Manganese (Mn) toxicity in plants a comprehensive overview: a review
Автор: Chetry Poonam, Konwar Tashmi
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 1 т.21, 2025 года.
Бесплатный доступ
Manganese (Mn), an essential micronutrient necessary for plant growth and development, has serious phytotoxic effects when present in surplus concentrations. The higher concentration of Mn manifests as a reduction in photosynthetic activity and a gradual up regulation of oxidative stress, which results in reduced yield. Interestingly, Mn toxicity is a serious issue in acid soil, which is mainly encountered in sizable parts of the soil across the globe. In order to mitigate the detrimental influence of Mn on crop productivity, it is of significance to comprehend the diverse physiological aspects of Mn. Thus, such information is crucially important for the identification and development of Mn-tolerant genotypes. Hence, this review article precisely discusses the diverse physiological aspects of Mn toxicity in plants.
Acid soil, manganese, metal ion uptake, phytotoxicity, tolerance
Короткий адрес: https://sciup.org/143183771
IDR: 143183771
Текст обзорной статьи Manganese (Mn) toxicity in plants a comprehensive overview: a review
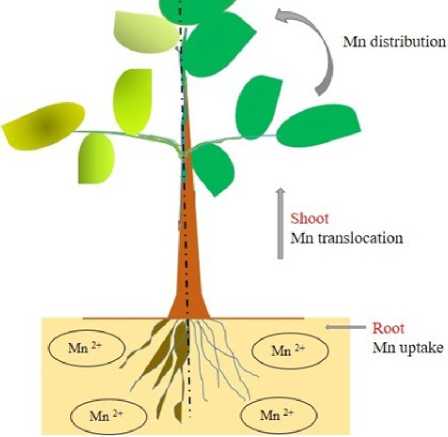
Plants experience an array of abiotic stresses that seriously restrict their productivity and yield. In this context, the acidity of soils is a matter of serious concern which comprises about 40–50% of the total ice-free land, primarily in humid climates (Von Uexküll & Mutert, 1995). The low soil pH directly or indirectly affects the mineral uptake in plants, drastically limiting agriculture activities around the world. Moreover, acid soil results in the toxicity of plants on account of enhanced availability of metal ions (Fe, Mn, Al) and the deficiency of crucial elements required for plant growth (Ca, P, Mg, K, Mo, B) (Kochian et al ., 2004; Chetry & Sharma, 2023). Mineral nutrients which have specific and essential functions in plant metabolism are classified as macronutrients and micronutrients. Manganese (Mn) is one of the 17 essential micronutrients required for the normal growth and development of plants. This metalloenzyme (Mn) cluster serves as an activator for several enzymes necessary for chlorophyll synthesis and the proper functioning of photosynthesis (Terry & Ulrich, 1974). However, Mn becomes toxic in poorly drained soil with low pH (Marschner, 1995; Pittman, 2005). Unlike aluminium (Al), excess Mn generally affects shoots more than roots in low-pH soil (Foy et al ., 1978), as the accumulation of Mn has been predominantly reported in shoot rather than root systems (Page & Feller, 2005). The thresholds of Mn toxicity and tolerance vary significantly among plant species and among cultivars within a species (Foy et al ., 1988). For example, rice is considered a Mn-tolerant species among the cereal crops, especially flooded or paddy rice (Lindon, 2001). Moreover, rice species have been reported to accumulate Mn in their leaves at concentrations as high as 5000 µg g-1 DW without showing any toxic symptoms, which is remarkably high when compared with the Mn concentration recorded in barley with toxicity of 150 µg g-1 DW (Vlamis & Williams, 1964).
Mn (II) the ubiquitously available form for plants, which can be easily oxidised to Mn (III) and Mn (IV) in the presence of an acidic environment (Marschner, 2011). The bioavailability of Mn depends on a range of environmental factors, viz., soil acidity, redox potential, temperature and moisture, which gradually increase the concentration of Mn either individually or in a sequence (George et al., 2012). Mn toxicity results when the normal concentration of biologically available Mn is increased above the threshold level. Thus, the excess amount of Mn causes drastic physiological as well as biochemical changes in plants. Hence, an attempt was made to understand the phytotoxic effect of Mn in plants, which would provide insight into the physiological processes functioning during Mn toxicity as well as the detoxification mechanisms inbuilt in plants to sustain such abiotic stress.
Mn uptake in plants
The uptake of Mn depends on the soil pH as well as soil redox potential, as acid soil results in an increase in bioavailable Mn by promoting the reduction of soil-bound Mn (Mn3+ and Mn4+ to Mn2+) (Goulding, 2016). Moreover, the uptake of Mn has been reported to be an active process where H+-ATPases are used to create an electrochemical gradient across the plasma membrane of root cells (Rengel, 2000). The mechanism of Mn uptake has been depicted in two phases. (a) first phase consists of the absorption of Mn2+ via the negatively charged cell wall, which constitutes the apoplast of the root epidermal cells (b) second phase comprises Mn2+ transported to other parts via the symplastic pathway. It is interesting to note that the transporters associated with Mn uptake also compete with other divalent cations, such as Fe2+, Zn2+, Cu2+, Cd2+, Ca2+, Co2+ and Ni2+, because of their non-specificity and low requirement for Mn2+ for plant nutrition. Once Mn uptake from the soil takes place, diverse families of transport proteins are known to maintain Mn homeostasis, which have been classified as importers and exporters respectively. The importers mainly translocate Mn from the extracellular space into the cytosol, whereas the exporters are responsible for the exclusion of Mn from the cytosol into intracellular compartments. The natural resistance-associated macrophage protein (NRAMP) family, the zinc-regulated transporter/iron-regulated transporter (ZRT/IRT)-related protein (ZIP) family and the yellow stripe-like (YSL) family have members involved in the transport of Mn2+ into the cytosol. In contrast, the cation diffusion facilitator/metal transport protein (CDF/MTP)
family, the vacuolar iron transporter (VIT) family, the Ca2+/cationantiporter (CaCA) superfamily, the bivalent cation transporter (BICAT) family and the P 2A -type ATPase family have members involved in the transport of Mn2+ out of the cytosol (Alejandro et al ., 2020). The sequestration of surplus Mn in the vacuoles, endoplasmic reticulum, or Golgi bodies has key roles in Mn tolerance (Williams & Pittman, 2010). A variety of transporter proteins belonging to the family, viz., cation exchanger (CAX), cation diffusion facilitator (CDF) and P 2A -type ATPase, mediate these processes, particularly in Arabidopsis thaliana . (Hirschi et al ., 2000; Delhaize et al ., 2007; Wu et al ., 2002).
Phytotoxicity of Mn in plants
Mn toxicity limits plant productivity in acid soils after aluminium (Al), where it prevents the uptake and transport of various other essential plant nutrients because of their resemblance with the ionic radius and ligand binding ability (Clark, 1982). The common symptoms of Mn toxicity are marginal chlorosis and necrosis of leaves, which strongly vary depending on the plant species. For example, Mn restricts the number and size of nodules and causes bronze speckle in marigold or geranium, crinkle leaf necrosis in cotton, stem streak necrosis in potato, internal bark necrosis in apple, tip burn in carnation, and fruit cracking in muskmelon (Foy et al ., 1978; Foy, 1983). Although a low concentration of Mn is a basic requirement for plant growth, the excess Mn in the soil not only harms plant productivity but also influences their yield and quality. Additionally, the excess concentration of Mn represents an important factor in environmental contamination, which causes various phytotoxic effects (Figure 1) (Pitman, 2005). Higher (Mn) concentrations are responsible for oxidative bursts with the production of reactive oxygen species (ROS) like superoxide radicals (O 2 -), hydrogen peroxide (H 2 O 2 ), and hydroxyl radicals (OH·) (Demirevska-
Kepova et al ., 2004; Boojar & Goodarzi, 2008). To cope with these ROS, the combined action of enzymatic antioxidant systems like the production of superoxide dismutases, catalases, peroxidases and the synthesis of non-enzymatic antioxidants like ascorbate and glutathione is necessary (Sharma & Dietz, 2009). The threshold of Mn toxicity and the tolerance to excess Mn concentrations vary characteristically according to plant species and their cultivars (Foy et al ., 1988).
Mn toxicity typically causes the oxidation of excess Mn2+ to Mn3+ in the apoplast, which in turn results in strong oxidative damage of proteins and lipids (Fecht-Christoffers et al ., 2006). The visible symptoms of Mn toxicity, i.e., brown necrotic spots on the leaves, have been proposed to occur due to the accumulation of high levels of oxidised phenolics in the apoplast (Wissemeier & Horst, 1992). Mn toxicity in wheat results in reduced shoot fresh weight, leaf extension, and nodal root growth, which cause death of the seminal root system and early senescence of the lower leaves (Khabaz-Saberi et al ., 2006).
Effects of Mn toxicity on photosynthesis
carboxylation efficiency in various plant species. The decline in photosynthesis rate is considered one of the major mechanisms constituting the toxic effects of excess Mn in rice and wheat (Lidon et al ., 2004; Macfie & Taylor, 1992). Moreover, in the hyperaccumulator species Phytolacca acinosa, Mn affects photosynthetic activity, attributing the hyperaccumulator capacity of the species to efficient Mn complexing and not to abruptly modifying the chloroplast structures (Weng et al ., 2013). Thus, in a comprehensive way, it can be assumed that Mn toxicity hampers the photosynthesis process in plants, thereby restricting the growth of the plant in a concerted manner (Figure 2).
Toxicity
Tolerance
-
✓ Chlorosis of leaf
^ Generation of brown roots
-
✓ Trigger oxidative stress
^ Disnipt photosynthesis
^ ABC transporter and GSH metabolic pathway activates
-
✓ Organic acid formation and translocation
S Antioxidant enzymes produce
МАРК signaling pathway activates
J Membrane lipid peroxidation
Figure 1 . Schematic representation of toxicity and strategies of tolerance adopted by plants towards Mn. Uptake of Mn from soil and thereby translocation and distribution of Mn towards aerial parts.
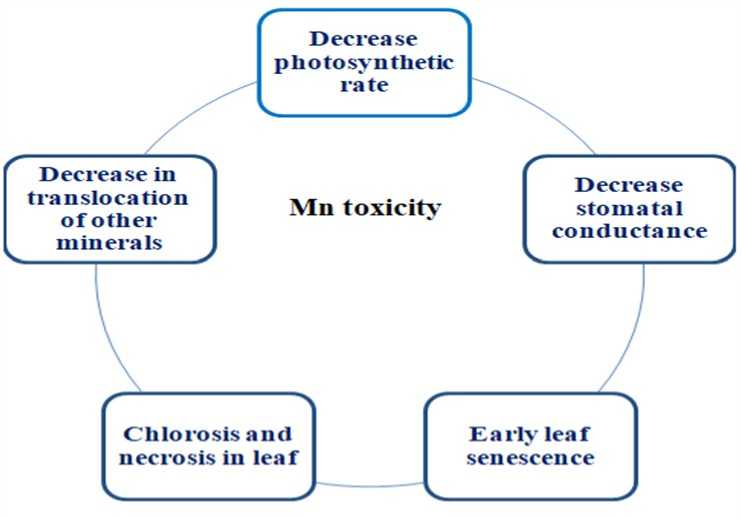
Figure 2. Effect of Mn toxicity in photosynthesis leading to decline plant growth.
Table 1: Tolerance mechanism adopted by Mn hyperaccumulator plant species.
|
Plants/family |
Mechanism |
References |
|
Celosia argentea (Amarantheaceae) |
Plant transport Mn from root to shoot, where ABC transporter and GSH metabolic pathway help in the detoxification. |
Yu et al . (2023), Liang et al . (2024) |
|
Chengiopanax sciadopylloide (Araliaceae) |
Carboxylic acids, viz., malate or citrate, play an important role in the hyperaccumulation of Mn. |
Fernando et al . (2010) |
|
Eucalyptus grandis ⨰ Eucalyptus urophylla (Myrtaceae) |
Plants detoxify Mn by forming complexes with high-molecular-weight proteins and low-molecular-weight organic acids. |
Xie et al . (2015) |
|
Phytolacca americana (Phytolaccaceae) |
Mn is removed from the root surface by precipitation of the phosphate to form Mn phosphate crystals in rhizosphere. |
Dou & Qi (2023) |
|
Polygonum hydropiper (Polygonacceae) |
Enzymes like sulfhydryl group (-SH) and glutathion (GSH) play a major role in detoxification. |
Yang et al . (2016) |
|
Polygonum lapathifolium (Polygonacceae) |
Sulphate regulates Mn uptake and translocation in this plant. |
Liu et al . (2021a) |
|
Polygonum perfoliatum (Polygonacceae) |
This plant tolerates Mn stress through the production and transportation of organic acids and membrane lipid peroxidation. |
Xue et al . (2018) |
|
Polygonum pubescens (Polygonacceae) |
Antioxidant enzymes play a vital role in alleviating Mn stress. |
Liu et al . (2021b) |
|
Schima superba (Theaceae) |
Under stress condition, mitogen-activated protein kinase (MAPK) signalling regulates Mn stress. |
Liaquat et al . (2022) |
Interaction of Mn with other elements
applications, which could lead to serious nutritional imbalance because Mg also interferes with Ca uptake. As compared to other nutrient elements, the absorption of K is slightly affected by increasing Mn concentrations (Heenan & Campbell, 1981). High K levels in the shoots of Mn-tolerant 'Lee' soybean alleviated the harmful effects of high internal Mn concentrations (Brown & Jones, 1977). Abundant evidence shows that a soluble source of Si in the growth medium can protect plants against Mn toxicity (Bowen, 1972). The higher absorption of Si by monocots than by dicots may help explain the higher tolerance of monocots to Mn toxicity (Foy et al ., 1978). Si reduced or prevented Mn toxicity in barley, rice, rye, ryegrass, and sorghum (Vlamis & Williams, 1967; Galvez et al ., 1989). Plant tolerance to soluble Mn may also be affected by the concentration of S, Al, Zn and Cu in the medium. Additional S may lower the pH of the growth medium and increase the availability of Mn to plants. Thus, all the elements behave diversely to increase the internal concentration of Mn in plants.
Mn detoxification mechanisms
Hyperaccumulator of Mn
Some rare plants accumulate trace elements in extreme concentrations and are known as hyperaccumulator plants. Cuba, an island country, has the highest number of plant hyperaccumulators, accounting for 128 species (Reeves et al ., 1999). Several Mn hyperaccumulator plant species, along with their specific tolerance strategies, have been enlisted in Table 1. In an experiment with Mn hyperaccumulators ( Phytolacca americana , P. perfoliatum, and P. hydropiper), it was found that P. perfoliatum has superior Mn accumulation and tolerance abilities (Liu et al ., 2010). However, P. americana is a common weedy species and has no specific association with high Mn soils. It was suggested that P. americana secretes acids into the rhizosphere as a means of acquiring P, which might coincidentally increase Mn uptake.
Conclusion and future prospects
Mn toxicity hampers plant growth and yield worldwide, particularly in acidic soils. Mn, however, is necessary for plant growth in trace amounts, but the surplus availability of this metal ion consequently leads to phtotoxicity. Thus, in the global scenario, it is imperative to study the response of diverse agroecosystems to surplus Mn concentrations. Furthermore, a clearer understanding of the mechanisms of Mn toxicity and tolerance among different plants is of the utmost necessity for future sustainable agriculture. In this context, the Mn hyperaccumulator species are likely to serve as an important genotype for understanding the tolerance strategies adopted by those plants to survive the Mn surplus condition. In addition, the interactive influence of Mn with other co-occurring factors in the acidic soils, such as other heavy metals as well as Al stress and surplus Fe concentrations, needs to be appropriately addressed. Moreover, cereal crops must be genetically engineered in order to develop Mn-tolerant crops that can be grown in soil with low pH conditions to maintain the production of food grains with low yield losses.
ACKNOWLEDGMENT
The authors are grateful to respective Department of Agricultural Biotechnology, Assam Agricultural University and Department of Botany, School of Life Sciences, Sikkim University. The award of Senior Research Fellowship to PC by Council of Scientific and Industrial Research (CSIR), New Delhi is also thankfully acknowledged.
CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest.
Список литературы Manganese (Mn) toxicity in plants a comprehensive overview: a review
- Alejandro, S., Holler, S., Meier, B., and Peiter, E. (2020). Manganese in plants: from acquisition to sub cellular allocation. Front. Plant Sci, 11, 300.
- Arya, S. K., and Roy, B. K. (2011). Manganese induced changes in growth, chlorophyll content and antioxidants activity in seedlings of broad bean (Vicia faba L.). J. Environ Biol, 32(6), 707.
- Baldisserotto, C., Ferroni, L., Anfuso, E., Pagnoni, A., Fasulo, M. P., and Pancaldi, S. (2007). Responses of Trapa natans L. floating laminae to high concentrations of manganese. Protoplasma, 231(1-2), 65-82.
- Blair, L. M., and Taylor, G. J. (1997). The nature of interaction between aluminum and manganese on growth and metal accumulation in Triticum aestivum. manganese toxicity. Plant Soil, 91, 171-180.
- Boojar, M. M. A., and Goodarzi, F. (2008). Comparative evaluation of oxidative stress status and manganese availability in plants growing on manganese mine. Ecotoxicol Environ Saf, 71(3), 692-699.
- Bowen, J. E. (1972). Manganese-silicon interaction and its effect on growth of sudan grass. Plant Soil, 37(3), 577-588.
- Brown, J. C., and Jones, W. E. (1977). Fitting plants nutritionally to soils. II. cotton 1. Agron J, 69(3), 405409.
- Chetry, P., and Sharma, S. S. (2023). Variability in seed germination and seedling growth of some rice (Oryza sativa L.) landraces from Sikkim Himalaya: a-amylase activity and response to gibberellic acid. J Indian Bot. Soc, 101(03), 156-162.
- Clark, R. B. (1982). Plant response to mineral element toxicity and deficiency. In breeding plants for less favourable environments, Edited by: Christiansen, M.N. and Lewis, C.F. 71 - 142. New York, NY: John Wiley and Sons.
- Clemens, S. (2001). Molecular mechanisms of plant metal tolerance and homeostasis. Planta, 212, 475486.
- Davis, J. G. (1996). Soil pH and magnesium effects on manganese toxicity in peanuts. J. Plant Nutr, 19(3-(2001). Developmental and induced responses of nickel-based and organic defenses of the nickel-hyperaccumulating shrub, Psychotria douarrei. New. Phytol, 150, 49-58.
- Delhaize, E., Gruber, B. D., Pittman, J. K., White, R. G., Leung, H., Miao, Y., Jiang, L., Ryan, P. R., and Richardson, A. E. (2007). A role for the AtMTP11 gene of Arabidopsis in manganese transport and tolerance. Plant J, 51(2), 198-210.
- Demirevska-Kepova, K., Simova-Stoilova, L., Stoyanova, Z., Hölzer, R., and Feller, U. (2004). Biochemical changes in barley plants after excessive supply of copper and manganese. Environ Exp Bot, 52(3), 253-266.
- Dou, C., and Qi, C. (2023). Rhizospheric precipitation of manganese by phosphate: a novel strategy to enhance Mn tolerance in the hyperaccumulator Phytolacca americana. Toxics, 11(12), 977.
- Dou, C., Fu, X., Chen, X.,Shi, J., and Chen, Y. (2008). Accumulation and detoxification of manganese in hyperaccumulator Phytolacca americana. Plant Biol, 11,664-670.
- Ducic, T., and Polle, A. (2005). Transport and detoxification of manganese and copper in plants. Braz. J. Plant Physiol, 17, 103-112.
- Ducic, T., and Polle, A. (2007). Manganese toxicity in two varieties of Douglas fir (Pseudo tsuga menziesii var. viridis and glauca) seedlings as affected by phosphorus supply. Funct. Plant Biol, 34, 31-40.
- Elamin, O. M., and Wilcox, G. E. (1986). Effect of magnesium and manganese nutrition on muskmelon growth and manganese toxicity. J. Amerc Soc. Hort Sci, 111(4), 582-587.
- Fecht-Christoffers, M. M., Fuhrs, H., Braun, H. P., and Horst, W. J. (2006). The role of hydrogen peroxide-producing and hydrogen peroxide-consuming peroxidases in the leaf apoplast of cowpea in manganese tolerance. Plant Physiol, 140(4), 14511463.
- Fernando, D. R., and Lynch, J. P. (2015). Manganese phytotoxicity: new light on an old problem. Ann. Bot, 116(3), 313-319.
- Fernando, D. R., Mizuno, T., Woodrow, I. E., Baker, A. J. M., and Collins, R. N. (2010). Characterization of foliar manganese (Mn) in Mn (hyper) accumulators using X-ray absorption spectroscopy. New Phytol, 1014-1027.
- Environ Exp Bot., 37(1), 25-37. Blarney, F., Joyce, D., Edwards, D., and Asher, C. (1986). Role of trichomes in sunflower tolerance to 4), 535-550.
- Davis, M.A., Pritchard, S.G., Boyd, R.S., Prior, S.A. Foy, C. D. (1983). The physiology of plant adaptation to mineral stress. 355-391.
- Foy, C. D., Chaney, R. T., and White, M. C. (1978). The physiology of metal toxicity in plants. Annu. Rev. Plant Physiol, 29(1), 511-566.
- Foy, C. D., Scott, B. J., and Fisher, J. A. (1988). Genetic differences in plant tolerance to manganese toxicity. In manganese in soils and plants. 293-307.
- Foy, C.D., Weil, R.R., and Coradetti, C.A. (1995). Differential manganese tolerances of cotton genotypes in nutrient solution. J. Plant Nutr, 18, 685706.
- Galvez, L., Clark, R. B., Gourley, L. M., and Maranville, J. W. (1989). Effects of silicon on mineral composition of sorghum grown with excess manganese. J. Plant Nutr, 12(5), 547-561.
- George, E., Horst, W. J., and Neumann, E. (2012). Adaptation of plants to adverse chemical soil conditions. In Marschner's mineral nutrition of higher plants. Acad Pres, 409-472.
- González, A., and Lynch, J. P. (1999). Subcellular and tissue Mn compartmentation in bean leaves under Mn toxicity stress. Funct Plant Biol, 26(8), 811-822.
- Goulding, K. W. T. (2016). Soil acidification and the importance of liming agricultural soils with particular reference to the United Kingdom. Soil Use Manage, of water: an overview. Philos. Trans. R. Soc. B, Biol. Sci, 357(1426), 1369-1381.
- Heenan, D. P., and Campbell, L. C. (1981). Influence of potassium and manganese on growth and uptake of magnesium by soybeans (Glycine max (L.) Merr. cv.
- Wagner, G. J. (2000). Expression of Arabidopsis CAX2 in tobacco. Altered metal accumulation and increased manganese tolerance. Plant Physiol, 124(1), 125-134.
- Horiguchi, T. (1987). Mechanism of manganese toxicity and tolerance of plants. II. Deposition of oxidized manganese in plant tissues. J. Soil Sci. Plant Nutr, 33, 595-606.
- Horst, W. J. and Marschner, H. (1990). Decrease in nitrate uptake and increase in proton release in zinc deficient cotton, sunflower and buckwheat plants. Plant Soil, 129(2), 261- 268.
- Horst, W. J., and Marschner, H. (1978). Effect of excessive manganese supply on uptake and translocation of calcium in bean plants (Phaseolus vulgaris L.). Zeitschrift fur Planzenphysiologie. 87, 137-148.
- Horst, W. J., Fecht, M., Naumann, A., Wissemeier, A. H., and Maier, P. (1999). Physiology of manganese toxicity and tolerance in Vigna unguiculata (L.) Walp. J. Plant Nutr. Soil Sci, 162, 263-274.
- Huang, H., Zhao, Y., Xu, Z., Zhang, W., and Jiang, K. (2019). Physiological responses of Broussonetia papyrifera to manganese stress, a candidate plant for phytoremediation. Ecotoxicol Environ Saf, 181, 18-25.
- Kazda, M., and Zvacek, L. (1989). Aluminium and manganese and their relation to calcium in soil solution and needles in three Norway spruce (Picea abies, L. Karst.) stands of Upper Austria. Plant Soil, 114(2), 257-267.
- Khabaz-Saberi, H., Setter, T. L., and Waters, I. (2006). Waterlogging induces high to toxic concentrations of iron, aluminum and manganese in wheat varieties on acidic soil. J. Plant Nutr, 29(5), 899-911.
- Kitao, M., Lei, T. T., and Koike, T. (1997). Effects of manganese toxicity on photosynthesis of white birch (Betula platyphylla var. japonica) seedlings. Physiol Plant, 101(2), 249-256.
- Kochian, L. V., Hoekenga, O. A., and Pineros, M. A. (2004). How do crop plants tolerate acid soils?-mechanisms of aluminium tolerance and phosphorous efficiency. Annu. Rev. Plant Biol, 55(1), 459-493.
- Liang, L., Ze, M., Yang, J., Xu, Q., Du, C., Hu, X.....and Qi, T. (2024). Physiological and transcriptomic response reveals new insight into manganese tolerance of Celosia argentea Linn. J. Hazard. Mater, 465, 133079.
- Liaquat, F., Munis, M. F. H., Arif, S., Manzoor, M. A., Haroon, U., Shah, I. H.....and Qunlu, L. (2022). 32(3), 390-399.
- Goussias, C., Boussac, A., and Rutherford, A. W. (2002). Photosystem II and photosynthetic oxidation Bragg). Plant Soil, 61, 447-456.
- Hirschi, K. D., Korenkov, V. D., Wilganowski, N. L., and Reprisal of Schimasuperba to Mn stress and exploration of its defense mechanism through transcriptomic analysis. Front. Plant Sci, 13, 1022686.
- Lidon F (2001). Tolerance of rice to excess manganese in the early stages of vegetative growth. Manganese accumulation in rice: implications for photosynthetic functioning. J. Plant Physiol. 161(11), 1235-1244.
- hyperaccumulators or accumulators. Plant Soil, 335, 385-395.
- Liu, K., Li, C., Dai, C., Qin, R., Liang, X., Li, Y., and Yu, F. (2021a). A novel role of sulfate in promoting Mn phytoextraction efficiency and alleviating Mn stress in Polygonum lapathifolium Linn. Ecotoxicol Environ Saf, 213, 112036.
- Liu, K., Xu, J., Dai, C., Li, C., Li, Y., Ma, J., and Yu, F. (2021b). Exogenously applied oxalic acid assists in the phytoremediation of Mn by Polygonum pubescens Blume cultivated in three Mn-contaminated soils. Front. Environ. Sci. Eng, 15, 113.
- Macfie, S. M., and Taylor, G. J. (1992). The effects of excess manganese on photosynthetic rate and concentration of chlorophyll in Triticum aestivum grown in solution culture. Physiol Plant. 85(3), 467475.
- Marschner, H. (1995). Mineral nutrition of higher plants. Acad Press., London.
- Marschner, H. (Ed.). (2011). Marschner's mineral nutrition of higher plants. Acad Press., London.
- Morgan, P. W., Taylor, D. M., and Joham, H. E. (1976). Manipulation of IAA-oxidase activity and auxin-deficiency symptoms in intact cotton plants with manganese nutrition. Physiol Plant, 37(2), 149-156.
- Nogueira, M. A., Magalhaes, G. C., and Cardoso, E. J. (2004). Manganese toxicity in mycorrhizal and phosphorus-fertilized soybean plants. J. Plant Nutr, 27(1), 141- 156.
- Osawa, T., and Ikeda, H. (1976). Heavy metal toxicites in vegetable crops. J. Jap SocHort Sci, 45(1), 50-58.
- Page, V., and Feller, U. R. S. (2005). Selective transport of zinc, manganese, nickel, cobalt and cadmium in the root system and transfer to the leaves in young wheat plants. Ann. Bot, 96(3), 425-434.
- Pittman, J. K. (2005). Managing the manganese: molecular mechanisms of manganese transport and homeostasis. New Phytol, 167(3), 733-742.
- Rees, W. J., and Sidrak, G. H. (1961). Inter-relationship of aluminium and manganese toxicities towards plants. Plant Soil, 101-117.
- Reeves, R. D., Baker, A. J. M., Borhidi, A., and Berazain, R. (1999). Nickel hyperaccumulation in the serpentine flora of Cuba. Ann. Bot, 83, 1-10.
- Rengel, Z. (2000). Uptake and transport of manganese in plants. Metal ions in biological systems. Marcel Dekker, New York, 57-87.
- Rosas, A., Rengel, Z., and Mora, M. (2007). Manganese supply and pH influence growth, carboxylate exudation and peroxidase activity of ryegrass and white clover. J. Plant Nutr, 30, 253- 270.
- Sarkar, D., Pandey, S. K., Sud, K. C., and Chanemougasoundharam, A. (2004). In vitro characterization of manganese toxicity in relation to phosphorus nutrition in potato (Solanum tuberosum L.). Plant Sci, 167(5), 977-986.
- Sharma, S. S., and Dietz, K. J. (2009). The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci, 14(1), 43-50.
- Shuman, L. M., and Anderson, O. E. (1976). Interactions of Mn with other ions in wheat and soybeans. Commun. Soil Sci. Plant Anal, 7(6), 547-557.
- Subrahmanyam, D., and Rathore, V. S. (2000). Influence of manganese toxicity on photosynthesis in ricebean (Vigna umbellata) seedlings. Photosynthetica, 38(3), 449-453.
- Taylor, G. J., Blarney, F. P. C., and Edwards, D. G. (1998). Antagonistic and synergistic interactions between aluminum and manganese on growth of Vigna unguiculata at low ionic strength. Physiol Plant, 104(2), 183-194.
- Characterization of manganese accumulation. J. Plant Physiol, 158, 1341- 1348. Lidon, F. C., Barreiro, M. G., and Ramalho, J. C. (2004).
- Liu, P., Tang, X., Gong, C., and Xu, G. (2010). Manganese tolerance and accumulation in six Mn
- Terry, N., and Ulrich, A. (1974). Effects of magnesium deficiency on the photosynthesis and respiration of leaves of sugar beet. Plant Physiol, 54(3), 379-381.
- Vlamis, J., and Williams, D. E. (1964). Iron and manganese relations in rice and barley. Plant Soil, 221-231.
- Vlamis, J., and Williams, D. E. (1967). Manganese and silicon interaction in the gramineae. Plant Soil, 131140.
- Von Uexkull, H. R., and Mutert, E. (1995). Global extent, development and economic impact of acid soils. Plant Soil, 171(1), 1-15.
- Vose, P. B., and Jones, D. G. (1963). The interaction of manganese and calcium on nodulation and growth in varieties of Trifolium repens. Plant Soil, 372-385.
- Weng, X. Y., Zhao, L. L., Zheng, C. J., and Zhu, J. W. (2013). Characteristics of the hyperaccumulator plant Phytolacca acinosa (Phytolaccaceae) in response to excess manganese. J. Plant Nutr, 36(9), 1355-1365.
- Williams, L. E., and Pittman, J. K. (2010). Dissecting pathways involved in manganese homeostasis and stress in higher plant cells. In cell biology of metals and nutrients Springer, Berlin, Heidelberg, 95-117.
- Wissemeier, A. H., and Horst, W. J. (1992). Effect of light intensity on manganese toxicity symptoms and callose formation in cowpea (Vigna unguiculata (L.) Walp.). Plant Soil, 143(2), 299-309.
- Wu, Z., Liang, F., Hong, B., Young, J. C., Sussman, M. R., Harper, J. F., and Sze, H. (2002). An endoplasmic reticulum-bound Ca2+/Mn2+ pump, ECA1, supports plant growth and confers tolerance to Mn2+ stress. Plant Physiol, 130(1), 128-137.
- Xie, Q., Li, Z., Yang, L., Lv, J., Jobe, T. O., and Wang, Q. (2015). A newly identified passive hyperaccumulator Eucalyptus grandisx E. urophylla under manganese stress. PLoS One, 10(9), e0136606.
- Xue, S., Wang, J., Wu, C., Li, S., Hartley, W., Wu, H.....and Cui, M. (2018). Physiological response of Polygonum perfoliatum L. following exposure to elevated manganese concentrations. Environ Sci Pollut Res, 25, 132-140.
- Yang, Z. B., You, J. F., Xu, M. Y., and Yang, Z. M. (2009). Interaction between aluminum toxicity and manganese toxicity in soybean (Glycine max). Plant Soil, 319(1-2), 277- 289.
- Yang, X. J., Deng, D. M., Liu, K. H., and Yu, F. M. (2016). Response of enzymatic and non-enzymatic antioxidant defense systems of Polygonum hydropiper to Mn stress. J. Cent South Univ, 23(4), 793-797.
- Yu, G., Ullah, H., Wang, X., Liu, J., Chen, B., Jiang, P., Lin, H., Sunahara, G.I., You, S., Zhang, H., and Shahab, A. (2023). Integrated transcriptome and metabolome analysis reveals the mechanism of tolerance to manganese and cadmium toxicity in the Mn/Cd hyperaccumulator Celosia argentea Linn. J. Hazard. Mater, 443, 130206.


