Magnetic characteristics of iron nanoclusters
Автор: Kveglis L.I., Makarov I.N., Noskov F.M., Nasibullin R.T., Nyavro A.V., Cherepanov A.N., Olekhnovich A.E., Saprykin D.N.
Журнал: Siberian Aerospace Journal @vestnik-sibsau-en
Рубрика: Technological processes and material science
Статья в выпуске: 3 vol.22, 2021 года.
Бесплатный доступ
The study of the nanocrystalline state, which significantly changes most of the physical characteristics of sub-stances, is very relevant. Of great practical interest are the works devoted to the study of the magnetic character-istics of nanocrystals of ferromagnetic substances. It has already been shown that the size of iron nanocrystals significantly affects the magnitude of their magnetization. Nevertheless, an adequate model of the structure of nanocrystalline formations consisting of a different number of iron atoms, which allows us to describe the exper-imentally detected changes in the magnetic characteristics, has not yet been presented. In this paper, we analyze nanocrystalline iron clusters that are different in configuration and number of their constituent atoms. Spatial models of clusters are constructed using a three-dimensional modeling program, and the coordinates of individual atoms in the cluster are determined. The proposed structures of nanocrystals are based on tetrahedrally close-packed cluster assemblies of iron atoms. The electron state density spectra were con-structed for the proposed clusters. For this purpose, the theory of the electron density functional was used, the calculation was carried out by the method of scattered waves in accordance with the band theory of crystals. It is shown that the appearance of magnetization in tetrahedral densely packed cluster formations is associat-ed with the excited electronic states of the atoms located on the surface of the nanocluster. Excited atoms have an increased electron density, that is, electrons are able to transition to states with higher energy, approaching the Fermi energy. In this case, the Stoner criterion necessary for the occurrence of magnetization is fulfilled. The con-figurations of electrons with spin up and down differ, which is why uncompensated magnetic moments appear. It is confirmed that the proposed models of iron nanoclusters satisfactorily correspond to the known experimental data.
Iron nanocluster, tetrahedrally close-packed structures, magnetic properties
Короткий адрес: https://sciup.org/148329585
IDR: 148329585 | DOI: 10.31772/2712-8970-2021-22-3-526-535
Текст научной статьи Magnetic characteristics of iron nanoclusters
It is known that the nanocrystalline state changes not only the structure, but also many physical characteristics of substances [1]. The paper [2] presents studies of the magnetic properties of iron cluster particles with different numbers of atoms. It is shown that the ferromagnetic properties of iron clusters depend on the number of atoms in them. For clusters smaller than 50 atoms, the magnetic moments reach 3.1 Bohr magnetons. As the number of atoms increases to 500–700 in the cluster, the magnetic moments approach the magnetization of bulk samples, which is 2.2 Bohr magnetons (Fig. 1).
The authors [3] found the presence of magnetization in cluster formations even in the absence of ferromagnetic atoms in them. Hysteresis loops confirming magnetization were obtained for CeO 2 , GaN, Al 2 O 3 , and other clusters. Ferromagnetism is also observed in thin films of HfO 2 , TiO 2 , ZnO [4; 5].
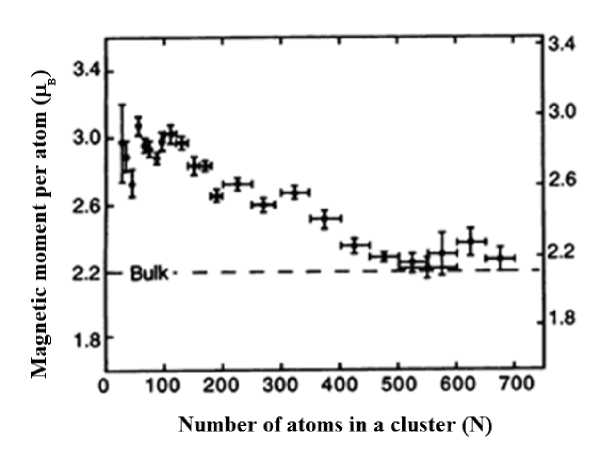
Рис. 1. Зависимость среднего магнитного момента на атом для кластеров железа от числа атомов в нём при 120 К [2]
Fig. 1. Dependence of the average magnetic moment per atom for iron clusters on the number of atoms in it at 120 K [2]
Cluster models are the most objective representation of the structure of metals with a small number of atoms [6]. In addition, structures corresponding to cluster structures also occur in the volume of metals, at interfaces, fracture surfaces, etc. When modeling nanocluster formations, we use sets of close-packed tetrahedron assemblies that most adequately correspond to the nanocrystalline state [7].
Purpose of work
The purpose of work is to explain the nature of the magnetization increase of iron in nanocluster formations containing different numbers of atoms.
Objectives of the work
-
1. Construction of three-dimensional cluster models of tetrahedral close-packed iron nanoclusters with different numbers of atoms.
-
2. Construction of electron density spectra of the investigated nanoclusters.
Methods
To determine the coordinates of the atoms, spatial models of the clusters were constructed using a threedimensional modeling program. The program has the necessary tools to determine the coordinates of atoms of the studied clusters. The cluster models were based on theoretical values of iron crystal lattice parameters: a ГЦК = 3.656 Å, а ОЦК = 2.866 Å [8].
The density spectra of electron states were constructed within the framework of the density functional theory (DFT) [9] using the basis of plane waves and ultrasoft pseudopotentials. The calculation was carried out by the scattered wave method (SW) in accordance with the zone theory of crystals, an analogue of the Korringa – Kohn – Rostoker method, adapted for cluster models. The SW method is based on the formalism of multiple scattering of electron waves on a system of potentials. It allows us to perform calculations based on atomic nuclei coordinates for clusters of several atoms and large systems. The interaction of electrons is described by averaged values of periodic fields described by the MT-potential [10]. The QuantumEspresso software package [11] was used for the calculations.
Results
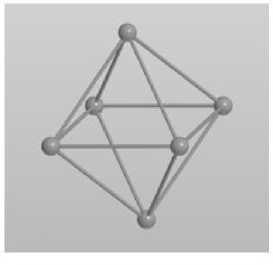
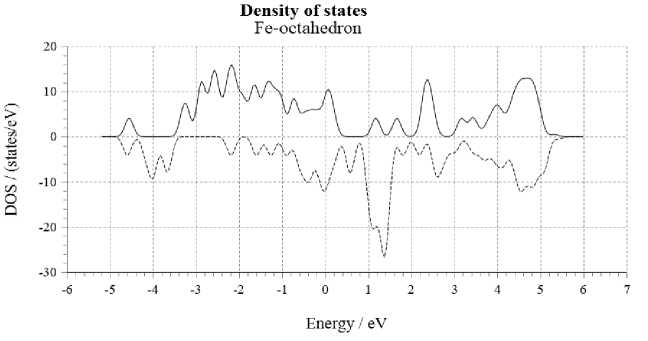
At the first stage, a model of octahedral cluster for iron of face-centered crystal lattice modification including six atoms was constructed (Fig. 2, a ). Further, the probability density diagram of electron states for the above cluster was obtained (Fig. 2, б ).
а
б
Рис. 2. Октаэдрический кластер ГЦК-железа из шести атомов:
а – общий вид; б – зависимость плотности вероятности распределения электронных состояний от энергии электронов со спином вверх и вниз
-
Fig. 2. Octahedral cluster of fcc iron of six atoms:
a – general view; b – dependence of the probability density of the distribution of electronic states on the energy of electrons with spin up and down
According to the spectrum data (Fig. 2, б ), we can conclude that the electron moments with opposite spins are uncompensated. The calculated average effective magnetic moment per atom was 1.67 μ B /atom. This result contradicts the data obtained for the magnetization of austenitic steels having a FCC lattice, which do not exhibit ferromagnetic properties for massive samples [8].
An octahedral cluster of six atoms can be transformed into a cluster formation consisting of close-packed tetrahedrons. According to [12], an octahedron can be transformed into three bound tetrahedrons by switching one chemical bond between two atoms out of six (Fig. 3).
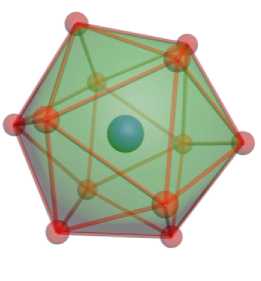
Tetrahedral assemblies can be organized in different ways. The best known example is the Franck – Kasper structure FK-12 [13], which corresponds to an icosahedron. Such an assembly can be constructed from twenty close-packed tetrahedrons (Fig. 4, a ).
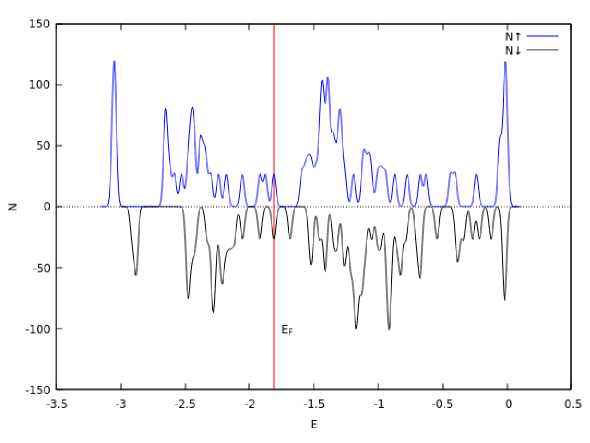
Calculation of the density of electronic states of the icosahedral cluster (Fig. 4, б ) showed that the average magnetic moment is 0.21 μ B /atom.
It is known that the structure FK-12 along with FK-14 appears in Hadfield steel 110Г13Л during plastic deformation on the deformation or fracture surface. Hadfield steel is also austenitic, but exhibits non-zero magnetization under mechanical action [14].
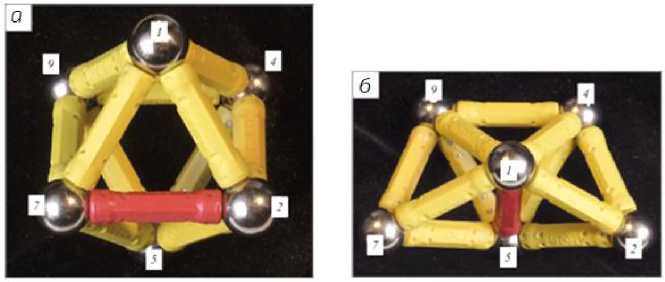
Рис. 3. Преобразование нанокластеров:
а – модель откаэдрического кластера; б – модель кластера из трёх тетраэдров [12]
-
Fig. 3. Transformation of nanoclusters:
a – model of a hexagonal cluster; b – model of a cluster of three tetrahedra [12]
а
б
Рис. 4. Икосаэдрический кластер железа из 13 атомов:
а – общий вид; б – зависимость плотности вероятности распределения электронных состояний от энергии электронов со спином вверх и вниз; вертикальная линия соответствует энергии Ферми
-
Fig. 4. Icosahedral iron cluster of 13 atoms:
a – general view; b – dependence of the probability density of the distribution of electronic states on the energy of electrons with spin up and down; the vertical line corresponds to the Fermi energy
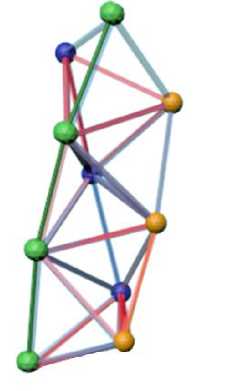
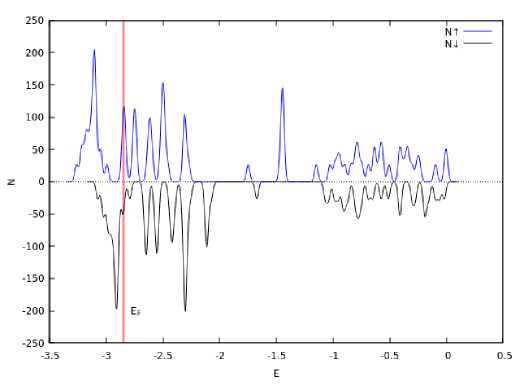
There are various possibilities of packing tetrahedrons in a nanocluster. Thus, the formation of a cluster in the form of a tetrahedral helix for compounds Ni 4 Ti 3 , Al 4 C 3 , etc. was shown in [12; 15; 16]. The formation of such a structure is related to the possibility of transition of an octahedral cluster into a group of three tetrahedral ones (see Fig. 3). Fig. 5, a shows a nanocrystalline iron cluster representing a helix of seven tetrahedrons. A spin-polarized density of electron states was plotted for such a tetrahedral assembly (Fig. 5, б ); the average magnetic moment per atom was 0.26 μ B /atom.
When further coordination spheres are constructed (Fig. 6) using tetrahedral clusters, the number of atoms on the surface increases with geometric progression. The central atom in the center is common to all spirals. The first row of tetrahedrons is located on top and has 12 generalized atoms between them on the surface. The second row has 20 atoms on the surface, the third has 32 atoms, the fourth has 52 atoms, etc. (Fig. 6).
However, according to Delaunay's rule [6], it is impossible to realize in three-dimensional space a close-packed structure of an icosahedron containing more than 2 coordination spheres.
а
б
Рис. 5. Спиральный кластер железа, составленный из семи тетраэдров: а – общий вид; б – зависимость плотности вероятности распределения электронных состояний от энергии электронов со спином вверх и вниз
-
Fig. 5. Spiral iron cluster, composed of seven tetrahedra: a – general view; b – dependence of the probability density of the distribution of electronic states on the energy of electrons with spin up and down
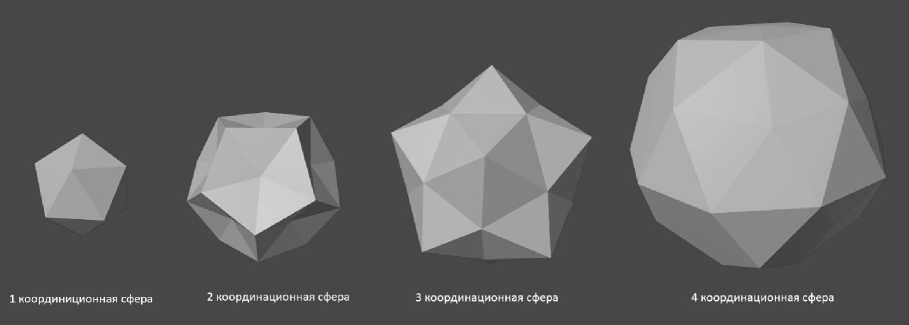
Рис. 6. Эволюция координационных сфер из тетраэдрических кластеров: от первой до четвёртой
Fig. 6. Evolution of coordination spheres from tetrahedral clusters: from the first to the fourth
The next stage of modeling was the combination of a spiral and icosahedral cluster. When twenty tetrahedral spirals are close-packed (Fig. 7), an icosahedron containing 13 atoms is formed in the first coordination sphere. The second coordination sphere contains 20 atoms. Here the first 3 tetrahedrons from each of the 20 spirals are connected in a coordinated manner. Then each spiral grows independently in the direction from the center perpendicular to the faces of the first coordination sphere. Each helix has 10 atoms, 6 of which, packed in 3 tetrahedrons, are in 2 coordination spheres. The remaining 4 atoms of each helix are packed into "tails" of 4 tetrahedrons. The total number of atoms in such positions is 4 × 20 = 80. Thus, the total number of atoms in the cluster packed with 20 tetrahedral spirals is 33 + 80 = 113 atoms (Fig. 6).

а
Рис. 7. Комбинация икосаэдрического и спиралевидного кластеров железа: а – неполная модель из бумаги; б – трехмерная компьютерная модель
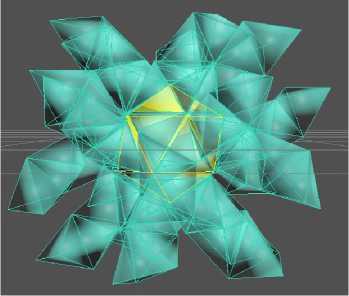
б
Fig. 7. Combination of icosahedral and spiral iron clusters: a – incomplete paper model; b – three-dimensional computer model
Fig. 7 shows a structure built of 20 tetrahedral ten-atom spirals with one common atom in the center. The second coordination sphere also forms an icosahedron containing 20 atoms. Here we get 33 atoms in total in the first and second coordination spheres. This structure corresponds to the experimentally obtained result with the maximum magnetization shown in Fig. 1.
Conclusion
The appearance of magnetization in cluster formations is associated with excited electronic states of those atoms that are on the surface of a nanoparticle or nanofilm. Stressed nonequilibrium electronic states appear in such systems. For small-sized cluster models, most of the atoms are on the surface and have open bonds. The excited atoms have increased electron density, i.e., the electrons are able to move into states with higher energy, approaching the Fermi energy. In this case, the Stoner criterion necessary for the emergence of magnetization is fulfilled [17]. The configurations of electrons with spin up and down are different, resulting in uncompensated magnetic moments. Thus, the proposed models of iron nanoclusters correspond quite satisfactorily to the experimental data of [1].
Список литературы Magnetic characteristics of iron nanoclusters
- Mir materialov i tekhnologiy. Nanomaterialy. Nanotekhnologii. Nanosistemnaya tekhnika : mirovye dostizheniya za 2005 g. [The world of materials and technologies. Nanomaterials. Nanotechnology. Nanosystem technology: world achievements in 2005: collection of articles] ed. P. P. Maltsev. Moscow, Tekhnosfera Publ., 2006, 149 p.
- Billas I. M. L., Chatelain A., Walt A. H., Magnetism from the Atom to the Bulk in Iron, Cobalt, and Nickel Clusters. Science. 1994, Vol. 265, P. 5179. 10.1126/science.265.5179.1682.
- Sundaresan A., Rao C. N. R. Ferromagnetism as a universal feature of inorganic nanoparticles. Nano Today. 2009, No. 4. Doi:10.1016/j.nantod.2008.10.002.
- Venkatesan M., Fitzgerald C. B., Coey J. M. D. Nature. 2004. Vol. 430, P. 630.
- Hong N. H., Sakai J., Poirot N., Brize V. Phys. Rev. 2006. No. B 73. P. 132404.
- Bul’yonkov N. A., Tytik D. L. [Modular design of icosahedral metal clusters]. Izv. AN: Ser Chemical. 2001, No. 1, P. 1–19 (In Russ.).
- Kveglis L. I., Noskov F. M., Volochaev M. N., Nyavro A. V., Filarowski A. Magnetic Properties of Nickel-Titanium Alloy during Martensitic Transformations under Plastic and Elastic Deformation. Sym-metry. 2021, No. 13(4), P. 665. https://doi.org/10.3390/sym13040665.
- Trushin Yu. V. Fizicheskie osnovy materialovedeniya. T. 3. [Physical foundations of materials science. Volume 3]. SPb., Izd-vo Akadem. un-ta Publ., 2015, 356 p.
- Hohenberg P., Kohn W. Inhomogeneous electron gas. Phys. Rev. 1964. No. B. 136, P. 864. https://doi.org/10.1103/PhysRev.136.B864.
- Nyavro A. V. Evolyutsiya elektronnykh sostoyaniy: atom – molekula – klaster – kristall. [Evolution of Electronic States: atom – molecule – cluster – crystal]. Tomsk, Izdat. dom Tomskogo gos. un-ta Publ., 2013, 268 p.
- Giannozzi P., Baroni S., Bonini N., Calandra M., Car R., Cavazzoni C., Wentzcovitch R. M., Quan-tum espresso: a modular and open-source software project for quantum simulations of materials. Journal of Physics: Condensed Matter. 2009, P. 21–39. 10.1088/0953-8984/21/39/395502.
- Kraposhin V. S., Talis A. L., Demina E. D., Zaitsev A. I. [Crystal-geometric mechanism of spinel and manganese sulfide intergrowth into a complex non-metallic inclusion]. MiTOM. 2015, P. 4. (In Russ.).
- Frank F. C., Kasper J. S. Complex alloy structures regarded as sphere packings. II. Analysis and clas-sification of representative structures. Acta Crystall. 1959. Vol. 1, P. 483–499.
- Kveglis L. I., Abylkalykova R. B., Noskov F. M., Arhipkin V. G., Musikhin V. A., Cherepanov V. N., Niavro A. V. Local electron structure and magnetization in β-Fe86Mn13C Superlattices and Microstruc-tures. 2009. Vol. 46, No. 1–2, P. 114–120.
- Lipscomb W. N. Framework rearrangement in boranes and carboranes. Science. 1966, Vol. 153(3734), P. 373-8. Doi: 10.1126/science.153.3734.373.
- Bakir M., Jasiuk I. Novel Metal-Carbon Nanomaterials: A Review On Covetics. Advanced Materials Letters. 2017, No. 8, P. 884–890. 10.5185/amlett.2017.1598.
- Tikadzumi S. Fizika ferromagnetizma. Magnitnye kharakteristiki i prakticheskie primeneniya [Phys-ics of ferromagnetism. Magnetic characteristics and practical applications ]. Moscow, Mir Publ., 1987, 419 p.


