assigned to the Fe2+/3+ oxidations. The aliphatic and aromatic substituents in ribbed chelate α-dioximate fragments of these 4-pyridinylboron-capped iron(II) clathrochelates have significant no effect on this redox process except that of six strong electron-withdrawing chlorine substituents in Fe(Cl 2 Gm) 3 (B-4-Py) 2 which oxidizes at a much higher oxidation potential value (Tab. 1). The differences ΔE between the peaks of direct and backward waves for these complexes are from 90 to 110 mV and are characteristic of quasi-reversible redox processes. The cathodic range of CVs for all the clathrochelate studied contain thewaves of themetal-centered quasi-reversible Fe2+/+ reductions; so, the anionic clathrochelate species formed undergo only slight structural changes during this reduction and are stable in the CV time scale. The reduction potential value is substantially shifted to the anodic range in the case of the hexachloroclathrochelate Fe(Cl 2 Gm) 3 (B-4-Py) 2 . Note that the variation of a scan rate in a wide range from 50 to 1000 mV s-1 does not affect the current peak ratio that is equal to 1. In this case, the reduced anionic 19-electron cage iron(I) complex is rather thermodynamically stable and undergoes no substantial chemical and structural transformations in the CV time scale. Therefore, this iron hexachloroclathrochelate seems to be an efficient electrocatalyst for hydrogen production from H+ ions
–40 1---------------'---------------1---------------1---------------1---------------'---------------1---------------'---------------1
– 2000 –1500 –1000 –500 0
E, mV
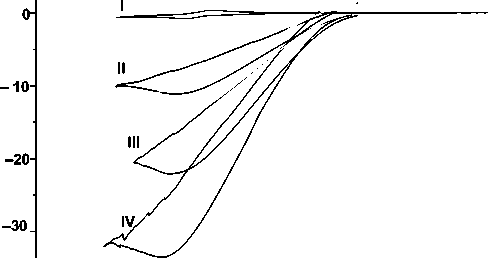
Fig. 2. CVA for 0,5 mM acetonitrile solutions of FeNx 3 (B-4-Py) 2 on a glassy carbon electrode in the absence and (top–bottom) in the presence of 5, 10, 15 and 25 mM of CF 3 COOH.
We studied the electrocatalytic properties of the clathrochelates obtained in HER using CF 3 COOH as a source of H+ ions. The addition of CF 3 COOH to their acetonitrile solutions caused a formation of the catalytic waves at the potentials of the redox M2+/+ couples (Fig. 2). An increase in the concentration of H+ ions leads to slight catodic shifts of the catalytic waves accompanying the increase in their intensities caused by the electrocatalytic H+ reduction (as follows from GC data). The complexes obtained efficiently electrocatalyze hydrogen evolution (Fig. 1) from an acid solution in acetonitrile as well as from aqueous buffers with low pH (1 – 4). NMR spectroscopy data confirmed that protonation of both the pyridine moieties occurred, thus giving protonated species that were stable in these conditions. Titration of an acetonitrile solution of a clathrochelate with an acid resulted in a significant increase of the current assigned to the Fe2+/+ pair, while the peak of the reverse process vanished. Kinetic parameters and overall efficiency of the catalytic process depended greatly on the nature of ribbed substituents in chelate fragments of these clathrochelates. Based on the dependence of the rate of the electrocatalytic process 2Н+/Н 2 on the pH value of the aqueous solution, a limiting step was identified and a catalytic mechanism elucidated experimentally. All proton reduction reactions were first order in the catalyst’s concentration and second order in the acid’s concentration; for all of them, rate constants and activation energies have been obtained.
Conclusions. Pyridinyl-functionilized iron (II) clathrochelates emerge as first homogeneous electrocatalysts of hydrogen evolution at low pH; varying their ribbed substituents allows designing electrocatalysts with better performance and stability in harsh near-industrial conditions.
Список литературы Клатрохелатные комплексы железа (II) — высокоэффективные электрокатализаторы получения водорода при низких значениях pH
- Lewis N. S., Nocera D. G. Powering the planet: chemical challenges in solar energy utilization // Proc Natl Acad Sci USA. - 2006. -Vol. 103. - P. 15729-15735. EDN: KRQJJI
- Merki D., Hu X. Recent developments of molybdenum and tungsten sulfides as hydrogen evolution catalysts // Energy Environ Sci. - 2011. -Vol. 4. - P. 3878- 3888. EDN: PNLJJH
- Helm M. L., Stewart M. P., Bullock R. M., Rakowski DuBois M., DuBois D. L. Synthetic nickel electrocatalyst with a turnover frequency above 100,000 s-1 for H2 production // Science. - 2011. -Vol. 333. - P. 863-866.
- Wang M., Chen L., Sun L. Recent progress in electrochemical hydrogen production with earth-abundant metal complexes as catalysts // Energy Environ Sci. - 2012. - Vol. 5. - P. 6763-6778. EDN: YCKXKF


