Localization of osteocalcin in bone healing treated by local application of collagen and beta-tricalcium phosphate in rats
Автор: Hussein B.J., Ghani B.A.
Журнал: Гений ортопедии @geniy-ortopedii
Рубрика: Оригинальные статьи
Статья в выпуске: 6 т.30, 2024 года.
Бесплатный доступ
Introduction Bone repair is a complex and multifaceted process that generally happens naturally unless complicated by situations such as substantial bone defects. The bone healing process is typically divided into three stages: inflammation, repair, and remodeling. Beta-tricalcium phosphate (β-TCP) renowned for its abundant reserves of calcium and phosphorus, easily assimilated by the body. Its exceptional biocompatibility assists in the formation of an absorbable interlinked structure at the injury site, contributing to the advancement of the healing process.Purpose This study aimed to estimate the effects of a scaffold of collagen/β-tricalcium phosphate (Coll/βTCP) on bone construction to evaluate its latent usage as a bone auxiliary to repair bone defects.Material and Methods The experiment was performed on 20 adult male albino rats. Four holes were surgically created on each animal, two in each femur; two holes were treated separately with Coll or β-TCP, one hole with their combination. The untreated hole served as a control. Animals were scarified after twoand four-week treatment periods (10 rats for each). Immunohistochemical analysis of bone marrow stromal cells, osteocytes, osteoblasts and osteoclasts using polyclonal antibodies to osteocalcin was performed.Result Immunohistochemical results discovered strong positive expression of osteocalcin in bone healing in the group of combined treatment (β-TCP and collagen) as compared to other groups. Highly significant differences were seen between the combination of collagen with β-TCP and the control group at both timepoints of the experiment.Discussion The marker osteocalcin is unique to osteoblasts, specifically to osteoblasts that are actively forming new osteoid or remodeling bone. The obtained findings showed that mean values of osteocalcin expression were greater in the experimental groups than in the control group.Conclusion The combination of collagen with β-TCP showed the greatest efficacy in accelerating bone healing and increasing osteogenic capacity due to increased osteocalcin immunoreactivity.
Bone defect, collagen, β-tcp, osteocalcin
Короткий адрес: https://sciup.org/142243887
IDR: 142243887 | УДК: 616.718.4-004.8-089.844-74:577.112:661.635.41:616.71-003 | DOI: 10.18019/1028-4427-2024-30-6-881-888
Текст научной статьи Localization of osteocalcin in bone healing treated by local application of collagen and beta-tricalcium phosphate in rats
Bone repair is a complex and multifaceted process that generally happens naturally unless complicated by situations such as substantial bone defects. The bone healing process is typically divided into three stages: inflammation, repair, and remodeling [1]. However, the system of healing of bone defect is time intense, and generation of original bone takes place gradually as a result of the size of defects or unbalanced biomechanical possessions, uncomplimentary environment of wound, suboptimal surgical preparation, metabolic aspects, hormones, nutrition, and functional stress [2]. The two primary tissue compartments that make up a bone are called trabecular bone (also known as cancellous or spongy bone) and cortical bone (also known as thick or compact bone). The structural makeup of bone is a combination of inorganic substances like hydroxyapatite and whitlockite with organic collagen nanoparticles [3]. With hydroxyapatite (HA) and within the collagen matrix, there is an existing bone material called whitlockite. It plays a vital role via earlier stages of bone development. It is present in short-range order and is difficult to identify in the bone, when compared to HA mineral that cover 80 % of the bone inorganic phase. It has similar structural analogy with β-tricalcium phosphate (β-TCP), however detailed structural and crystallographic analyses of bone have shown that β-TCP is merely a synthetic analog of bone whitlockite, having the same crystalline structure whereas different chemically. Whitlockite contain magnesium at Ca(IV), Ca(V) positions, and HPO42- on a threefold axis in a rhombohedral crystal lattice. Its bio-compatibility, functionality, negative surface charge, mechanical strength, and stability in physiological solvents make it an ideal bone substitute as compared to hydroxyapatite (HA) and β-TCP [4].
Beta-tricalcium phosphate (β-TCP) also called bone ash is a tertiary calcium phosphate [Ca3(PO4)2], renowned for its abundant reserves of calcium and phosphorus, easily assimilated by the body. Its exceptional biocompatibility assists in the formation of an absorbable interlinked structure at the injury site, contributing to the advancement of the healing process [5]. β-TCP is widely acknowledged for its ability to support new bone growth and induce bone formation, making it a highly effective material in managing bone defects in orthopedic and maxillofacial surgery [6]. It facilitates the process of bone renewal and gradually disintegrates inside the body, paving the way for the development of new bone tissue, thereby ensuring the effective repair of the injury [7]. Collagen (Coll), a crucial protein naturally synthesized in the body, serves as the primary structural component in the skin, tendons, and bones. Renowned for its favorable biocompatibility and minimal immune response, collagen has been extensively researched for its potential applications in various biomedical products, including cosmetics and pharmaceuticals [8]. Collagen, a reliable biomaterial, has been widely utilized in tissue manufacturing and clinical contexts, serving as an essential component in dental compounds, regeneration of skin templates, and biodegradable conditions. Its usefulness extends to various medical fields, including cardiovascular operation, plastic surgery, orthopedics, urology, neurology, and ophthalmology [9]. When employed as a scaffold, collagen not only provides a structural foundation for cells to adhere to and proliferate, but also impacts cellular activity. Studies have confirmed that collagen-based biomaterials effectively facilitate bone regeneration when inserted into bone defectiveness [10]. Collagen sponge promotes wound healing by allowing blood vessels and fibroblasts from the surrounding tissue to infiltrate the sponge inside and form granulation tissue. Furthermore, the collagen sponge device is required for the healing of dental extraction sockets [11]. Osteocalcin (OCN) serves as an exclusive protein in bones, serving as an indicator of fully developed osteoblast function. This protein acting a crucial role in the process of bone restructuring, the creation of novel bone tissue, and the strengthening of bone minerals [12]. Naturally pro-osteoblastic, or bone-building, osteoblasts secrete osteocalcin and it is believed to have a role in the regulation of the body metabolism. Moreover, calcium ion homeostasis and bone mineralization are related to it [13]. Osteocalcin is a helpful indicator of bone turnover that is especially beneficial for patients who are receiving treatment for bone disease [14]. Serum osteocalcin levels are an indicator of osteoblast activity and bone turnover. Osteoblasts secrete OCN, which has a strong affinity for the bone hydroxyapatite matrix [15]. The primary sources of OCN are chondrocytes, cementoblasts, odontoblasts, mature osteoblasts, and osteocytes. OCN is essential for bone mineralization [16].
Identification of bone marrow mesenchymal stromal cells (BMSCs), firstly by Frieden in 1976 [17], as peri-cytes comprising the hematopoietic niche, which are a group of heterogeneous cells composed of multi-potent stem cells, involving osteo-chondral and adipocyte progenitors [18]. They are forming immunomodulatory ability with niche, which of great clinical significance and are widely explored in biological engineering and the treatment of autoimmune disorders [19, 20]. BMSCs have limited applications because of less well-defined [21], however the traditional RNA sequencing can only obtain the average data of cells, which fail to reflecting cellular heterogeneity [22]. Recently, by lineage tracing and single-cell sequencing, many new subgroups of BMSCs and their roles in normal physiological and pathological conditions have been clarified [17].
Purpose This study aimed to estimate the effects of a scaffold of collagen/β- tricalcium phosphate (Coll/ β-TCP) on bone construction to evaluate its latent usage as a bone auxiliary to repair the defects of bone.
MATERIALS AND METHODS
Study design
Twenty adult male albino rats weighing approximately 250–350 g and aged 3–4 months were used in this study. Rats were given an intramuscular injection, with a dosage of 50 mg ketamine hydrochloride per 1 ml per kilogram of body weight, combined with 2 % xylazine at a rate of 0.2 ml per kilogram of body weight. Sterile conditions were maintained during the surgical procedure by making an incision on the skin and underlying fascia. Then, reflection was performed to expose the rat femurs. Intrabony holes approximately 3 mm in depth and 2 mm in width were induced in both femurs of each animal (Fig. 1), with intermittent drilling and constant cooling with normal saline using a micro engine that was set at a rotary speed of 2500 rpm. The operation sites were washed with normal saline to remove debris. Then they were subdivided into:
-
1. Group A (control group); 20 holes were left untreated for spontaneous healing.
-
2. Group B; 20 holes were filled with collagen.
-
3. Group C; 20 holes were filled with β-TCP.
-
4. Group D (20 holes) were filled with combination of Coll and β-TCP material in a ratio of 1:1.
Finally, animals were sacrificed by administering an excessive amount of anesthesia, two and four weeks after surgery (10 rats for each healing interval).
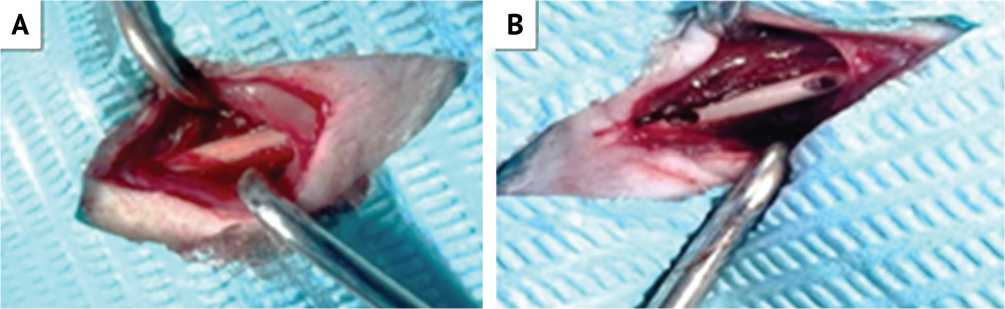
Fig. 1. A: Exposure of rat femur; B: Holes prepared
Immunohistochemical preparation
Collected bone specimens were fixed for 24 hours using 10 % freshly made formalin; then the process of decalcification was done by using 10 % formic acid for 2–3 days; afterwards, they were embedded in paraffin wax. A microtome that operated automatically divided the blocks for serial slices of 4 μm, which were positioned on charged slide. After immunohistochemical staining, osteocalcin localization by bone cells and bone marrow stromal cells (BMSCs) was analyzed for all collected bone specimens at 2- and 4-weeks.
Statistical analysis
Descriptive analysis of mean, standard deviation (SD), minimum (Min), and maximum (Max) values of the immunoreactive score (IRS) of OCN by bone cells (OB, OC, and OCL) and bone marrow stromal cells (BMSC) at 2- and 4-week duration was done for all investigated groups. Immunohistochemical scoring of OCN. Employing an objective lens with a power of ×40, the procedure was carried out, taking into account scoring systems were used as follows. To estimate the immunostaining of the antibodies, positively stained cells were calculated at 5 representative fields (×40) for 2- and 4-weeks periods of healing. Within the chosen fields, the percentage of cells that tested positive for OCN was scored and evaluated visually. Calculation of the ratio of stained cells to the total cell count and multiplying it by 100 offers an approximation of the percentage of cells that display positive staining. The scores were: 0 (no stain), 1 (< 25 %), 2 (25–50 %), 3 (> 51–80 %), 4 (> 80 %) stained cells in two sections and scoring the intensity of stain as: 0 (no clear stain), 1 (mild stain), 2 (moderate stain), 3 (intense stain) and calculating the immunoreactive score (IRS), which is obtained by multiplying the of positive cells (0–4) by the staining intensity score, has a range of {0–12} [23].
RESULTS
Immunohistochemical results for expression of osteocalcin after 2 and 4 weeks
Two weeks
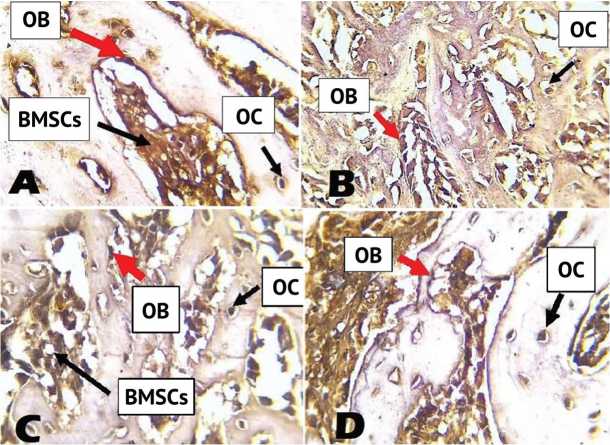
Immunohistochemical localization of ocsteocalcin in the control group showed strong positive expression in osteoblasts, osteocytes and negative staining is evident within the bone (Fig. 2, A). Moderate positive expression of immunohistochemical localization of osteocalcin in collagen group was shown in osteoblasts and osteocytes (Fig.2, B).In the β-tricalciumphosphate group, immunohistochemical localization of osteocalcin revealed positive expression in osteoblasts, osteocytes and bone marrow stromal cells, with negative expression seen in trabecular bone (Fig. 2, C). Positive immunohistochemical localization of osteocalcin in bone section postoperatively in the combination group was detected in osteocytes and osteoblasts, while trabecular bone had a negative expression (Fig. 2, D).
BMSCs
Fig. 2. A: View of the control group after 2 weeks shows positive localization of osteocalcin in osteoblasts (OB), osteocytes (OC) and bone marrow stromal cells (BMSCs); B: Collagen group shows positive expression of OCN after 2 weeks in OBs and OCs; C: Positive localization of osteocalcin in the β-TCP group after 2 weeks is seen in OBs, OCs and BMSCs; D: View of combination group after 2 weeks shows a strong positive expression of osteocalcin in OCs and OBs. (DAB stain with counter stain hematoxylin ×40)
Four weeks
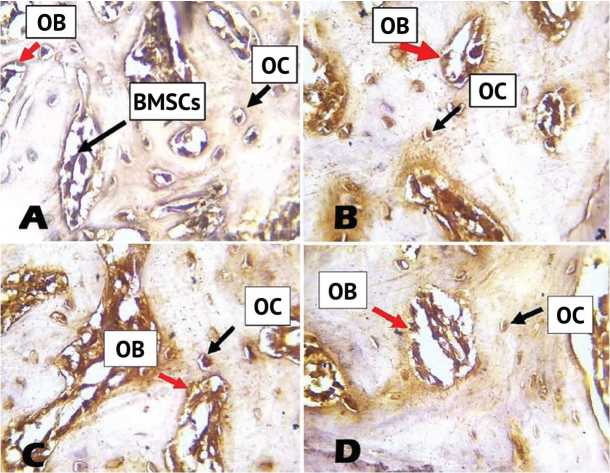
Immunohistochemical localization of osteocalcin antibody in bone sections of the control group showed positive expression in bone marrow stromal cells, osteoblasts, osteocytes; bone was negatively stained (Fig. 3, A). The collagen group revealed positive expression of osteocalcin seen in osteocytes and osteoblasts, negative expression noticed in bone (Fig. 3, B). Positive expression of osteocalcin seen in osteoblasts, osteocytes, bone was negatively stained in β-TCP group (Fig. 3, C). Immunohistochemical localization of osteocalcin in the combination group shows positive expression in osteoblasts and osteocytes (Fig. 3, D).
BMSCs
Fig. 3. A: View of the control group after 4 weeks shows strong positive expression of osteocalcin in osteocytes (OC), osteoblasts (OB), bone marrow stromal cells (BMSC); B: View of the collagen group after 4 weeks shows strong positive expression of osteocalcin in OCs and OBs; C: View of the β-TCP group after 4 weeks shows a strong positive expression of osteocalcin in OCs and OBs; D: View of the combination group after 4 weeks shows positive expression of osteocalcin in OCs and OBs. (DAB stain with counter stain hematoxylin × 40)
Descriptive data analysis for percentages of positively expressed bone cells for OCN in the studied groups at 2 and 4 weeks
The descriptive data of mean, SD, minimum and maximum values of the immunoreactive score (IRS) for expression of OCN by bone cells (osteoblasts, osteocytes, osteoclast) and bone marrow stromal cells at 2 and 4 weeks in all studied groups are illustrated in Table 1 and Figure 4. The mean value of IRS of osteocalcin by OB, OC and BMSCs decreased with time in all studied groups, the highest values were detected in the combination group at both time-points. Regarding OCs, increase in mean values of IRS of osteocalcin was seen in all groups and highest values were noticed after 4 weeks in the combination group.
Descriptive mean values of IRS of OCN in bone cells and BMSC in the studied groups at 2 and 4 weeks
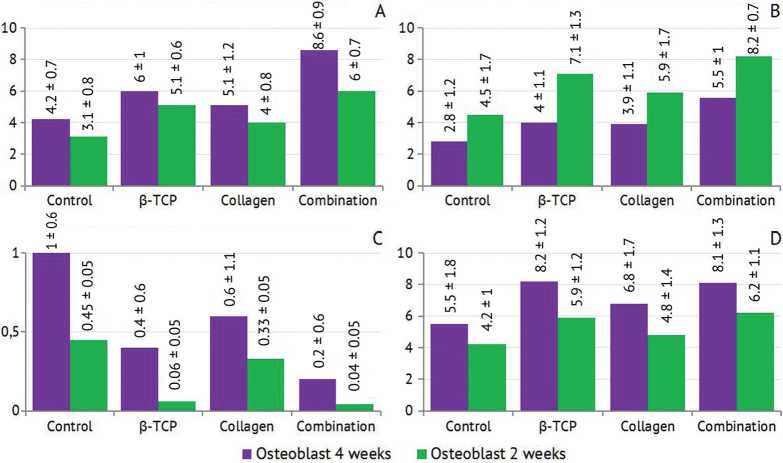
Table1
|
Variable |
Duration |
A(Control) group |
B (Collagen) group |
C (β-TCP) group |
D Collagen/ β-TCP (combination) group |
||||||||||||
|
Mean |
SD |
Min. |
Max. |
Mean |
SD |
Min. |
Max. |
Mean |
SD |
Min. |
Max. |
Mean |
SD |
Min. |
Max. |
||
|
Osteoblasts |
2 weeks |
4.2 |
0.7 |
2 |
5.8 |
5.1 |
1.2 |
3.3 |
6.2 |
6 |
1 |
3.5 |
8 |
8.6 |
0.9 |
6.5 |
10.5 |
|
4 weeks |
3.1 |
0.8 |
0 |
4.3 |
4 |
0.8 |
0.8 |
5.5 |
5.1 |
0.6 |
3.8 |
7 |
6 |
0.7 |
3 |
8.8 |
|
|
Osteocytes |
2 weeks |
2.8 |
1.2 |
0 |
4 |
3.9 |
1.1 |
0 |
5 |
4 |
1.1 |
1 |
5.8 |
5.5 |
1 |
4 |
6.8 |
|
4 weeks |
4.5 |
1.7 |
1.3 |
5.3 |
5.9 |
1.7 |
3.5 |
8 |
7.1 |
1.3 |
3 |
8 |
8.2 |
0.7 |
6.5 |
10 |
|
|
Osteoclasts |
2 weeks |
1 |
0.6 |
0 |
1.5 |
0.6 |
1.1 |
0 |
3 |
0.4 |
0.6 |
0 |
1.5 |
0.2 |
0.6 |
0 |
0.8 |
|
4 weeks |
0.45 |
0.1 |
0 |
0.6 |
0.33 |
0.05 |
0 |
0.5 |
0.06 |
0.05 |
0 |
0.1 |
0.04 |
0.05 |
0 |
0.09 |
|
|
BMSCs |
2 weeks |
5.5 |
1.8 |
4 |
8.3 |
6.8 |
1.7 |
4 |
8.3 |
8.2 |
1.2 |
4 |
9.8 |
9.1 |
1.3 |
6 |
11.5 |
|
4 weeks |
4.2 |
1 |
3 |
5.3 |
4.8 |
1.4 |
1.8 |
5.9 |
5.9 |
1.2 |
1.5 |
6.5 |
6.2 |
1.1 |
3 |
8.3 |
|
Fig. 4. A: Mean value of IRS for OCN in OB after 2- and 4-weeks healing intervals;
-
B: Mean value of IRS for OCN in OC after 2-and 4-weeks healing intervals;
-
C: Mean value of IRS for OCN in OC after 2-and 4-weeks healing intervals;
-
D: Mean value of IRS for OCN in BMSCs after 2- and 4-weeks healing intervals
Group comparison for bone cells after 2 and 4 weeks
The group comparison of IRS expression of OCN for bone cells (OB, OC, OCL) and BMSCs illustrated in Table 2 using ANOVA test, a highly significant difference was found between all investigated groups at 2 and 4 weeks.
Group comparison for bone cells and BMSCs for OCN expression at 2 and 4 weeks
Table 2
|
Variable |
Duration |
Group comparison |
|
|
F test |
P value |
||
|
Osteoblasts |
2 weeks |
6.256 |
0.000** |
|
4 weeks |
6.025 |
0.000** |
|
|
Osteocytes |
2 weeks |
4.873 |
0.003** |
|
4 weeks |
5.719 |
0.000** |
|
|
Osteoclasts |
2 weeks |
5.767 |
0.000** |
|
4 weeks |
5.016 |
0.000** |
|
|
BMSCs |
2 weeks |
6.124 |
0.000** |
** Highly significant result
DISCUSSION
When there is a loss of bone substance, it is referred to as a bone defect. This can be caused by a variety of congenital disorders, infections, degenerative diseases, trauma, bone tumors, and infections [24]. Bone reconstruction involves the reconfiguration of various bone cells to maintain bone strength and regulate mineral balance in response to environmental adjustments. This complex procedure can be categorized into four phases: initiation, maintenance, reversal, and growth through absorption, carried out by osteoclasts and osteoblasts, respectively [25].
The marker osteocalcin is unique to osteoblasts, specifically to osteoblasts that are actively forming new osteoid or remodeling bone. The obtained findings showed that mean values of IRS of osteocalcin expression were greater in the experimental groups than in the control group, except for OCL where values were greater in the control group at both time-points and that at 2 weeks they were greater than at 4 weeks. This coincides with A.L. Alpan et al. study [26] that showed an early detection of strong positive expression for osteocalcin recorded as new bone matrix and the surrounding region of the resorptive lacunae suggesting active bone matrix production.
Mean values of IRS elevated with the time in osteocytes and this phenomena is in line with P. Sananta et al. study [27] that found that the mean value of positive expression for osteocalcin in osteoblasts reduced, whereas for osteocytes raised at 4 weeks of healing duration in a bony defect in experimental rats.
Immunohistochemical findings for IRS of osteocalcin monoclonal antibodies positive expression was detected in bone cells and BMSCs in both groups with high significant difference which is similar to B.H. Al-Molla et al. study [28] that mentioned osteocalcin expression was strongly positive in the active mitotic osteoblast and progenitor cells in amelogenin and amelogenin-propolis coated implants and negative expression in an uncoated group at one-week interval.
Moreover, the immunohistochemical findings seem to be as same as the conclusions of the survey where the bone defect was treated with Eucommia ulmoides [29].
Bone defects treated with Coll/β-TCP combination have upward mean values of IRS of OCN expression which may indicate an elevating in osteoblasts activity in the early period of bone healing which was noticed at 2 weeks. These were supported by Abeas and Al-Azawy [30] who reported that the experimental group dental cells and their progenitors expressed a strong immunoreaction for osteocalcin, which is crucial for promoting and speeding up bone formation and regeneration through raising the activity of osteogenic cells which is an essential role in the orthodontic treatment.
The findings supported that the osteocalcin-positive osteoblasts and osteocytes indicated greater bone tissue maturity which was significant in the experimental group treated with alendronate as an anti-resorptive drug [31]. Moreover, it was found that immunohistochemical analysis of osteocalcin expression at the boneimplant site showed active osteoblasts scattered during an early bone deposition suggesting that OCN appears to play a part in the early phases of bone formation and that OCN regulates osteoblast activity and acts as a chemotactic agent for osteoclasts [32].
CONCLUSION
The combination of collagen with β-TCP promotes and accelerates the bone defect healing process through raised osteocalcin expression.
Conflict of interest Not declared.
Funding Not declared.
Ethical Approval This work was approved and received a permission from the Ethical Reviewer Board Committee of College of Dentistry, University of Baghdad (No. 429721 in 27 Dec 2021).
Список литературы Localization of osteocalcin in bone healing treated by local application of collagen and beta-tricalcium phosphate in rats
- Othman Jassim H, Al-Ghaban NMH. Effect of Eucommia Ulmoides on Healing of Bon Defect Using Histological and Histomorphometric Analysis in Rat: in vivo Study. Arch Razi Inst. 2023;78(2):651-657. doi: 10.22092/ ARI.2022.359483.2434
- Majeed SS, Ghani BA. Effect of topical application of flavonoids extract of Hibiscus sabdariffa on experimentally induced bone defect. JBagh Coll Dent. 2018;30(1):33-38. doi: doi: 10.12816/0046309
- Mohamed IF, Ghani BA, Fatalla AA. Histological Evaluation of the Effect of Local Application of Punica granatum Seed Oil on Bone Healing. Int JBiomater. 2022;2022:4266589. doi: 10.1155/2022/4266589
- Batool S, Liaqat U, Babar B, Hussain Z. Bone whitlockite: synthesis, applications, and future prospects. J. Korean Ceram. Soc. 2021;58(5):530-547. doi: 10.1007/s43207-021-00120-w
- Mohseni M, Jahandideh A, Abedi G, et al Assessment of tricalcium phosphate/collagen (TCP/collagene) nanocomposite scaffold compared with hydroxyapatite (HA) on healing of segmental femur bone defect in rabbits. Artif Cells NanomedBiotechnol. 2018;46(2):242-249. doi: 10.1080/21691401.2017.1324463
- AL-Mashhadi ZAj, AL-Ghaban NMH. Local Evaluation of Chitosan and B-Tricalcium Phosphate Alone and Combination in Bone Defect of Rabbit by Histological and Histomorphometric Analysis. J Res Med Dent Sci. 2022;10(9):171-178.
- Rejab AF, Minwah BS, Ameen YA. Histological evaluation for the use of ß-tricalcium phosphate as a bone substitute in accelerating bone healing: an experimental study on rabbits. Al-Rafidain Dental Journal. 2014;14(2):205-211. doi: 10.33899/RDEN.2014.160900
- Gelse K, Pöschl E, Aigner T. Collagens--structure, function, and biosynthesis.AdvDrugDelivRev. 2003;55(12):1531-1546. doi: 10.1016/j.addr.2003.08.002
- Wang H. A Review of the Effects of Collagen Treatment in Clinical Studies. Polymers (Basel). 2021;13(22):3868. doi: 10.3390/polym13223868
- Klimek K, Ginalska G. Proteins and Peptides as Important Modifiers of the Polymer Scaffolds for Tissue Engineering Applications-A Review. Polymers (Basel). 2020;12(4):844. doi: 10.3390/polym12040844
- Kuroyanagi Y, Suzuki R, Kuroyanagi M. Design of Collagen-Based Sponge Device for Use in Oral Surgery. Open Journal of Regenerative Medicine. 2021;10(3):31-49. doi: 10.4236/ojrm.2021.103003
- Ferron M, Lacombe J. Regulation of energy metabolism by the skeleton: osteocalcin and beyond. Arch Biochem Biophys. 2014;561:137-46. doi: 10.1016/j.abb.2014.05.022
- Lee NK, Sowa H, Hinoi E, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130(3):456-469. doi: 10.1016/j.cell.2007.05.047
- Pittas AG, Harris SS, Eliades M, et al. Association between serum osteocalcin and markers of metabolic phenotype. J Clin Endocrinol Metab. 2009;94(3):827-832. doi: 10.1210/jc.2008-1422
- Lee AH, Huttenlocker AK, Padian K, Woodward HN. Analysis of Growth Rates. In: Padian K, Lamm E-T, eds. Bone histology of fossil tetrapods: advancing methods, analysis, and interpretation. Berkeley: University of California Press; 2013:217-251.
- Smane L, Pilmane M. Osteopontin, osteocalcin, and osteoprotegerin expression in human tissue affected by cleft lip and palate. SHS Web Conf. 2016;30. doi: 10.1051/shsconf/20163000008
- Gao Q, Wang L, Wang S, et al. Bone Marrow Mesenchymal Stromal Cells: Identification, Classification, and Differentiation. Front CellDevBiol. 2022;9:787118. doi: 10.3389/fcell.2021.787118
- Wolock SL, Krishnan I, Tenen DE, et al. Mapping Distinct Bone Marrow Niche Populations and Their Differentiation Paths. Cell Rep. 2019;28(2):302-311.e5. doi: 10.1016/j.celrep.2019.06.031
- Amini AR, Laurencin CT, Nukavarapu SP. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng. 2012;40(5):363-408. doi: 10.1615/critrevbiomedeng.v40.i5.10
- Laranjeira P, Pedrosa M, Pedreiro S, et al. Effect of human bone marrow mesenchymal stromal cells on cytokine production by peripheral blood naive, memory, and effector T cells. Stem Cell Res Ther. 2015;6(1):3. doi: 10.1186/scrt537
- Azadniv M, Myers JR, McMurray HR, et al. Bone marrow mesenchymal stromal cells from acute myelogenous leukemia patients demonstrate adipogenic differentiation propensity with implications for leukemia cell support. Leukemia. 2020;34(2):391-403. doi: 10.1038/s41375-019-0568-8
- Tang X, Huang Y, Lei J, et al. The single-cell sequencing: new developments and medical applications. Cell Biosci. 2019;9:53. doi: 10.1186/s13578-019-0314-y
- Fedchenko N, Reifenrath J. Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue - a review. DiagnPathol. 2014;9:221. doi: 10.1186/s13000-014-0221-9
- Alsaeed MA, Al-Ghaban NMH. Chitosan Nanoparticle/Simvastatin for Experimental Maxillary Bony Defect Healing: A Histological and Histomorphometrical Study. Biomimetics (Basel). 2023;8(4):363. doi: 10.3390/biomimetics8040363
- AL-Ghaban NMH, Jasem GH. Histomorphometric evaluation of the effects of local application of red clover oil (Trifolium pratense) on bone healing in rats. JBagh College Dentistry. 2020;32(2):26-31. doi: 10.26477/jbcd.v32i2.2891
- Alpan AL, Toker H, Ozer H. Ozone Therapy Enhances Osseous Healing in Rats With Diabetes With Calvarial Defects: A Morphometric and Immunohistochemical Study. JPeriodontol. 2016;87(8):982-889. doi: 10.1902/jop.2016.160009
- Sananta P, Dradjat RS, Rosandi RD, Sugiarto MA. Bone tissue engineering application on fracture healing with bone defect as assessed through osteocalcin and bone morphogenetic protein-2 (BMP-2) biomarker examination: experimental study on murine models. F1000Research. 2022;11:596. doi: 10.12688/f1000research.110867.1
- Al-Molla BH, Al-Ghaban NM, Taher A. In Vivo Immunohistochemical investigation of Bone Deposition at Amelogenin Coated Ti Implant Surface. Smile Dental Journal. 2014;9(1). doi: 10.12816/0008316
- Jassim HO, AL-Ghaban NMH. Evaluation of Local Application of Eucommia Ulmoides Extract on Bone Healing in Rats by Histomorphometrically and Immunohistochemical Study on Osteocalcin. Autoref. M.Sc. College of Dentistry, University of Baghdad. 2022. Available at: https://codental.uobaghdad.edu.iq/wp-content/uploads/sites/14/2022/11/%D8%AD%D9%8A %D8%AF%D8%B1-%D8%B9%D8%AB%D9%85%D8%A7%D9%86-%D8%AC%D8%A7%D8%B3%D9%85.pdf. Accessed Oct 15, 2024.
- Abeas KA, Al-Azawy AM. Immunohistochemical Evaluation of Osteocalcin Expression with Application of LIPUS During Relapse Phase of Orthodontic Therapy. J University of Babylon. 2017;25(2):620-629.
- Ramalho-Ferreira G, Faverani LP, Momesso GAC, et al. Effect of antiresorptive drugs in the alveolar bone healing. A histometric and immunohistochemical study in ovariectomized rats. Clin Oral Investig. 2017;21(5):1485-1494. doi: 10.1007/s00784-016-1909-x
- Al-Ghani B, Al-Hijazi A, AL-Zubaydi T. In vivo immunohistochemical investigation of bone deposition at collagen-coated Ti implant surface. J Bagh Coll Dent. 2011;23:10-15. doi: 10.12816/0008316


