Legume-rhizobial symbiosis: progress and prospects
Автор: Glyanko A.K.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 2 т.14, 2018 года.
Бесплатный доступ
Data on the role of the legume-rhizobial symbiosis (LRS) in national economic and a brief history of the fundamental study of this unique biological phenomenon are summarized. The features of the formation of root nodules of determinant and indeterminant types are described. The physiological role of the rhizobial Nod factor in suppressing the defense system of a legume plant and the role of the plant's immune systems (MTI and ETI) in the rhizobial infection and the formation of LRS are discussed. Signal systems of a legume plant (Ca2+, NO-synthase, NADPH oxidase) and their components (ROS, RNS) and other signaling molecules involved and interacting in LRS onset are described. The necessity of studying the local and systemic resistsnce of the legume plant to the rhizobial infection is emphasized.
Rhizobiaceae family, legume plants (fabaceae), legume-rhizobial symbiosis, root nodules, nod factor, plant immune systems (mti, eti), ros, rns, ca2+, h2o2, no, salicylic acid, local and systemic resistance, biotic stress
Короткий адрес: https://sciup.org/143165190
IDR: 143165190
Текст обзорной статьи Legume-rhizobial symbiosis: progress and prospects
Legume plants ( Fabaceae ) numbered more than 1500 species set a large group of angiospermous and a significant part of fodder and food crops grown in the world. They are cultivated on 12-15% of arable land on the planet and account for more than 25% of the world's crop production which is 247 million tonnes of grains per year (Graham, Vance, 2003). Their main feature is the ability to restore atmospheric nitrogen thanks to a symbiotic association with soil nodule bacteria of Rhizobiaceae family (rhizobia). This feature allows growing legumes on nitrogen-deficient soils, thereby saving expensive nitrogen fertilizers and protecting water sources from contamination with harmful mineral nitrogen. These plants are often used as “green fertilizer” to improve nitrogen fertility, chemical and physical properties of the soil (Shevchuk, 1979). Legumes include the main food crops: soybeans, peas, clover, alfalfa, mung beans, chickpeas (Bengal gram) and others. In addition to food and fodder crops, such legumes as soybeans ( Glycine max L. Merr.) and pongamia ( Pongamia pinnata L.) are regarded as possible sources of biofuel due to the high content of oil in their seeds (Scott et al. , 2008).
The total level of biological nitrogen fixation on the Earth is 175 – 320 million tonnes of nitrogen per year and the use of mineral fertilizers in agriculture is 110 – 140 million tonnes per year (Sidorova et al. , 2006). However, covering the demand for agriculture in the nitrogen deficit in soils due to the production of mineral fertilizers is irrational due to large energy inputs for their production. Therefore, the increase in the efciency of the biological nitrogen fixation process, mainly due to the symbiotic nitrogen fixation of legumes, sets the main strategic task of the biological science (Mishustin, 1972).
The family Rhizobiaceae includes gram-negative bacteria from various genera: Rhizobium, Azorhizobium,
Allorhizobium, Bradyrhizobium, Mezorhizobium and Sinorhizobium . Gram-positive soil nitrogen-fixing bacteria of the genus Frankia (the family Actinomycetes ) can also form mutualistic associations with non-leguminous woody and shrubby plants (for example, alder and sea-buckthorn). In contrast to rhizobia, these soil bacteria fix atmospheric nitrogen in the soil in a free state; while penetrating into the tissues of the roots of woody plants, they (like rhizobia) form root nodules where atmospheric N2 is reduced to ammonium used by the plant for the synthesis of amino acids, protein and other N-containing compounds (Mishustin, Shilnikova, 1973).
The discovery of nodule bacteria as symbiotic partners of legume plants was in the middle and the end of the 19th century. The special role in these studies belongs to such foreign and native scientists as: J. - B. Bussengo, E. Lahman, G. Gelrigel, M. Beyerink, M.S. Voronin, D.N. Pryanishnikov and others (Mishustin, Shilnikova, 1968). It is noteworthy that in the beginning of the 20th century, blocking or inhibiting of the process of symbiotic nitrogen fixation by legume plants by the presence of sufcient quantities of mineral nitrogen absorbed by the plant in the soil was discovered (Fred, Graul, 1916). That speaks for the legume-rhizobial symbiosis as a possible “emergency system” functioning under extreme conditions – when there is a shortage of mineral nitrogen absorbed by the plant.
At present, mutants of legume plants (for example, peas) have been made with the help of chemical mutagenesis. They are diferently able for forming nodules on the roots: non-nodulating, supernodulating and hypernodulating (Sidorova et al. , 2003, 2006). Of these, there are mutants irresponsive to high doses of soil nitrates which unequally infuence the accumulation of nitrogen in plants and the productivity of grain
(Sidorova et al. , 2010). The cited authors conclude that the obtained forms (lines) of pea are not inferior to commercial ones in terms of productivity, but they considerably exceed them in terms of accumulation of the root mass and aerial parts of plants and the content of nitrogen in them. That makes such forms promising for their use as siderates, the positive outcome of which is expressed in afterefect. Moreover, they represent valuable genetic material for the nurture of new high-yield commercial samples (Sidorova, Shumnyj, 1999).
Biological science has accumulated a large body of scientific information on the legume-rhizobial fixation of atmospheric nitrogen from various scientific positions: physiological, biochemical, genetic, morphological, agronomic and others (Mishustin and Shilnikova, 1968, 1973; Djordjevic et al. , 1987; Rolfe, Gresshof , 1988; Brewin, 1991; Hirsch, 1992; Franssen et al. , 1992; Spaink, 1995; Denarie, Debelle, 1996; Vorob’ev, 1998; Long, 2001; Ferguson, Mathesius, 2003; Gage, 2004; Sidorova et al. , 2006; Oldroyd, Downie, 2008; Tikhonovich, Provorov, 2009; Krugova, 2009; Ferguson et al. , 2010; Oldroyd et al. , 2011; Provorov, Vorob’ev, 2012, etc.). Accumulated scientific results on the mechanisms of legume-rhizobial symbiosis allow us to raise the question of the possibility of constructing a nitrogen-fixing apparatus (on the basis of symbiosis) in other (non-legume) plants, primarily in cereals for bread making (Charpentier, Oldroyd, 2010, Beatty, Good, 2011).
The enormous general biological and economic importance of the Rhizobiaceae family bacteria attracts specialists of various profiles from various agrochemical and plant physiologists to molecular biologists and geneticists. The collective monograph: “Rhizobiaceae. Molecular biology of bacteria interacting with plants” published in 2002 in the publishing house “Biont” (S.-Petersburg, 567 p.) is an example of a broad generalization of research results on this problem. The book represents a translation of the original edition of “The Rhizobiaceae. Molecular biology of model plant-associated bacteria” (published by “Kluwer Acad. Press”, 1998). The monograph “Genetic foundations of the evolution of plant-microbial symbiosis” by N.A. Provorov and N.I. Vorob’ev, the employees of the All-Russian Research Institute for Agricultural Microbiology (S.-Petersburg, 2012, 400 p.) is another example in this aspect. The book presents a summary of current knowledge about the patterns of the establishment of mutualistic relationships between plant and microbial organisms with the analysis and generalization of scientific data on genetic, physiological, biochemical, morphological, ecological and other features of plant microbial communities. Significant place in the monograph is given to the legume-rhizobial symbiosis (LRS) with the consideration of such issues as ontogeny and regulation of nodule development, specificity, signal interactions and metabolic integration (Glyan’ko, 2014a). The staf of the Institute of Cytology and Genetics of the Siberian Branch of the Russian Academy of Sciences (Novosibirsk) made a significant contribution to the study of symbiotic nitrogen fixation (Sidorova 1981, Shumny et al., 1991). Among the publications of the post-Soviet space in recent years, a fundamental four-volume monograph “Biological fixation of nitrogen” issued by the Institute of Plant Physiology and Genetics of the National Academy of Sciences of Ukraine in cooperation with other institutions is worth noticing (Kiev: Logos, 2010, 2011). In this monograph, the process of nitrogen fixation is analyzed from interdisciplinary positions: separate volumes are devoted to the physiological, biochemical, microbiological aspects of legume-rhizobial symbiosis and plant-microbial associations as well as to questions of the genetics of nitrogen fixation. In particular, the authors consider transposon mutagenesis among the perspectives that allow solving complex problems of increasing the efciency of plant-microbial symbiosis functioning (Kots et al., 2010, 2011).
Features of the formation of root nodules in legume plants. Root nodules are the main ecological, physiological and biochemical niche in legumes for nitrogen fixation and symbiosis functioning. Rhizobia are capable of forming nitrogen-fixing nodules on certain types of legumes and are classified into two groups: fast growing varieties ( Rhizobium ) and slow growing ones ( Bradyrhizobium ). As a rule, infection with nodule bacteria Rhizobium is typical for limited species of legume plants, although there are strains with a wide range of hosts. Thus, the following cross-inoculation groups were identified: Rhizobium (Rh.) trifolii (cow clover), Rh. leguminosarum (peas, vetch, lentil), Rh. phaseoli (bean), Rh. meliloti (alfalfa, sweet clover), Rh. japonicum (soybean), Rh. lupine (lupine), Rh. cicer (gram), Bradyrhizobium japonicum (soya, vigna), Rhizobium spp (strains BR816, NGR234, MPIK1030 with a wide range of host plants), Sinorhizobium fredii (strain USDA257 with a wide range of hosts). Although Rhizobium is a member of the family Rhizobiaceae which includes the known plant pathogen Agrobacterium , they are usually not considered as plant pathogens or parasitic bacteria (Djordjevic et al. , 1987). However, plants infected with rhizobia do not lose their innate defense systems which can be directed against rhizobia in case of violation of symbiotic relationships (Glyan’ko, Ischenko, 2017).
Thanks to the nodule, nutrients are exchanged between the organisms: the bacteria supply the plant with reduced nitrogen (ammonium) and the plant supplies them with carbohydrates (dicarboxylic acids). After genetic transformations and signaling processes involving plant favonoids and bacterial lipopolysaccharides (the so-called Nod factors), bacteria enter the root hairs through newly synthesized specific plant structures – infection threads (ITs), initiating meristematic activity in the cortical cells of the root and formation of nodule primordum. Bacteria multiply in infection threads of root hairs. Moving along them to the cells of the host plant with the help of endocytosis they form organelle-like structures – symbiosomes. The symbiosomes diferentiate in the nodule into bacteroides where the fixation of atmospheric nitrogen occurs with the participation of the bacterial nitrogenase enzyme complex (Kretovich, 1997).
There are two main morphological types of nodules in legumes: determinate and indeterminate (Hirsch, 1992). Formed indeterminate nodules typical for example for peas, clover and alfalfa have a cylindrical shape, a permanent meristem and include four diferentiated zones: meristematic, infectious, N2 fixing and a senescence zone in mature nodules (Timmers et al. , 2000). Mature indeterminate nodules contain a heterogeneous population of nitrogen fixing bacteroides.
Legumes with determinate nodules are mainly tropical and subtropical species. In particular, they include soybean (Glycine max), pongamia (Pongamia pinnata), bean (Phaseolus vulgaris), vigna (Vigna Savi) as well as some temperate climate species, for example, Lotus japonicus. The determinate nodules are spherical and do not form a permanent meristem. They grow by cell stretching faster compared to cell division. The central zone in these nodules is occupied by infected and uninfected host cells where atmospheric nitrogen fixation occurs (Franssen et al., 1992). It should be added that the bacterial nitrogenase in the nodules is irreversibly inhibited by “trace” amounts of oxygen; consequently, the nodule development occurs under varying conditions – from normal conditions (normoxis) in the formation of symbiotic nodules to microoxic ones in functioning nodules (Appleby, 1992). This is one of the intensively studied features of rhizobia functioning in nodules (Yakovleva, 1975; Tsyganova et al., 2011). There exist plants that form nodules on roots and stems at the same time. In particular, such plants include the subtropical and tropical legume plant sesbania of the Sesbania Scop. genus, numbered more than 50 species. Sesbania is widely used in the countries of southern Asia as a siderat and as an ornamental plant (Vul’f, Maleeva, 1969).
Formation and functioning of legume-rhizobial symbiosis. The initial stages of LRS include infection of the roots of legumes and the formation of symbiotic structures: infection threads (ITs) for the multiplication and transport of bacteria to cortex cells and root nodules for enzymatic fixation of atmospheric nitrogen. These complex processes involve both symbionts and represent molecular dialogue between the two partners resulting in profound genetic, metabolic, structural and other changes in the plant organism that lead to bacterial invasion and nodule formation (Oldroyd, Downie, 2008; Oldroyd et al.., 2011). The main role in these processes is played by Nod factors which represent bacterial lipohitooligosaccharides interacting on the plasmalemma of epidermal cells with receptorlike kinases (RLK). That leads to the activation of symbiotic pathways in the host plant (Murray, 2011). In the initial stage, rhizobia in the rhizosphere or ones adsorbed on the root surface recognize plant favonoids specific to them (daidzen, genistein, couestrol, naringenin, etc.) and activate the transcription of the nod-genes that are required for infection, nodule formation and choice of a specific host plant (Denarie, Debelle, 1996). The products of interaction of favonoids with rhizobial genes lead to the synthesis of various compounds including rhizobial NFs (Long, 1996). The basic NF skeleton (chitooligosaccharide) has modifications in various species of rhizobia. The skeletom mainly modifies according to the length and level of saturation of the side fatty acid residues (C16 – C20) as well as according to the presence of radicals – acetyl, sulfate, carbamoyl, fucosyl (Gough, Cullimore, 2011). The side NFs chains specifically interact with plant receptors (RLK) and carry out signal transduction to establish a mutualistic cohabitation of the two organisms causing a variety of responses in the host plant: a change in the membrane potential, depolarization of the root hair plasmalemma and initiation of nodule primordum (Heidstra, Bisseling, 1996; Glyan’ko, 2014). The complex NFs structure determines the specificity of rhizobia to the legume host plants. In this case, the polyspecificity of rhizobia to the host plant is due to the variety of their synthesis of NFs. An example of the broad specificity of rhizobia to legume host plants is the rhizobial strain NGR234 capable of infecting and nodulating 112 species of legume plants as well as the non-leguminous plant Parasponia andersonii Miq. from the hemp family (Cannabaceae) (Skorpil, Broughton, 2005).
The physiological role of Nod factor (NF) and plant receptors (RLK) in the symbiosis
Molecular interaction of NFs and RLK is the main condition for the start of programs of genetic infection and nodule formation (nodulation) of the roots of legume plants. These events are preceded by the preinfective responses of the host plant to the action of NF: the alteration of the cytoskeleton, the swelling and twisting of the tip of the root hair, and the formation of radically directed cytoplasmic bridges in the outer cortical cells – preinfection threads. A bacterial microcolony carrying out local hydrolysis of the cell wall of the root hair is formed in these threads. As a result, the root hair ceases external growth and begins to grow inward forming a tubular invagination of the cell wall of the infection thread where the bacteria multiply and move to the cells of the cortex. The participation of NF-signaling in the formation of IT is proved in experiments with mutant organisms (Gough, Cullimore, 2011). The role of NF in the activation of a number of genes and transcription factors (proteins: NIN, RPG, ERN, CYCLOPS, CERBERUS) is shown. That is associated with infection and nodule primordum formation (Ferguson et al., 2010).
Plant hormone cytokinin, the receptor of which is histidine kinase (MtCRE1 / LjHK1), takes part in the initiation of cortical cell division and morphogenesis of the nodule primordum; it is activated by Ca2+-calmodulin-dependent kinase (CCaMK) (Glyan’ko, 2015; Glyan’ko, Ischenko, 2015). In this case, the hormonal status of the cells shifts toward increasing the content of cytokinin and reducing the content of auxin. However, the relationship of these hormones and their role in nodule formation can significantly change in the future (Akimova, Sokolova, 2012; Ferguson, Mathesius, 2014).
The role of Nod factors in suppressing the defense systems of the host plant
In addition to initiating symbiotic functions, NFs are involved in the inactivation of the defense systems of the macrosymbiont (Glyan’ko, 2016; Glyan’ko, Ischenko, 2017). Plant receptors (for example, LysM RLK) “recognize” rhizobial NFs and inhibit the innate MTI-defense plant system by an unknown mechanism with the simultaneous initiation of a cascade of symbiotic reactions (Liang et al. , 2013). However, this process can be violated with unfavorable conditions for symbiosis. That leads to the activation of plant defense mechanisms such as the synthesis and accumulation of ROS, RNS and signaling compounds (O2•-, H2O2, NO, Ca2+) including defense mechanisms. To initiate these mechanisms, the host plant uses two ways of the innate immune system: MTI (MAMP-triggered immunity) and ETI (efector-triggered immunity) (Figure 1).
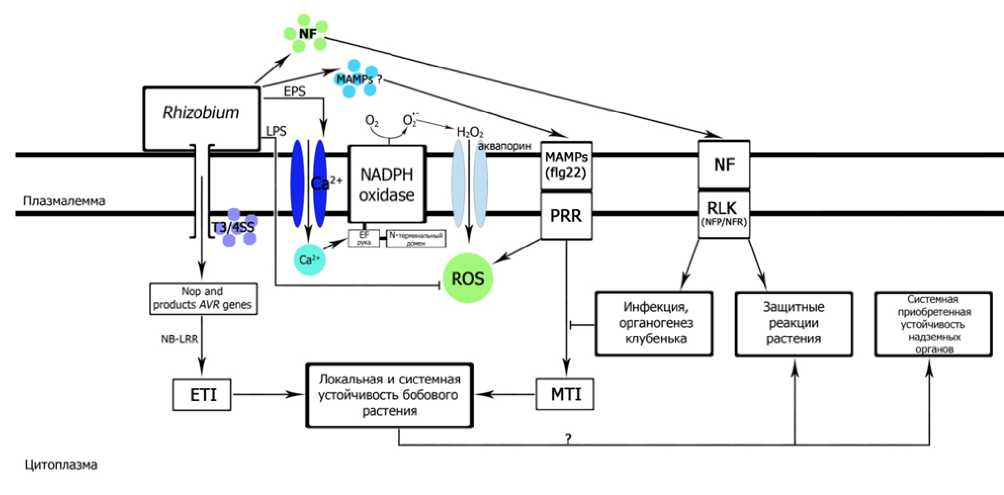
Figure 1. Scheme of immune reactions in a legume plant infected with rhizobia (Glyan’ko, Ischenko, 2017a).
Translation of Russian terms to Fig.1 in English. Плазмалемма – Plasmalemma ; Цитоплазма – Сytoplasm ; Локальная и системная устойчивость бобового растения – Local and systemic resistance of a legume plant ; Инфекция, органогенез клубенька – Infection, nodule organogenesis ; Защитные реакции растения – Plant defense reactions ; Системная приобретенная устойчивость надземных органов – Systemic acquired resistance of aerial organs ; Аквопорины — Aquaporins.
Explanations. Rhizobium – nodule bacteria (rhizobia); NF – rhizobial Nod factor; MAMPs – molecular microbial patterns; EPS – rhizobial exopolysaccharides; LPS – rhizobial lipopolysaccharides; ROS – reactive oxygen species; NADPH oxidase – favin-containing enzyme involved in the generation of ROS on the plasmalemma; fg22 – active determinant of MAMP; PRR – plant receptor; RLK (NFP / NFR) – plant receptors-like kinases; T3 / 4SS – bacterial secretion systems of types 3 and 4; Nop and products Avr -genes (Nodulational protein Nop L and products of Avr -genes) bacterial compounds secreted into a plant cell; NB-LRR – the product of plant R-genes; ETI –intracellular immune system of the plant associated with bacterial efectors; MTI – the plant's nonspecific immune system associated with MAMPs; aquaporin – water protein channel; N-terminal domain – a site of an enzyme that undergoes phosphorylation with Ca2+-dependent protein kinases; EF-hand – enzyme motifs that bind two calcium ions.
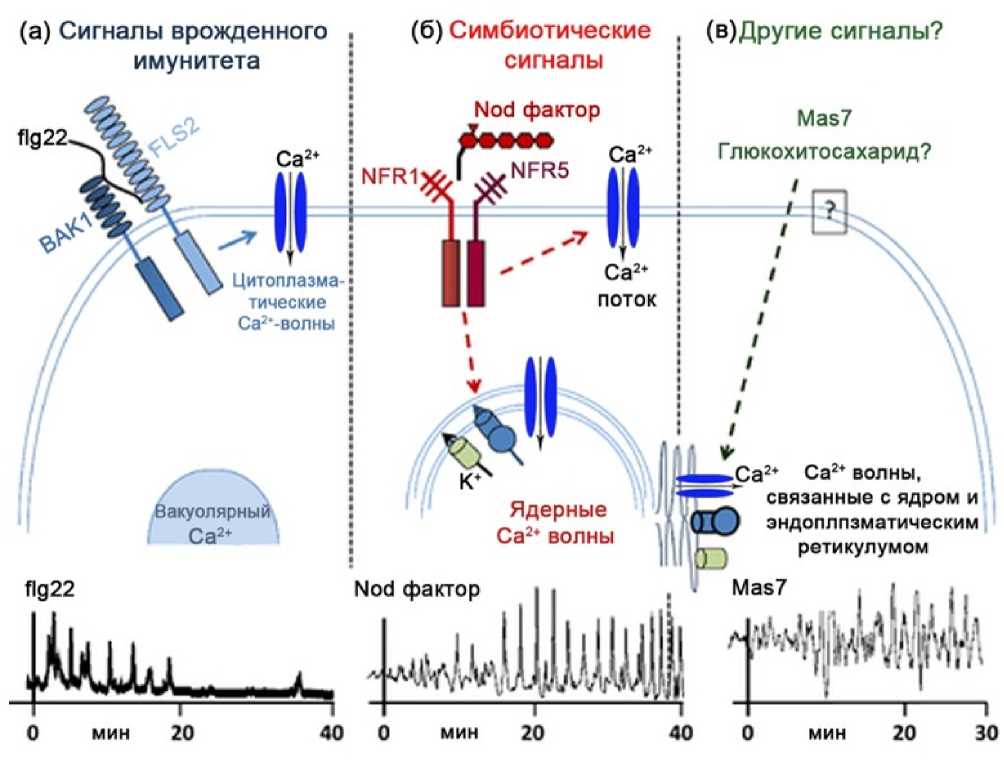
fig22
NFR1
Вакуолярный Ca2*
, (в)Другие сигналы?
Nod фактор
Ca2’
NFR5
Mas?
Пд22
Nod фактор
Цитоплазма тические Са5*-волны
Са2* поток
(б) Симбиотические сигналы
Mas?
Глюкохитосахарид?
j I I I i I * ►Са2* Са2* волны, ■^ связанные с ядром и эндоплпзматическим ретикулумом
О мин 20
--Г 0 мин 20
(а) Сигналы врожденного имунитета
--г 0 мин 20
К
Ядерные
Са2‘ волны
Figure 2. Са2+-oscillations in the plant cell depending on the signaling stimulus (Modification: Downie, 2014)
Explanations. (a) – innate immune signaling (fg22), (b) – symbiotic signaling (Nod factor), (c) – other signaling types (Mas7, glucochitosaccharides). (a) Interaction of the bacterial MAMP-compound flg22 (fagellin) with the surface receptors of the plant cell (leucine-rich FLS2 and BAK1) causes activation of Са2+ fux into the cytoplasm and calcium oscillations in the cytoplasm. (b) In a symbiotic signaling, the Nod factor (NF) interacts on the plasmalemma with the plant receptors NFR1 or NFR5 causing there through an increase in Са2+ fux into the cytoplasm and calcium oscillations in the nucleus. (c) Interaction of the peptide Mas7 (Mastoparan7) with the plant cell causes the appearance of calcium oscillations in the nucleus and is associated with the endoplasmic reticulum but does not cause an increase in Са2+ fux from the extracellular space. It should be noted that diferent biological stimuli ( flg22, NFF, Maas2 ) cause Са2+oscillations diferent in the amplitude, frequency and duration. However, the nature of Са2+- spiking in mycorhizal and rhizobial symbiosis is the same in all plant species (Granqvist et al. , 2015).
The first way is based on the use of transmembrane plant receptor kinases (RKs) or proteins (RLPs) and microbial compounds MAMPs (microbial-associated molecular patterns) or PAMPs (pathogen-associated molecular patterns). The second way is intracellular, where polymorphic NB-LRR (nucleotide binding - leucine rich repeat) plays the main role. These defense systems act when infected with both rhizobia and pathogens. Moreover, the functioning of these systems is not excluded in the initial stages of rhizobial infection and in the functioning of LRS (Gourion et al. , 2015; Glyan’ko, Ischenko, 2017).
MTI activity has been proven to be suppressed by high concentrations of NFs (Liang et al. , 2013). Such inhibition of MTI defense system activity is independent of nodulation as it was observed in soybean mutants unable to synthesize NF receptors (Liang et al. , 2013). The ability of NF at high doses (10-6 to 10-9 M) to suppress the activity of the defense systems of the host plant can be a part of the mechanism by which nodulation is regulated on the feedback principle (Bufard et al. , 1996). Thus, NFs can both block and activate the plant defense system and participate in reactions related to the nodule organogenesis.
At the earliest stages of invasion, rhizobia behave like pathogens and, apparently, initiate systemic acquired resistance in plant organs that are not subjected to rhizobial infection. Due to this, it is proposed to distinguish between the local and systemic resistance of the legume plant to the infection with rhizobia (Glyan’ko, Ischenko, 2017). Local resistance localized in the roots (and in some cases, in stems) is suppressed by rhizobia; systemic resistance prevents rhizobial infection of the plant aerial organs (Fig. 1).
Signaling systems of rhizobia and legume plants in LRS formation
Plants, like animals, perceive signals of a diferent nature coming from the environment and react to them with the help of a genetic apparatus activating defense mechanisms and changing the metabolism conciliatorily to new external or internal conditions. Bacterial NF-signaling closely interacts with plant signal systems: calcium, NO-synthase, NADPH oxidase (Glyan’ko, 2015). Expression of the symbiotic nuclear genes of the host plant is carried out with the participation of these signal systems and their components (ROS, RNS) as intermediates (secondary messengers) in LRS formation.
The role of calcium in symbiosis. The Са2+ signal system in symbiosis (as in other cases) is associated with the appearance of oscillations, the so-called calcium spiking as a result of rhythmic increase and decrease in intracellular calcium concentration (Granqvist et al. , 2015). Са2+ oscillations are characterized by amplitude, frequency and duration (Fig. 2). It is proven that the calcium oscillation system is a highly conserved part of the symbiotic signaling pathway that determines the role of calcium in the genetic efect on LRS formation (Granqvist et al. , 2015). In case of LRS, the fast fux into the cytoplasm is replaced by a decrease in its content in the cytoplasm (Shaw, Long, 2003). Oscillations of cytosolic calcium are observed approximately 10 – 15 min after exposure of rhizobia or the purified NF to the calcium fux into the cytoplasm. They cause the phosphorylation of proteins – transcription factors – with the participation of CCaMK (Са2+-calmodulin kinases). Further, they cause the expression of symbiotic genes in the nucleus. However, the mechanism for decoding symbiotic calcium oscillations is still the recurring problem. CCaMK capable of binding 4 calcium molecules is assumed to be involved in this process (Singh, Parniske, 2012). The
Са2+-spiking mutation inhibits nodules organogenesis in Lotus japonicus , but inhibition is removed by a preactivated form of CCaMK (Hayashi et al. , 2010).
The role of ROS in LRS formation. Molecular oxygen acts as the precursor in the formation of reactive oxygen species (О2•-, Н2О2, •ОН, ОН1, О21). The intensive ROS formation in plants occurs in the photosystem, peroxisomes, mitochondrial respiratory chain and in other cell structures where one-electron O2 reduction occurs. The peroximal and NADPH oxidase of plasmalemma is one of the most important ROS sources in phytopathogenesis (Tarchevsky, 2002; Maksimov, Cherepanova, 2006). The close connection between ROS production with the release of calcium ions into the cytoplasm under the action of stressors is proved and the importance of Са2+ and ROS as key components of the single signal system is emphasized (Kolupaev, 2007). The membrane NADPH oxidase which binds Са2+ and is activated by it plays an important role in this process (Sagi, Fluhr, 2006).
ROS formation during the development of symbiotic interaction is the main factor in the similarity of early plant responses to infection with pathogens and symbiotrophs (Baron, Zambryski, 1995; Deakin, Broughton, 2009). However, the role of ROS, in particular, hydrogen peroxide (Н2О2) and superoxide anion radical (О2•-) in the symbiotic relationship between the two organisms is not fully understood. In contrast to pathogenesis, when ROS perform a toxic role for the pathogen, in LRS rhizobia inhibits the signaling pathways leading to the development of plant defense systems (Bufard et al., 1996). The content of Н2О2 increases in the first day after the inoculation of the pea roots with a compatible rhizobia strain (Vasil’eva et al, 2001). It has been shown that the rhizobial antioxidant enzyme SOD (superoxide dismutase) catalyzing the dismutation reaction of О2•- in Н2О2 is necessary for the normal rhizobial infection and nodulation (Santos et al., 2001). According to Lohar et al. (2007), a change in the morphology of the root hairs in response to the rhizobial infection is accompanied by temporary fuctuations in Н2О2 concentration. The authors attribute this to the need for ROS for the processes leading to the twisting of the root hairs and the subsequent invasion of nodule bacteria. The transient increase in ROS in root hair cells when treating bean root hairs with the purified NF is specific for the early stage of the interaction between rhizobia and legume since it difers from the dynamics of ROS in the action of pathogen elicitor characterized by a constant increase in the ROS content (Cardenas, Quinto, 2008). On the other hand, the treatment of alfalfa seedlings with the purified NF leads to inhibition of Н2О2 formation which is associated with the defense response of the plant (Shaw, Long, 2003). Thus, the literature data indicate ROS either as a negative regulator of LRS formation or as a positive regulator of this process. Consequently, the rhizobial infection is able to regulate the content of cytotoxic compounds such as Н2О2 and NO (Glyan’ko et al., 2014). However, certain defense macrosymbiont responses are induced during the development of the nodule (Vasse et al., 1993; Gamas et al., 1998).
Nitric oxide (NO) in the symbiosis. NO belongs to the group of active forms of nitrogen (RNS) in which there are other low-molecular N-compounds: OONO-, NO2-, NO-, NO+. Nitrotyrosine-containing and other proteins modified with NO and OONO- S-nitrosothiols are considered as compounds of the RNS group participating in the nitrosative stress (Klatt, Lamas, 2000). The role of RNS in the processes of LRS formation and functioning is one of the least studied parties. In studying this issue, the focus is on the NO molecule which has a wide range of biological efects (Meilhoc et al., 2011). Nitric oxide is already detected in the first hours after the interaction of rhizobia and a legume in IT, tissues of the nodule meristem, in nitrogen-fixing nodules (Baudouin et al., 2006; Meilhoc et al., 2011; Glyan’ko, 2013). There is evidence that NO can participate in the regulation of nodule formation on the roots of legumes (Herouart et al., 2002), but high NO concentrations prevent the infection of plants with rhizobia and mycorrhiza (Meyer et al., 2005). According to Mitanova et al. (2006), the exogenous NO (in the form of the NO donor – sodium nitroprusside) adversely afected the adhesion and penetration of rhizobia into the roots of peas. In these experiments, the addition of hemoglobin from the erythrocytes of a horse to the medium with sodium nitroprusside reduced the inhibitory efect of NO on these processes by a factor of 1.5. That is explained by NO binding by hemoglobin. The efect of the rhizobial infection on the NO content in the susceptible for rhizobia zone of the pea root has been revealed depending on the strain of nodule bacteria that difer in the degree of N2-fixing capacity and virulence (Glyan’ko et al., 2012; 2014). These facts indicate the participation of NO in the processes of LRS formation.
NO synthesized in bean nodules is bound by leghemoglobin with nitrosylleghemoglobin formation (Yamamoto et al. , 1990; Yamamoto, Kanayama, 1990). This can lead to the blocking of the nitrogen fixation process by inhibiting the transfer of oxygen into nodules bacteroides. The question of the role of non-symbiotic hemoglobin in LRS is important. It is believed that the role of non-symbiotic hemoglobin in uninfected cells can consist in detoxifying NO which has a negative efect on the formation of the symbiosis as well in modulating the NO level (Simoda et al. , 2005). It can be concluded that
NO functions as a multipurpose regulator of various mechanisms of the symbiotic process: recognition of the host plant; modulation of the defense reactions of the macrosymbiont; formation of the primordium and nodule organogenesis; nitrogen fixation and carbon metabolism in nodules; nodule aging. NO interacts with other biological molecules, for example, phytohormones and ROS (Hichri et al. , 2015).
The role of salicylic acid in LRS. Systemic plant resistance (SAR) is formed with the participation of salicylic acid (SA) and hydrogen peroxide (Ryals et al, 1996; Molodchenkova, 2001; Mostofa et al. , 2015) and accompanied by the increase in the SA content, its derivatives as well as by the increased expression of SA dependent genes (Shah, Zeier, 2013). The interaction of SA and Н2O2 in the metabolism of plants has been shown. A change in the intracellular concentration of one of these compounds afects the content of the other (Leon et al. , 1995; Rao et al. , 1997). Enzymes of the redox cycle (catalase, peroxidase, NADPH oxidase, SOD, etc) function in these processes. According to Kolupaev et al. (2016), ROS, Са2+, NO are involved in the signal transduction of the exogenous SA into the genetic apparatus. There is evidence of the participation of SA and its derivatives (methyl salicylate) in a signal transfer chain for long distances during SAR formation (Park et al. , 2007). However, earlier results do not confirm the role of SA as a distant signal (Vernooij et al. , 1994; Pallas et al. , 1996).
Thus, SA is a component of protection from the pathogenic invasion and a component of increase of resistance to anthropogenic factors of plant cells. Such SA functions can impede the formation and functioning of LRS. Indeed, the exogenous SA had a negative efect on LRS (Shumnyj et al. , 1991; Martinez-Abarka, 1998; Glyan’ko et al, 2005). Some literature data confirm that
SA synthesis by the host plant is regulated at the gene level with the participation of the rhizobial Nod factor (Glyan’ko, 2014). This is confirmed by the results of significant accumulation of SA in the roots of the rhizobial NF mutant and during the inoculation of alfalfa with an incompatible rhizobial strain (Blilou et al. , 1999). Nod factor is suggested to block the SA-signaling mechanism associated with the defense responses of the host plant by reducing the synthesis of SA (Bueno et al. , 2001). On the other hand, it was found that a decrease in the endogenous level of SA by the expression of the transgenic enzyme salicylate hydrolase positively infuenced the processes of infection and nodulation in a number of legumes (Stacey et al. , 2006). These results indicate the important role of SA in the defense reactions of the macrosymbiont in the formation of LRS.
Signaling functions of ROS and RNS and their interaction in the formation of LRS
The important role of ROS and RNS in the response of plants to biotic and abiotic stresses is undoubted (Corpas et al. , 2013; Yu et al. , 2014; Baxter et al. , 2014; Karpets, Kolupaev 2017). Synthesis of these molecules is associated with programmed cell death (PCD) which is an important mechanism for regulating the growth and development of plants and eliminating damaged or infected cells under stress (Wang et al. , 2013). In the study of LRS, the mechanisms of the interaction of the bacterial NF and signal plant molecules (Са2+, ROS, RNS, SA) attract attention first of all (Scheler et al. , 2013). It has been proved that NO and ROS (H2O2) can regulate each other's synthesis (Figure 3). During a hypersensitive reaction NO can have an efect on the synthesis of H2O2 through the S-nitrosylation of plasmalemma NADPH oxidase (AtRbohD) (Yun et al. , 2011). On the other hand, the accumulation of H2O2
induces NO synthesis by enhancing the expression of nitrate reductase. In summary action it subsequently leads to cell death (Wang and al., 2013). However, NO accumulation in cells is preceded by the release of Са2+ ions into the cytosol, the activation of NADPH oxidase and the enhancement of the synthesis of hydrogen peroxide. Thus, the cross-interaction of NO and Н2О2 is an important feature of the activity of these molecules.
It is known that NO in the nitrosylation reaction can react with the reduced glutathione (GSH) with the formation of S-nitrosoglutathione (GSNO) which is a mobile reservoir of the bioactive NO (Barraso et al. , 2013; Kubienova et al. , 2014; Yu et al. , 2014). On the other hand, peroxynitrite (ONOO-) – a powerful oxidation-nitrating molecule – forms in the result of the reaction between О•- and NO (Corpas, Barroso, 2014).
As a result of the presence of NO, GSNO and ONOO- in plant tissues, a post-translational modification of proteins occurs, similar to S-nitrosylation and nitration (Romero-Puertas et al. , 2013). Catalase and glycolate oxidase are inhibited by the S-nitrosylation reaction in peroxisomes; that can regulate the cellular level of Н2О2 (Ortega-Galisteo et al. , 2012). On the other hand, ONOO- generation can induce nitration of plant proteins and nitrosative damage to plant cells, although endogenous nitration can perform a regulatory function (Barraso et al. , 2013).
The mechanisms of interaction between ROS and RNS are well studied in phytopathogenesis, when in systemic acquired resistance the main roleplay ShF (Super-high Frequency) and the process of programmed cell death (PCD) as a result of the rapid synthesis of these molecules (Bolwell, 1999). At present, it has been proved that the ratio of NO, O2•-, H2O2 and ONOO- in the cell determines ShF under the infuence of biotic and abiotic factors (Zaninotto et al., 2006; Zhao et al., 2007). It should be noted that the information on the role of these molecules in the formation and functioning of LRS is insufcient. It especially concerns the local and systemic resistance of a legume plant to the rhizobial invasion (Glyan’ko, 2016). Thus, from the above, it can be concluded that ROS and RNS in LRS can play a dual role: as signaling molecules and as toxic compounds. However, in both cases their role is associated with the rhizobial infection, the formation of symbiotic structures and their functioning.
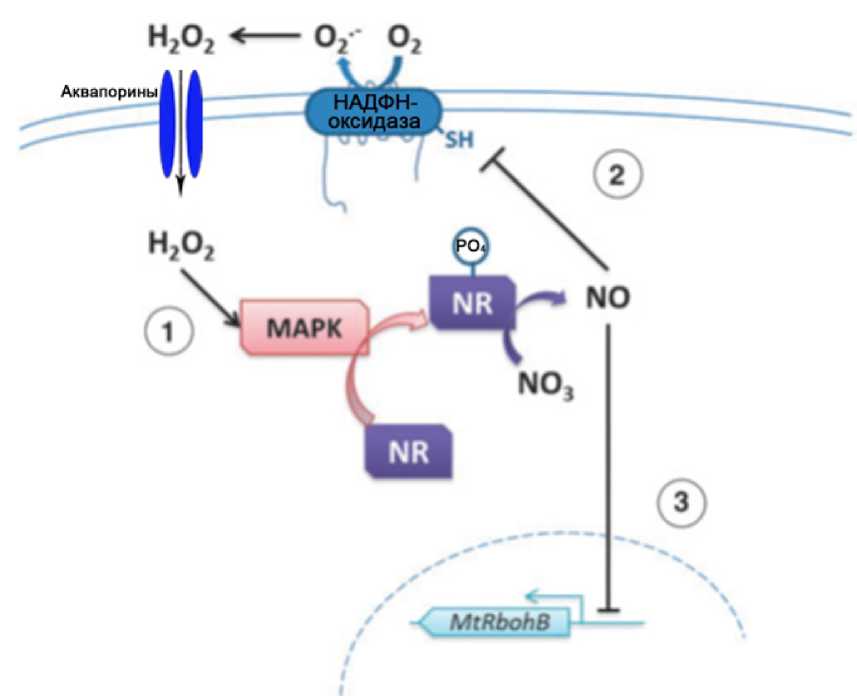
Figure 3. Diagram of interaction between ROS and NO in the legume-rhizobial symbiosis (Modification: Puppo et al. , 2013). 1. Synthesis of Н2О2 with the participation of NADPH oxidase localized on the membrane is shown; activation of MAP kinase (MAPK) and, respectively, nitrate reductase (NR) by hydrogen peroxide (Wang et al. , 2010). 2. Inhibition of NADPH oxidase (RBOH) by NO (nitrosylation) (Yun et al. , 2011). 3. Possible repression of the MtRbohB gene of the RBOH enzyme by NO expression in the symbiotic interaction of alfalfa and Sinorhizobium meliloti (Boscari et al. , 2013).
Translation of Russian terms to Fig.3 in English: НАДФН-оксидаза – NADPH oxidase; Аквопорины - Aquaporins
Rhizobium
NO
НАДФН-оксидаза
NO синтаза, нитратредуктаза и др.
Активность белков - Экспрессия генов
Развитие инфекционной нити
Развитие клубенька
------2^*--------
Функционирование клубенька
- нитратное дыхание - старение. ...
LysM RLK
\ЛПС
X
ONOO
Дифференциация бактероидов
Ог /Н2О2 генерирующие системы
NO генерирующие системы
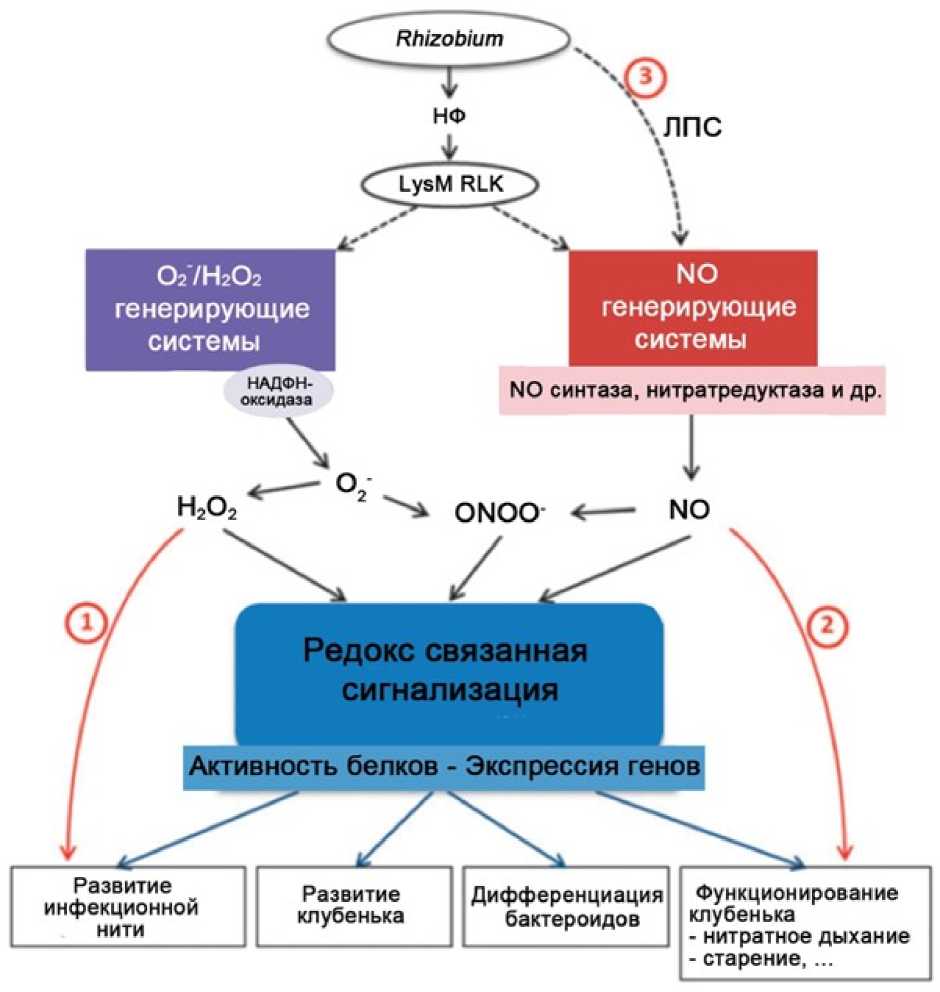
Figure 4. Scheme of the summing role of Н2О2 and NO in the processes of nodulation (Modification: Puppo et al. , 2013). The efect of the rhizobial nod factor (NF) in its interaction with the plant receptor (LysM RLK) on systems generating Н2О2 and NO, as well as on the formation of O 2•- and ONOO-was shown. The efect of these molecules on signaling based on the redox reactions (sulfonylation, nitrosylation and nitration of proteins) and leading to gene expression and protein activation is shown below. As a result, these processes are associated with the formation of infection threads (ITs) (1), with the nodule organogenesis, diferentiation of bacteroides, nodules functioning, nitrate respiration and nodules aging (2). The participation of Н2О2 in the formation of ITs and the role of NO in mature nodules are shown separately. The participation of rhizobial lipopolysaccharides (LPS) in the formation of NO (3) is presented (Hichri et al. , 2015).
Translation of Russian names to Fig.4 in English: Генерирующие системы (НАДФН –оксидаза, NO –синтаза, нитратредуктаза и др.) – Generating systems (NADPH oxidase, NO-synthase, nitrate reductase, ets ) ;
Редокс связанная сигнализация – Redox associated signalling ; Активность белков – Protein activity ; Экспрессия генов – Gene Expression ; Развитие инфекционной нити – Infection thread development ; Развитие клубенька – Nodule development ; Дифференциация бактероидов – Diferentiation of bacteroides ; Функционирование клубенька, нитратное дыхание, старение – Nodule functioning, nitrate respiration, aging.
Fig. 4 shows the summing role of Н2О2 and NO in the processes of nodulation. Rhisobial NFs interact with plant receptors (LysM RLK) and infuence the generation of О2•-, Н2О2, NO, ONOO- with the participation of NADPH oxidase, NO synthase, nitrate reductase, nitrite reductase and others. The formed signal molecules form redox-based signaling which afects the activity of the modified proteins and the genes expression. All these processes are associated with the formation and functioning of LRS: formation of infection threads, nodules development, and diferentiation of bacteroides in nodules, N2 fixation, nitrate respiration in nodules, and the aging and decay of nodules.
It should be noted that small signal molecules – ROS and RNS – are not the only compounds actively participating in the process of LRS. An important role, for example, belongs to phytohormones that initiate the morphogenesis of the nodule (auxin and cytokinin) and regulate the rhizobial infection (abscisic and jasmonic acids) (Glyan’ko, 2015). .
CONCLUSIONS
Microorganisms are an integral part of the life on The Earth. Growth, development of living beings, including plants, is impossible without them in most cases. Often, interaction between organisms occurs on the basis of parasitic or mutalistic symbiosis. In the first case only a microorganism – a parasite that harms the host plant – benefits. The second case is beneficial to both symbionts. Undoubtedly, nodule bacteria and legume plants underwent a long evolution before their interaction became useful to the host plant in the form of atmospheric nitrogen reduced in rhizobia in planta. In this case, the rhizobia's vital activity in the root nodules is carried out due to the energy materials supplied by the plant. The question that has to be answered is why rhizobia chose legumes for their activities (with rare exceptions). The knowledge of this unique biological feature in the long term will lead to the creation of a nitrogen fixing apparatus in non-legume plants and will solve the problem of plant nutrition with environmentally friendly nitrogen (Beatly, Good, 2011, Provorov, Vorob’ev, 2012). The fundamental knowledge obtained at the present time provides the basis for solving this problem. However, there are still many unanswered questions requiring their resolution. These include: the resistance of the legume plant to the rhizobia invasion and the involvement of the innate immune systems of the plant in the processes of infection and the functioning of the N2-fixing apparatus in nodules; the role of rhizobial Nod factors in the suppression of defense systems of the host plant. It should be noted that the formation of systemic (acquired) resistance in plants occurs with the participation of phytohormones, the physiological efect of which is realized with the participation of signal mediators (Kolupaev et al., 2016). Signal mediators and phytohormones undoubtedly play an important role in LRS infuencing the processes of infection, morphogenesis of the nodule and its functioning. However, the currently established facts are often phenomenological in nature and do not disclose the mechanisms and sequence of signaling into the genome. This is far from fully explored area of research of LRS.
The innate immune systems of a legume plant whose participation in the legume-rhizobial symbiosis is stated (Gourion et al. , 2015) have also not been adequately studied. The question of the efect of a legume plant infected with rhizobia on the invasion of other microorganisms including parasitic plants is not clear. In other words, does the rhizobial infection facilitate plant infection with other microorganisms? (Gourion et al. , 2015). Due to this, the data on the root nodule as a microbiome where other bacteria (except rhizobia) function and where the physiological role in cohabitation of these bacteria with rhizobia is still not clear is of interest (Kuznetsova et al. , 2015; Martinez-Hidalgo, Hirsch, 2017). The study of these issues is extremely important for understanding the resistance of a legume plant to the rhizobial infection as well as for the role of signal system components in LRS onset.
ACKNFOWLEDGEMaENFT.
The author is grateful to Ishenina A.S. for the translation of the article into English.
REFERENFCES
Akimova G.P., Sokolova M.G. (2012) Cytokinin content during early stages of legume-rhizobium symbiosis and efect of hypothermia. Russ. J. Plant Physiol 59 , 656-661.
Appleby C.A. (1992) The origin and functions of haemoglobin in plants. Science Progress . 26 , 365398.
Baron C., Zambryski P.C. (1995) The plant response in pathogenesis, symbiosis, and wounding: variations on a common theme? Annu. Rev. Genet. 29 , 107129.
Barraso J.B., Valderrama R., Corpas F.J. (2013) Immmunolocalization of S-nitrosoglutathione, S-nitrosoglutathione reductase and tyrosine nitration in pea leaf organelles. Acta Physiol. Plant. 35, 2635-2640.
Baudouin E., Pieuchot L., Engler G., Pauly N., Puppo A . (2006) Nitric oxide is formed in Medicago truncutula –Sinorhizobium meliloti functional nodules. Mol. Plant-Microbe Interac. 19, 970-975.
Baxter A., Mittler R., Suzuki N. (2014) ROS as key players in plant stress signaling. J. Exp. Bot. 65 , 1229-1240.
Beatly P.H., Good A.G. (2011) Future prospects for cereals that fix nitrogen. Science . 333 , 416-417.
Bellin D., Asai S., Delledonne M., Yoshioka H. (2013) Nitric oxide as mediator for defense responses. Mol. Plant-Microbe Interac . 26 , 271-277.
Blilou I., Ocampo J., Garcia-Garrido J. (1999) Resistance of pea root to endomycorrhizal fungus or Rhizobium correlates with enhanced levels of endogenous salicylic acid. J. Exp. Bot. 50 , 16631668.
Brewin N.J. (1991) Development of the legume root nodules. Annu. Rev. Cell Biol. 2 , 191-226.
Boscari A., Del Giudice J., Ferrarini A., Venturini L., Zafni A.L., Delledonne M., Puppo A. (2013) Expression dynamics of the Medicago truncatula transcriptome during the symbiotic interaction with Sinorhizobium meliloti : which role for nitric oxide? Plant Physiol . 161 , 425-439.
Bueno P., Soto M.J., Rodriguez-Rosales M.P., Sanjuan J., Olivares J., Donaire J.P. (2001) Time-course of lipoxygenase, antioxidant enzyme activities and H 2 O 2 accumulation during the early stages of Rhizobium -legume symbiosis. New Phytol. 152 , 9196.
Bufard D., Esnault R., Kondorosi A. (1996) Role of plant defense in alfalfa during symbiosis. W ord J. Microbiol. Biotechnol. 12 , 175-188.
Corpas F.J., Barroso J.B. (2014). Peroxynitrite (ONOO-) is endogenously produced in Arabidopsis peroxisomes and is over producer under cadmium stress. Annals Bot . 113 , 87-96.
Cardenas L., Quinto C. (2008) Reactive oxygen species (ROS) as early signals in root hair cells responding to rhizobial nodulation factors. Plant Signal. Behav . 3 , 1101-1102.
Charpentier M., Oldroyd G. (2010) How close are we to nitrogen-fixing cereals? Curr. Opin. Plant Biol. 13 , 556-564.
Downie J.A. (2014) Calcium signals in plant immunity: a spiky issue. New Phytol. 204 , 733-735.
Deakin W.J., Broughton W.J. (2009) Simbiotic use of phatogenic strategies: rhizobial protein secretion systems. Nature Rev. Microbiol. 2 , 312-320.
Denarie J., Debelle F. (1996) Rhizobium lipochitooligosaccharide nodulation factors: signaling molecules mediating recognition and morphogenesis. Annu. Rev. Biochem . 65 , 503-535.
Ehrhard D.W., Atkinson E.M., Long S.R. (1996) Calcium spiking in plant root hairs responding to Rhizobium nodulation signals. Cell . 85(5) , 673-681.
Ferguson B.J., Mathesius U. (2003) Signaling interactions during nodule development. J. Plant Growth Regul . 22 , 47-72.
Ferguson B.J., Indrasumunar A., Hayashi S., Lin M-H., Lin Y-H., Reid D.E., Gresshof P.M. (2010) Molecular analysis of legume nodule development and autoregulation. J. Integr. Plant Biol. 52, 61-76.
Ferguson B.J., Mathesius U. (2014) Phytohormone regulation of legume-rhizobia interactions. J. Chemical Ecology . 40 , 770-790.
Franssen H.J., Vijn I., Yang W.C., Bisseling T. (1992) Developmental aspects of the Rhizobium -legume symbiosis. Plant Mol. Biol . 19 , 89-107.
Fred E.B., Graul J. (1916) The efect of soluble nitrogenous salts on nodule formation. J. Amer. Soc. Agron . 8 , 316-328.
Gough C., Cullimore J. (2011) Lipochitooligosaccharide signaling in endosymbiotic plant-microbe interactions. Mol. Plant-Microbe Interac . 24 , 867878.
Granqvist E., Sun J., den Camp R.O., Pujic P., Hill L., Normand P., Morris R.J., Downie J.A., Geurts R., Oldroyd G.E.D. (2015) Bacterial-induced calcium oscillations are common to nitrogen-fixing associations of nodulating legumes and nonlegumes. New Phytol. 202 , 551-558.
Graham P.H, Vance C.P. (2003) Legumes: importance and constraints to greater use. Plant Physiol. 131 , 872–877.
Glyan’ko A.K., Makarova LE., Vasil’eva G.G., Mironova N.V. (2005) Possible involvement of hydrogen peroxide and salicylic acid in the legume- Rhizobium symbiosis. Biology Bulletin. 32 , 245249.
Glyan’ko A.K., Mitanova N.B., Stepanov A.A. (2012) Infuence of environmental factors on the generation of nitric oxide in the roots of etiolated pea seedlings. Appl. Biochem. Microbiol . 48 , 83-89.
Glyan’ko A.K. (2013) Initiation of synthesis of nitrogen oxide (NO) in the roots of etiolated pea seedlings under the infuence of N-compounds // Biochemistry (Moskow) , 28 , 471-476.
Glyan’ko A.K., Ischenko A.A., Stepanov A.V. (2014) Infuence of calcium and rhizobial infection ( Rhizobium leguminosarum ) on the dynamics of the content of nitrogen oxide in the roots of etiolated pea seedlings ( Pisum sativum L.) Appl. Biochem. Microbiol. 50 , 652-657.
Glyan’ko A.K. (Review) (2014a). N.A. Provorov, N.I. Vorob’ev Genetic foundations of the evolution of plant-microbial symbiosis / Ed. I.A. Tikhonovich / Bulletin of the Society of Physiologists of Russia. 29 , 63-70 (in Russian).
Glyan’ko A.K. (2014) The role of Nod factor Rhizobium in the induction of plant signal systems during the formation of legume-rhizobial symbiosis. Bulletin of Kharkiv. nat. agrarian un-ty. Ser. Biology . 3 (33) , 614 (in Ukrainian).
Glyan’ko A.K. (2015) Phytohormones and nodule formation in legume plants. Bulletin of Kharkiv. nat. agrarian un-ty. Ser. Biology . 3 (36) , 6-19 (in Ukrainian)
Glyan’ko A.K., Ischenko A.A. (2015) The role of cytokinin and auxin in the regulation of the nodulation process in legume plants. Journal of Stress Physiology and Biochemistry . 11(2 ), 16-27 (in Russian).
Glyan’ko A.K. (2016) Defense systems of a legume plant in case of infection with rhizobia. Bulletin of Kharkiv. nat. agrarian un-ty. Ser. Biology. 1(32) , 6377 (in Ukrainian).
Glyan’ko A.K., Isсhenko A.A. (2017) Immunity of a leguminous plant infected by nodular bacteria Rhizobium spp. F. (Review). Appl. Biochem. Microbiol. 53 , 140-148.
Glyan’ko A.K., Ischenko A.A. (2017a) Reactive species of oxygen and nitrogen – possible mediators of systemic resistance in legumes under the infuence of the rhizobial infection. Bulletin of Kharkiv. nat. agrarian un-ty. Ser. Biology. 1(40), 9-20 (in Ukrainian).
Gage D.J. (2004) Infection and invasion of roots by symbiotic, nitrogen-fixing rhizobia during nodulation of temperate legumes. Microbiol. Mol. Biol. Rev . 68 , 280-300.
Gamas P., de Billy F., Truchet G. (1998) Symbiosisspecific expression of two Medicago truncatula nodulin genes, MtN1 and Mt13 , incoding products gomologous to plant defense proteins. Mol. PlantMicrobe Interac . 11 , 393-403.
Glyan’ko A.К., Ischenko A.A., Stepanov A.V. (2014) Infuence of calcium and rhizobial infections ( Rhizobium leguminosarum ) on the dynamics of nitric oxide (NO) content in roots of etiolated pea ( Pisum sativum L.) seedlings. Applied Biochem. Microbiol. 50 , 652-657.
Gourion B., Berrabah F., Ratet P., Stacey G. (2015) Rhizobium -legume symbioses: the crucial role of plant immunity. Trends Plant Sci. 20 , 186-194.
Hayashi T., Banda M., Kouchi H., Hayashi M., Imaizumi-Anraku H. (2010) A dominant function of CCaMK in intracellular accommodation of bacterial and fungal endosymbionts. Plant J. 63 , 141-154.
Heidstra R., Bisseling T. (1996) Nod factor-induced host responses and mechanisms of Nod factor perception. New Phytol. 133 , 25-43.
Herouart D., Baudouin E., Frendo P., Harrison J., Santos R., Jamet A., Van de Sype G., Touati D., Puppo A. (2002) Reactive oxygen species, nitric oxide and glutathione: key role in the establishment of the legume- Rhizobium symbiosis // Plant Physiol. Biochem. 40 , 619-624.
Hichri I., Bosscari A., Castella C., Rovere M., Puppo A., Brouquisse R. (2015). Nitric oxide: a multifaceted regulator of the nitrogen-fixing symbiosis. J. Exp. Bot. 66, 2877-2887.
Hirsch A.M. (1992) Developmental biology of legume nodulation. New Phytol. 122 , 211-237.
Klatt P., Lamas S. (2000) Regulation of protein function by S-glutathiolation in response to oxidative and nitrosative stress. Eur. J. Biochem. 262 , 49284944.
Kubienova L., Ticha T., Jahnova J., Luhova L., Mieslerova B., Pettrivalsky M. – (2014) Efect of abiotic stress stimuli on S-nitrosoglutathione reductase in plants. Planta . 239 , 139-146.
Kretovich V.L. (1997). Biochemistry of air nitrogen assimilation by plants. Moscow: Nauka, 486 p.
Karpets Yu.V., Kolupaev Yu.E. (2007) Functional interaction of nitrogen oxide with reactive oxygen species and calcium ions in the formation of adaptive plant reactions. Bulletin of Kharkiv. nat. agrarian un-ty. Ser. Biology. 2 (41) , 6-31 (in Ukrainian)
Kolupaev Yu.E. (2007) Calcium and stress reactions of plants. Bulletin of Kharkiv. nat. agrarian un-ty. Ser. Biology. 1(10) , 24-41 (in Ukrainian).
Kolupaev Yu.E., Karpets Yu.V., Yastreb T.O., Lugovaya A.A. (2016) Signal mediators in the realization of the physiological efects of stressful phytohormones. Bulletin of Kharkiv. nat. agrarian un-ty. Ser. Biology. 1(32) , 42-62 (in Ukrainian).
Kots S.Ya., Morgun V.V., Patyka V.F. et al. (2010, 2011) Legume-rhizobial symbiosis, Vol. 1, 508 p.;Vol. 2, 524 p.; Kiev: Logos (in Ukrainian).
Krugova E.D. (2009) Specific strategies of nodule and phytopathogenic bacteria in plant infection. Physiology and biochemistry of cult. plants . 41 , 315 (in Ukrainian).
Kuznetsova I.G., Sazonova A.L., Safronova V.I., Pinaev
A.G., Verkhozina A.V., Tikhomirova N.Yu., Osledkin Yu.S., Belimov A.A. (2015) Genetic diversity of microsymbionts of Baikal species of vetchling ( Lathyrus ), pea ( Vicia ), oxytrope ( Oxytropis ) and loco ( Astragalus ). Agricultural Biology (Sel’skohozyastvennaya Biologiya) . 50(3) , 345-352 (in Russian).
Long S.R. (2001) Genes and signals in the rhizobium-legume symbiosis. Plant Physiol . 125 , 69–72.
Leon J., Lawton M.A., Raskin I. (1995). Hydrogen peroxide stimulates salicylic acid biosynthesis in tobacco. Plant Physiol. 105 , 1673-1678.
Lohar D.P., Haridas S., Gantt J.S., VandenBosch K. A. (2007) A transient decrease in reactive oxygen species in roots leads to root hair deformation in the legume-rhizobia symbiosis. New Phytol. 123 , 39-49.
Liang Y., Cao Y., Tanaka K., Thibivilliers S., Wan J., Choi J., ho Kang C., Qiu J., Stacey G. (2013) Non legumes respond to rhizobial Nod factors by suppressing the innate immune response. Science . 341 , 1384-1387.
Long S.R. (1996) Rhizobium symbiosis: Nod factors in perspective. Plant Cell. 8 , 1885-1898.
Martinez-Abarka F., Herrera-Cervera J.A., Bueno P., Sanjuan J., Bisseling T., Olivares J. (1998) Involvement of salicylic acid in the establishment of the Rhizobium meliloti -alfalfa symbiosis. Mol. Plant Microbe Interac . 11 , 153-155.
Martinez-Hidalgo P., Hirsch A.M. (2017) The nodule microbiome: N2-fixing rhizobia do not live alone. Phytobiomes J. 1 (2), 70-82.
Meyer C., Lea U.S., Provan F., Kaizer W.M., Lillo C. (2005). Is nitrate reductase a major player in the plant NO (nitric oxide) game? Photosynth. Res. 83 , 181-189.
Meilhoc E., Boscan A., Bruand C., Puppo A.,Brouquisse R. (2011) Nitric oxide in legume-rhizobium symbiosis. Plant Science . 181 , 573-581.
Maksimov I.V., Cherepanov E.A. (2006) Pro/antioxidant system and plant resistance to pathogens. Biology Bulletin Reviews . 126 , 250-261.
Mishustin E.N., Shilnikova V.K. (1968) Biological fixation of atmospheric nitrogen. Moscow: Nauka, 531 p. (in Russian).
Mishustin E.N. (1972) Microorganisms and productivity of agriculture. Moskow: Nauka, 343 p. (in Russian).
Mishustin E.N., Shilnikova V.K. (1973) Nodule bacteria and inoculation process. Moscow: Nauka, 288 p. (in Russian).
Mitanova N.B., Glyan’ko A.K., Vasilieva G.G. (2006) Infuence of nitrogen compounds on the adhesion and penetration of nodule bacteria into root tissues and the growth of etiolated pea seedlings. Agrochemistry (Agrokhimiya). 10 , 52-55 (in Russian).
Molodchenkova O.O. (2001) Assumed functions of salicylic acid in plants. Physiology and biochemistry cult. plants . 33 , 463-473 (in Ukrainian).
Mostofa M.G., Fujita M., Tran L.S.P. (2015). Nitric oxide mediates hydrogen peroxide- and salicylic acid-induced salt tolerance in rice ( Oriza sativa ) seedlings. Plant Growth Regul . 22 , 265-277.
Murray J.D. (2011) Invasion by invitation rhizobial infection in legumes // Mol. Plant-Microbe Interac. 24, 631-639.
Ortega-Galisteo A.P., Rodriguez-Serrano M., Pazmino D.M., Gupta D.K., Sandalio L.M., Romero-Puertas M.S. (2012) S-Nitrosylated proteins in pea ( Pisum sativum L.) leaf peroxisomes: changes under abiotic stress. J. Exp. Bot. 63 , 2089-2103.
Oldroyd G.E.D., Murray J.D., Poole P.S., Downie A.
(2011) The rules of engagement in the legume-rhizobial symbiosis. Annu. Rev. Genet . 45 , 119144.
Oldroyd G.E.D., Downie J.A (2008) Coordinating nodule morphogenesis with rhizobial infection in legumes. Annu. Rev. Plant Biol . 59 , 519-546.
Park S.W., Kaimoyo E., Kumar D., Mosher S., Klessing D.F. (2007) Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science . 318 , 113-116.
Pallas J.A., Paiva N.L., Lamb C., Dixon R.A. (1996) Tobacco plants epigenetically suppressed in phenylalanine ammonia-lyase expression do not develop systemic acquired resistance in response to infection by tobacco mosaic virus. Plant J. 10 , 281-293.
Puppo A., Pauly N., Boscari A., Mandon K., Brouquisse R. (2013) Hydrogen peroxide and nitric oxide: key regulators of the legume – Rhizobium and mycorrhizal symbioses. Antioxidant Redox Signal . 18 , 2202-2219.
Provorov N.A., Vorob’ev N.I. (2012) Genetic foundations of the evolution of plant-microbial symbiosis. S. -Petersburg: Informnavigator, 400 p. (in Russian).
Rolfe B.G., Gresshof P.M. (1988). Genetic analysis of legume nodule initiation. Annu. Rev. Plant Physiol. Plant Mol. Biol. 39 , 297-319.
Rao M.V., Paliyath G., Ormrod D.P., Murr D.P., Watkins C.B. (1997) Infuence of salicylic acid on H2O2 production, oxidative stress, and H2O2-metabolizing enzymes. Salicylic acid-mediated oxidative damage requires H2O2. Plant Physiol 115 , 137-149.
Ryals J.A., Neuenschwander U.H., Willits M.G., Molina A., Steiner H.Y., Hunt M.D. (1996) Systemic acquired resistance. Plant Cell. 8 , 1809-1819.
Rhizobiaceae (2002) Molecular biology of bacteria interacting with plants. S.-Petersburg: Biont, 567 p. (in Russian).
Shaw S.L., Long S.R. (2003) Nod factor elicits two separable calcium responses in Medicago truncatula root hair cells. Plant Physiol. 131 , 976984.
Spaink H.P. (1995) The molecular basis of infection and nodulation by rhizobia: the ins and outs of sympathogenesis. Annu. Rev. Phytopathol. 33 , 345-368.
Skorpil P., Broughton W.J. (2005) Molecular interaction between Rhizobium and legumes. In: Molecular Basis of Symbiosis. Ed. J. Overmann. Berlin-Heidelberg: Springer-Verlag, P. 143-165.
Stacey G., McAlvin C.B., Sung-Yong Kim, Olivares J., Sato M.J. (2006) Efect of endogenous salicylic acid on nodulation in the model legumes Lotus japonicus and Medicago tranculata . Plant Physiol 141 , 1473-1481.
Santos R., Herouart D., Sigaud S., Touati D., Puppo A. (2001) Oxidative burst in alfalfa- Sinorhizobium meliloti symbiotic interaction. Mol. Plant-Microbe Interac. 14 , 86-89.
Sidorova K.K. (1981) Genetics of peas mutants. Novosibirsk: Nauka, 169 p. (in Russian).
Sidorova K.K., Shumnyj V.K. (1999) Genetics of symbiotic nitrogen fixation and selection bases for self-pollinating legumes (as in the case of Pisum sativum L.). Genetics (Genetika) . 35 , 1550-1557 (in Russian).
Sidorova K.K., Shumnyj V.K., Nazarov V.M. (2006). Symbiotic nitrogen fixation: genetic, selection and ecology-agrochemical aspects. Novosibirsk: Akad. publishing house “Geo”, 134 p. (in Russian).
Sidorova K.K., Shumnyj V.K. (2003) Creation and genetic study of the collection of symbiotic mutants of peas ( Pisum sativum L.). Genetics (Genetika) 39 , 501-509.
Sidorova K.K., Shumny V.K., Vlasova E.Yu., Glyanenko M.N., Mishchenko T.M., Maystrenko G.G. (2010) Symbiogenetics and selection of macrosymbiont for increasing nitrogen fixation as in the case of peas ( Pisum sativum L.) // VOGiS Herald. (Vestnik Vserossijskogo Obschestva Genetikov i Selektsionerov) 14 , 357-374 (in Russian).
Shevchuk V.Ye. (1979). Legumes and soil fertility. Irkutsk: East Siberian Book Publishing House, 98 p. (in Russian).
Shumnyj V.K., Sidorova K.K., Klevenskaya I.L. (1991) Biological fixation of nitrogen. Novosibirsk: Nauka, 270 p. (in Russian).
Sagi M., Fluhr R. (2006) Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol 141 , 336-340.
Scheler C., Durner J., Astier J. (2013) Nitric oxide and reactive oxygen species in plant biotic interactions. Curr. Opin. Plant Biol. 16 , 534-539.
Scott P., Pregelj L., Chen N., Hadler J., Djordjevic M., Gresshof P. (2008) Pongamia pinnata : an untapped resource for the biofuels industry of the future. BioEnergy Res . 1 , 2-11.
Shimoda Y., Nagata M., Suzuki A., Abe M., Sato S.,
Kato T., Tabata S., Higashi S., Uchiumi T. (2005) Symbiotic rhizobium and nitric oxide induce gene expression of non-symbiotic hemoglobin in Lotus japonicus . Plant Cell Physiol. 46 , 99-107.
Singh S., Parniske M. (2012) Activation of calcium – and calmodulin – dependent protein kinase (CCaMK), the central regulator of plant root endosymbiosis. Curr. Opin. Plant Biol. 15 , 444-453.
Timmers A.C., Soupene E., Auriac M.C., de Billy F., Vasse J., Boistard P., Truchet G. (2000) Saprophytic intracellular rhizobia in alfalfa nodules. Mol. Plant-Microbe Interac. 13 , 1204-1213.
Tarchevsky I.A. (2002) Signaling systems of plant cells. Moscow: Nauka, p.103-113 (in Russian).
Tikhonovich I.A., Provorov N.A. (2009) Symbiosis of plants and microorganisms. Molecular genetics of agrosystems of the future. S.-Petersburg: Publishing House of S- Petersburg University, 210 p. (in Russian).
Tsyganova A.V., Kitaeva A.B., Brevin N.J., Tsyganov V.E. (2011) Cellular mechanisms of the development of symbiotic nodules in legumу plants. Agrocultural Biology (Sel’skohozyastvennaya Biologiya). 3 , 34-41 (in Russian).
Vasse J., de Billy F., Truchet J. (1993). Abortion of infection during the Rhizobium meliloti -alfalfa symbiotic interaction is accompanied by hypersensitive reaction. Plant J. 4 , 555-566.
Vernooij B., Friedrich L., Morse A., Reist R., Kolditz-Jawhar R., Ward E. (1994) Salicylic acid is not the translocated signal responsible for inducing systemic acquired resistance. Plant Cell. 6 , 959965.
Vasil’eva G.G., Mironova N.V., Glyan’ko A.K., Shepot’ko L.N. (2001) Generation of superoxide radicals in pea seedlings during inoculation with nitrogen-fixing bacteria of diferent compatibility // Agricultural Biology (Sel’skohozyastvennaya Biologiya). 3, 7983 (in Russian).
Vorob’ev V.A. (1998) Symbiotic nitrogen fixation and temperature. Novosibirsk: Nauka, 126 p. (in Russian).
Vul'f E.V., Maleeva O.F. (1969) Directory. World resources of useful plants. Leningrad: Nauka, p. 221-222 (in Russian).
Wang P., Du Y., Ren D., Song C.P. (2010) Hydrogen peroxide-mediated activation of MAP kinase 6 modulates nitric oxide biosynthesis and signal transduction in Arabidopsis. Plant Cell. 22 , 29812998.
Yakovleva Z.M. (1975) Bacteroides of nodule bacteria. Novosibirsk: Nauka, 171 p. (in Russian).
Yun B.W., Feechan A., Yin M., Saidi N.B., Le Bihan T., Yu M., Moore J.W., Kang J.G., Kwon E., Spoel S.H., Pallas J.A., Loake G.J. (2011) S-nitrosylation of NADPH oxidase regulates cell death in plant immunity. Nature . 428 , 264-268.
Yamamoto Y., Kanayama Y. (1990) Inhibition of nitrogen fixation in soybean plants supplied with nitrate. II. Accumulation and properties of nitrosyllehemoglobin in nodules. Plant Cell Physiol 31 , 207-214.
Yamamoto Y., Watanabe I., Kanayama Y. (1990). Inhibition of nitrogen fixation in soybean plants supplied with nitrate. 1. Nitrite accumulation and formation of nitrosylleghemoglobin in nodules. Plant Cell Physiol. 31 , 341-346.
Yu M., Lamatina L., Spoel S.H., Loake G.J. (2014) Nitric oxide function in plant biology: a redox cue in deconvolution. New Phytol. 202, 1142-1156.
Zhao J., Fujita K., Sakai K. (2007) Reactive oxygen species, nitric oxide, and their interactions play diferent roles in Cupressus lusitanica cell death and phytoalexin biosynthesis. New Phytol. 125
215-229.
Zaninotto F., la Camera S., Polverari A., Delledonne M. (2006) Cross talk between reactive nitrogen and oxygen species during the hypersensitive disease resistance response. Plant Physiol. 141 , 379-383.
Список литературы Legume-rhizobial symbiosis: progress and prospects
- Akimova G.P., Sokolova M.G. (2012) Cytokinin content during early stages of legume-rhizobium symbiosis and effect of hypothermia. Russ. J. Plant Physiol. 59, 656-661
- Appleby C.A. (1992) The origin and functions of haemoglobin in plants. Science Progress. 76, 365-398
- Baron C., Zambryski P.C. (1995) The plant response in pathogenesis, symbiosis, and wounding: variations on a common theme? Annu. Rev. Genet. 29, 107-129
- Barraso J.B., Valderrama R., Corpas F.J. (2013) Immmunolocalization of S-nitrosoglutathione, S-nitrosoglutathione reductase and tyrosine nitration in pea leaf organelles. Acta Physiol. Plant. 35, 2635-2640
- Baudouin E., Pieuchot L., Engler G., Pauly N., Puppo A.(2006) Nitric oxide is formed in Medicago truncutula -Sinorhizobium meliloti functional nodules. Mol. Plant-Microbe Interac. 19, 970-975
- Baxter A., Mittler R., Suzuki N. (2014) ROS as key players in plant stress signaling. J. Exp. Bot. 65, 1229-1240
- Beatly P.H., Good A.G. (2011) Future prospects for cereals that fix nitrogen. Science. 333, 416-417
- Bellin D., Asai S., Delledonne M., Yoshioka H. (2013) Nitric oxide as mediator for defense responses. Mol. Plant-Microbe Interac. 26, 271-277
- Blilou I., Ocampo J., Garcia-Garrido J. (1999) Resistance of pea root to endomycorrhizal fungus or Rhizobium correlates with enhanced levels of endogenous salicylic acid. J. Exp. Bot. 50, 1663-1668
- Brewin N.J. (1991) Development of the legume root nodules. Annu. Rev. Cell Biol. 7, 191-226
- Boscari A., Del Giudice J., Ferrarini A., Venturini L., Zaffini A.L., Delledonne M., Puppo A. (2013) Expression dynamics of the Medicago truncatula transcriptome during the symbiotic interaction with Sinorhizobium meliloti: which role for nitric oxide? Plant Physiol. 161, 425-439
- Bueno P., Soto M.J., Rodriguez-Rosales M.P., Sanjuan J., Olivares J., Donaire J.P. (2001) Time-course of lipoxygenase, antioxidant enzyme activities and H2O2 accumulation during the early stages of Rhizobium-legume symbiosis. New Phytol. 152, 91-96
- Buffard D., Esnault R., Kondorosi A. (1996) Role of plant defense in alfalfa during symbiosis. Word J. Microbiol. Biotechnol. 12, 175-188
- Corpas F.J., Barroso J.B. (2014). Peroxynitrite (ONOO-) is endogenously produced in Arabidopsis peroxisomes and is over producer under cadmium stress. Annals Bot. 113, 87-96
- Cardenas L., Quinto C. (2008) Reactive oxygen species (ROS) as early signals in root hair cells responding to rhizobial nodulation factors. Plant Signal. Behav. 3, 1101-1102
- Corpas F.J., del Rio L.A., Barroso J.B. (2013) Protein tyrosine nitration in higher plants under natural and stress conditions. Front. Plant Sci. 4, 29 DOI: 10.3389/fpls.2013.00029
- Charpentier M., Oldroyd G. (2010) How close are we to nitrogen-fixing cereals? Curr. Opin. Plant Biol. 13, 556-564
- Downie J.A. (2014) Calcium signals in plant immunity: a spiky issue. New Phytol. 204, 733-735
- Deakin W.J., Broughton W.J. (2009) Simbiotic use of phatogenic strategies: rhizobial protein secretion systems. Nature Rev. Microbiol. 7, 312-320
- Denarie J., Debelle F. (1996) Rhizobium lipochitooligosaccharide nodulation factors: signaling molecules mediating recognition and morphogenesis. Annu. Rev. Biochem. 65, 503-535
- Djordjevic M.A., Gabriel D.W., Rolfe B.J. (1987) Rhizobium -the refined parasite avoid the host response? Annu.Rev. Phytopathol. 25, 145-168
- Ehrhard D.W., Atkinson E.M., Long S.R. (1996) Calcium spiking in plant root hairs responding to Rhizobium nodulation signals. Cell. 85(5), 673-681
- Ferguson B.J., Mathesius U. (2003) Signaling interactions during nodule development. J. Plant Growth Regul. 22, 47-72
- Ferguson B.J., Indrasumunar A., Hayashi S., Lin M-H., Lin Y-H., Reid D.E., Gresshoff P.M. (2010) Molecular analysis of legume nodule development and autoregulation. J. Integr. Plant Biol. 52, 61-76
- Ferguson B.J., Mathesius U. (2014) Phytohormone regulation of legume-rhizobia interactions. J. Chemical Ecology. 40, 770-790
- Franssen H.J., Vijn I., Yang W.C., Bisseling T. (1992) Developmental aspects of the Rhizobium-legume symbiosis. Plant Mol. Biol. 19, 89-107
- Fred E.B., Graul J. (1916) The effect of soluble nitrogenous salts on nodule formation. J. Amer. Soc. Agron. 8, 316-328
- Gough C., Cullimore J. (2011) Lipochitooligosaccharide signaling in endosymbiotic plant-microbe interactions. Mol. Plant-Microbe Interac. 24, 867-878
- Granqvist E., Sun J., den Camp R.O., Pujic P., Hill L., Normand P., Morris R.J., Downie J.A., Geurts R., Oldroyd G.E.D. (2015) Bacterial-induced calcium oscillations are common to nitrogen-fixing associations of nodulating legumes and non-legumes. New Phytol. 207, 551-558
- Graham P.H, Vance C.P. (2003) Legumes: importance and constraints to greater use. Plant Physiol. 131, 872-877
- Glyan’ko A.K., Makarova LE., Vasil’eva G.G., Mironova N.V. (2005) Possible involvement of hydrogen peroxide and salicylic acid in the legume-Rhizobium symbiosis. Biology Bulletin. 32, 245-249
- Glyan’ko A.K., Mitanova N.B., Stepanov A.A. (2012) Influence of environmental factors on the generation of nitric oxide in the roots of etiolated pea seedlings. Appl. Biochem. Microbiol. 48, 83-89
- Glyan’ko A.K. (2013) Initiation of synthesis of nitrogen oxide (NO) in the roots of etiolated pea seedlings under the influence of N-compounds//Biochemistry (Moskow), 78, 471-476
- Glyan’ko A.K., Ischenko A.A., Stepanov A.V. (2014) Influence of calcium and rhizobial infection (Rhizobium leguminosarum) on the dynamics of the content of nitrogen oxide in the roots of etiolated pea seedlings (Pisum sativum L.) Appl. Biochem. Microbiol. 50, 652-657
- Glyan’ko A.K. (Review) (2014a). N.A. Provorov, N.I. Vorob’ev Genetic foundations of the evolution of plant-microbial symbiosis/Ed. I.A. Tikhonovich/Bulletin of the Society of Physiologists of Russia. 29, 63-70
- Glyan’ko A.K. (2014) The role of Nod factor Rhizobium in the induction of plant signal systems during the formation of legume-rhizobial symbiosis. Bulletin of Kharkiv. nat. agrarian un-ty. Ser. Biology. 3 (33), 6-14 (in Ukrainian)
- Glyan’ko A.K. (2015) Phytohormones and nodule formation in legume plants. Bulletin of Kharkiv. nat. agrarian un-ty. Ser. Biology. 3 (36), 6-19 (in Ukrainian)
- Glyan’ko A.K., Ischenko A.A. (2015) The role of cytokinin and auxin in the regulation of the nodulation process in legume plants. Journal of Stress Physiology and Biochemistry. 11(2), 16-27
- Glyan’ko A.K. (2016) Defense systems of a legume plant in case of infection with rhizobia. Bulletin of Kharkiv. nat. agrarian un-ty. Ser. Biology. 1(37), 63-77 (in Ukrainian)
- Glyan’ko A.K., Isсhenko A.A. (2017) Immunity of a leguminous plant infected by nodular bacteria Rhizobium spp. F. (Review). Appl. Biochem. Microbiol. 53, 140-148
- Glyan’ko A.K., Ischenko A.A. (2017a) Reactive species of oxygen and nitrogen -possible mediators of systemic resistance in legumes under the influence of the rhizobial infection. Bulletin of Kharkiv. nat. agrarian un-ty. Ser. Biology. 1(40), 9-20 (in Ukrainian)
- Gage D.J. (2004) Infection and invasion of roots by symbiotic, nitrogen-fixing rhizobia during nodulation of temperate legumes. Microbiol. Mol. Biol. Rev. 68, 280-300
- Gamas P., de Billy F., Truchet G. (1998) Symbiosis-specific expression of two Medicago truncatula nodulin genes, MtN1 and Mt13, incoding products gomologous to plant defense proteins. Mol. Plant-Microbe Interac. 11, 393-403
- Glyan’ko A.К., Ischenko A.A., Stepanov A.V. (2014) Influence of calcium and rhizobial infections (Rhizobium leguminosarum) on the dynamics of nitric oxide (NO) content in roots of etiolated pea (Pisum sativum L.) seedlings. Applied Biochem. Microbiol. 50, 652-657
- Gourion B., Berrabah F., Ratet P., Stacey G. (2015) Rhizobium-legume symbioses: the crucial role of plant immunity. Trends Plant Sci. 20, 186-194
- Hayashi T., Banda M., Kouchi H., Hayashi M., Imaizumi-Anraku H. (2010) A dominant function of CCaMK in intracellular accommodation of bacterial and fungal endosymbionts. Plant J. 63, 141-154
- Heidstra R., Bisseling T. (1996) Nod factor-induced host responses and mechanisms of Nod factor perception. New Phytol. 133, 25-43
- Herouart D., Baudouin E., Frendo P., Harrison J., Santos R., Jamet A., Van de Sype G., Touati D., Puppo A. (2002) Reactive oxygen species, nitric oxide and glutathione: key role in the establishment of the legume-Rhizobium symbiosis//Plant Physiol. Biochem. 40, 619-624
- Hichri I., Bosscari A., Castella C., Rovere M., Puppo A., Brouquisse R. (2015). Nitric oxide: a multifaceted regulator of the nitrogen-fixing symbiosis. J. Exp. Bot. 66, 2877-2887
- Hirsch A.M. (1992) Developmental biology of legume nodulation. New Phytol. 122, 211-237
- Klatt P., Lamas S. (2000) Regulation of protein function by S-glutathiolation in response to oxidative and nitrosative stress. Eur. J. Biochem. 267, 4928-4944
- Kubienova L., Ticha T., Jahnova J., Luhova L., Mieslerova B., Pettrivalsky M. -(2014) Effect of abiotic stress stimuli on S-nitrosoglutathione reductase in plants. Planta. 239, 139-146
- Kretovich V.L. (1997). Biochemistry of air nitrogen assimilation by plants. Moscow: Nauka, 486 p
- Karpets Yu.V., Kolupaev Yu.E. (2007) Functional interaction of nitrogen oxide with reactive oxygen species and calcium ions in the formation of adaptive plant reactions. Bulletin of Kharkiv. nat. agrarian un-ty. Ser. Biology. 2 (41), 6-31 (in Ukrainian)
- Kolupaev Yu.E. (2007) Calcium and stress reactions of plants. Bulletin of Kharkiv. nat. agrarian un-ty. Ser. Biology. 1(10), 24-41 (in Ukrainian)
- Kolupaev Yu.E., Karpets Yu.V., Yastreb T.O., Lugovaya A.A. (2016) Signal mediators in the realization of the physiological effects of stressful phytohormones. Bulletin of Kharkiv. nat. agrarian un-ty. Ser. Biology. 1(37), 42-62 (in Ukrainian)
- Kots S.Ya., Morgun V.V., Patyka V.F. et al. (2010, 2011) Legume-rhizobial symbiosis, Vol. 1, 508 p.;Vol. 2, 524 p.; Kiev: Logos (in Ukrainian)
- Krugova E.D. (2009) Specific strategies of nodule and phytopathogenic bacteria in plant infection. Physiology and biochemistry of cult. plants. 41, 3-15 (in Ukrainian)
- Kuznetsova I.G., Sazonova A.L., Safronova V.I., Pinaev A.G., Verkhozina A.V., Tikhomirova N.Yu., Osledkin Yu.S., Belimov A.A. (2015) Genetic diversity of microsymbionts of Baikal species of vetchling (Lathyrus), pea (Vicia), oxytrope (Oxytropis) and loco (Astragalus). Agricultural Biology (Sel’skohozyastvennaya Biologiya). 50(3), 345-352
- Long S.R. (2001) Genes and signals in the rhizobium-legume symbiosis. Plant Physiol. 125, 69-72
- Leon J., Lawton M.A., Raskin I. (1995). Hydrogen peroxide stimulates salicylic acid biosynthesis in tobacco. Plant Physiol. 105, 1673-1678
- Lohar D.P., Haridas S., Gantt J.S., VandenBosch K. A. (2007) A transient decrease in reactive oxygen species in roots leads to root hair deformation in the legume-rhizobia symbiosis. New Phytol. 173, 39-49
- Liang Y., Cao Y., Tanaka K., Thibivilliers S., Wan J., Choi J., ho Kang C., Qiu J., Stacey G. (2013) Non legumes respond to rhizobial Nod factors by suppressing the innate immune response. Science. 341, 1384-1387
- Long S.R. (1996) Rhizobium symbiosis: Nod factors in perspective. Plant Cell. 8, 1885-1898.
- Martinez-Abarka F., Herrera-Cervera J.A., Bueno P., Sanjuan J., Bisseling T., Olivares J. (1998) Involvement of salicylic acid in the establishment of the Rhizobium meliloti-alfalfa symbiosis. Mol. Plant Microbe Interac. 11, 153-155
- Martinez-Hidalgo P., Hirsch A.M. (2017) The nodule microbiome: N2-fixing rhizobia do not live alone. Phytobiomes J. 1 (2), 70-82
- Meyer C., Lea U.S., Provan F., Kaizer W.M., Lillo C. (2005). Is nitrate reductase a major player in the plant NO (nitric oxide) game? Photosynth. Res. 83, 181-189
- Meilhoc E., Boscan A., Bruand C., Puppo A.,Brouquisse R. (2011) Nitric oxide in legume-rhizobium symbiosis. Plant Science. 181, 573-581
- Maksimov I.V., Cherepanov E.A. (2006) Pro/antioxidant system and plant resistance to pathogens. Biology Bulletin Reviews. 126, 250-261
- Mishustin E.N., Shilnikova V.K. (1968) Biological fixation of atmospheric nitrogen. Moscow: Nauka, 531 p.
- Mishustin E.N. (1972) Microorganisms and productivity of agriculture. Moskow: Nauka, 343 p.
- Mishustin E.N., Shilnikova V.K. (1973) Nodule bacteria and inoculation process. Moscow: Nauka, 288 p.
- Mitanova N.B., Glyan’ko A.K., Vasilieva G.G. (2006) Influence of nitrogen compounds on the adhesion and penetration of nodule bacteria into root tissues and the growth of etiolated pea seedlings. Agrochemistry (Agrokhimiya). 10, 52-55
- Molodchenkova O.O. (2001) Assumed functions of salicylic acid in plants. Physiology and biochemistry cult. plants. 33, 463-473 (in Ukrainian)
- Mostofa M.G., Fujita M., Tran L.S.P. (2015). Nitric oxide mediates hydrogen peroxide-and salicylic acid-induced salt tolerance in rice (Oriza sativa) seedlings. Plant Growth Regul. 77, 265-277
- Murray J.D. (2011) Invasion by invitation rhizobial infection in legumes//Mol. Plant-Microbe Interac. 24, 631-639
- Ortega-Galisteo A.P., Rodriguez-Serrano M., Pazmino D.M., Gupta D.K., Sandalio L.M., Romero-Puertas M.S. (2012) S-Nitrosylated proteins in pea (Pisum sativum L.) leaf peroxisomes: changes under abiotic stress. J. Exp. Bot. 63, 2089-2103
- Oldroyd G.E.D., Murray J.D., Poole P.S., Downie A. (2011) The rules of engagement in the legume-rhizobial symbiosis. Annu. Rev. Genet. 45, 119-144
- Oldroyd G.E.D., Downie J.A (2008) Coordinating nodule morphogenesis with rhizobial infection in legumes. Annu. Rev. Plant Biol. 59, 519-546
- Park S.W., Kaimoyo E., Kumar D., Mosher S., Klessing D.F. (2007) Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science. 318, 113-116
- Pallas J.A., Paiva N.L., Lamb C., Dixon R.A. (1996) Tobacco plants epigenetically suppressed in phenylalanine ammonia-lyase expression do not develop systemic acquired resistance in response to infection by tobacco mosaic virus. Plant J. 10, 281-293
- Puppo A., Pauly N., Boscari A., Mandon K., Brouquisse R. (2013) Hydrogen peroxide and nitric oxide: key regulators of the legume -Rhizobium and mycorrhizal symbioses. Antioxidant Redox Signal. 18, 2202-2219
- Provorov N.A., Vorob’ev N.I. (2012) Genetic foundations of the evolution of plant-microbial symbiosis. S. -Petersburg: Informnavigator, 400 p.
- Rolfe B.G., Gresshoff P.M. (1988). Genetic analysis of legume nodule initiation. Annu. Rev. Plant Physiol. Plant Mol. Biol. 39, 297-319
- Rao M.V., Paliyath G., Ormrod D.P., Murr D.P., Watkins C.B. (1997) Influence of salicylic acid on H2O2 production, oxidative stress, and H2O2-metabolizing enzymes. Salicylic acid-mediated oxidative damage requires H2O2. Plant Physiol. 115, 137-149
- Romero-Puertas M.S., Roddriguez-Serrano M., Sandalio L.M. (2013) Protein S-nytrosylation in plants under abiotic stress: an overview. Front. Plant Sci. 4: 373 DOI: 10.3389/fpls.2013.00373
- Ryals J.A., Neuenschwander U.H., Willits M.G., Molina A., Steiner H.Y., Hunt M.D. (1996) Systemic acquired resistance. Plant Cell. 8, 1809-1819
- Rhizobiaceae (2002) Molecular biology of bacteria interacting with plants. S.-Petersburg: Biont, 567 p.
- Shaw S.L., Long S.R. (2003) Nod factor elicits two separable calcium responses in Medicago truncatula root hair cells. Plant Physiol. 131, 976-984
- Spaink H.P. (1995) The molecular basis of infection and nodulation by rhizobia: the ins and outs of sympathogenesis. Annu. Rev. Phytopathol. 33, 345-368
- Skorpil P., Broughton W.J. (2005) Molecular interaction between Rhizobium and legumes. In: Molecular Basis of Symbiosis. Ed. J. Overmann. Berlin-Heidelberg: Springer-Verlag, P. 143-165
- Stacey G., McAlvin C.B., Sung-Yong Kim, Olivares J., Sato M.J. (2006) Effect of endogenous salicylic acid on nodulation in the model legumes Lotus japonicus and Medicago tranculata. Plant Physiol. 141, 1473-1481
- Santos R., Herouart D., Sigaud S., Touati D., Puppo A. (2001) Oxidative burst in alfalfa-Sinorhizobium meliloti symbiotic interaction. Mol. Plant-Microbe Interac. 14, 86-89
- Sidorova K.K. (1981) Genetics of peas mutants. Novosibirsk: Nauka, 169 p.
- Sidorova K.K., Shumnyj V.K. (1999) Genetics of symbiotic nitrogen fixation and selection bases for self-pollinating legumes (as in the case of Pisum sativum L.). Genetics (Genetika). 35, 1550-1557
- Sidorova K.K., Shumnyj V.K., Nazarov V.M. (2006). Symbiotic nitrogen fixation: genetic, selection and ecology-agrochemical aspects. Novosibirsk: Akad. publishing house "Geo", 134 p.
- Sidorova K.K., Shumnyj V.K. (2003) Creation and genetic study of the collection of symbiotic mutants of peas (Pisum sativum L.). Genetics (Genetika). 39, 501-509
- Sidorova K.K., Shumny V.K., Vlasova E.Yu., Glyanenko M.N., Mishchenko T.M., Maystrenko G.G. (2010) Symbiogenetics and selection of macrosymbiont for increasing nitrogen fixation as in the case of peas (Pisum sativum L.)//VOGiS Herald. (Vestnik Vserossijskogo Obschestva Genetikov i Selektsionerov) 14, 357-374
- Shevchuk V.Ye. (1979). Legumes and soil fertility. Irkutsk: East Siberian Book Publishing House, 98 p.
- Shumnyj V.K., Sidorova K.K., Klevenskaya I.L. (1991) Biological fixation of nitrogen. Novosibirsk: Nauka, 270 p.
- Sagi M., Fluhr R. (2006) Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol. 141, 336-340
- Shah J., Zeier J. (2013) Long-distance communication and signal amplification in the systemic acquired resistance. Front. Plant Sci. 4: 30 DOI: 10.3389/fpls.2013.0003
- Scheler C., Durner J., Astier J. (2013) Nitric oxide and reactive oxygen species in plant biotic interactions. Curr. Opin. Plant Biol. 16, 534-539
- Scott P., Pregelj L., Chen N., Hadler J., Djordjevic M., Gresshoff P. (2008) Pongamia pinnata: an untapped resource for the biofuels industry of the future. BioEnergy Res. 1, 2-11
- Shimoda Y., Nagata M., Suzuki A., Abe M., Sato S., Kato T., Tabata S., Higashi S., Uchiumi T. (2005) Symbiotic rhizobium and nitric oxide induce gene expression of non-symbiotic hemoglobin in Lotus japonicus. Plant Cell Physiol. 46, 99-107
- Singh S., Parniske M. (2012) Activation of calcium -and calmodulin -dependent protein kinase (CCaMK), the central regulator of plant root endosymbiosis. Curr. Opin. Plant Biol. 15, 444-453
- Timmers A.C., Soupene E., Auriac M.C., de Billy F., Vasse J., Boistard P., Truchet G. (2000) Saprophytic intracellular rhizobia in alfalfa nodules. Mol. Plant-Microbe Interac. 13, 1204-1213
- Tarchevsky I.A. (2002) Signaling systems of plant cells. Moscow: Nauka, p.103-113. Tikhonovich I.A., Provorov N.A. (2009) Symbiosis of plants and microorganisms. Molecular genetics of agrosystems of the future. S.-Petersburg: Publishing House of S-Petersburg University, 210 p.
- Tsyganova A.V., Kitaeva A.B., Brevin N.J., Tsyganov V.E. (2011) Cellular mechanisms of the development of symbiotic nodules in legumу plants. Agrocultural Biology (Sel’skohozyastvennaya Biologiya). 3, 34-41
- Vasse J., de Billy F., Truchet J. (1993). Abortion of infection during the Rhizobium meliloti-alfalfa symbiotic interaction is accompanied by hypersensitive reaction. Plant J. 4, 555-566
- Vernooij B., Friedrich L., Morse A., Reist R., Kolditz-Jawhar R., Ward E. (1994) Salicylic acid is not the translocated signal responsible for inducing systemic acquired resistance. Plant Cell. 6, 959-965
- Vasil’eva G.G., Mironova N.V., Glyan’ko A.K., Shepot’ko L.N. (2001) Generation of superoxide radicals in pea seedlings during inoculation with nitrogen-fixing bacteria of different compatibility//Agricultural Biology (Sel’skohozyastvennaya Biologiya). 3, 79-83
- Vorob’ev V.A. (1998) Symbiotic nitrogen fixation and temperature. Novosibirsk: Nauka, 126 p.
- Vul'f E.V., Maleeva O.F. (1969) Directory. World resources of useful plants. Leningrad: Nauka, p. 221-222
- Wang Y., Loake G.J., Chu C. (2013). Cross-talk of nitric oxide and reactive oxygen species in plant programmed cell death. Front. Plant Sci. 4: 314 DOI: 10.3389/fpls.2013.00314
- Wang P., Du Y., Ren D., Song C.P. (2010) Hydrogen peroxide-mediated activation of MAP kinase 6 modulates nitric oxide biosynthesis and signal transduction in Arabidopsis. Plant Cell. 22, 2981-2998
- Yakovleva Z.M. (1975) Bacteroides of nodule bacteria. Novosibirsk: Nauka, 171 p.
- Yun B.W., Feechan A., Yin M., Saidi N.B., Le Bihan T., Yu M., Moore J.W., Kang J.G., Kwon E., Spoel S.H., Pallas J.A., Loake G.J. (2011) S-nitrosylation of NADPH oxidase regulates cell death in plant immunity. Nature. 478, 264-268
- Yamamoto Y., Kanayama Y. (1990) Inhibition of nitrogen fixation in soybean plants supplied with nitrate. II. Accumulation and properties of nitrosyllehemoglobin in nodules. Plant Cell Physiol. 31, 207-214
- Yamamoto Y., Watanabe I., Kanayama Y. (1990). Inhibition of nitrogen fixation in soybean plants supplied with nitrate. 1. Nitrite accumulation and formation of nitrosylleghemoglobin in nodules. Plant Cell Physiol. 31, 341-346
- Yu M., Lamatina L., Spoel S.H., Loake G.J. (2014) Nitric oxide function in plant biology: a redox cue in deconvolution. New Phytol. 202, 1142-1156
- Zhao J., Fujita K., Sakai K. (2007) Reactive oxygen species, nitric oxide, and their interactions play different roles in Cupressus lusitanica cell death and phytoalexin biosynthesis. New Phytol. 175, 215-229
- Zaninotto F., la Camera S., Polverari A., Delledonne M. (2006) Cross talk between reactive nitrogen and oxygen species during the hypersensitive disease resistance response. Plant Physiol. 141, 379-383


