Metabolic processes that occur due to menopause in women with type 2 diabetes
Автор: Saliyeva Shahnozakhan Bakhtiyarjon, Yusupova Shahnoza Kadirzhanovna
Журнал: Re-health journal @re-health
Рубрика: Эндокринология
Статья в выпуске: 4 (12), 2021 года.
Бесплатный доступ
Menopause — and the years before it — may provide some challenges for women who have diabetes. Menopause is the phase of life after your periods have stopped and your estrogen levels decline. Menopause can also occur as a result of surgery, when the ovaries are removed for other medical reasons. Diabetes and menopause may team up for varied effects on body, including: Changes in blood sugar level, weight gain, infections, sleep problems and sexual problems. Usually some of the changes that occur in body during menopause put you at greater risk of type 2 diabetes: Metabolism slows down and organism don't burn calories as efficiently, which can lead to weight gain. Thus, in this article we will discuss the metabolic processes that take place in the body in cases of diabetes and menopause.
Menopause, diabetes, estrogen, ovaries, surgery, sugar, infection, calories, metabolic processes
Короткий адрес: https://sciup.org/14124620
IDR: 14124620
Текст научной статьи Metabolic processes that occur due to menopause in women with type 2 diabetes
QANDLI DIABET 2-TURIGA CHALINGAN AYOLLARDA MENOPAUZA TUFAYLI YUZAGA KELADIGAN METABOLIK JARAYONLAR
Menopauza undan oldingi yillar diabetga chalingan ayollar uchun ba'zi qiyinchiliklarni keltirib chiqarishi mumkin. Menopauza - bu hayz ko'rish to'xtatilgandan va estrogen darajasining pasayishidan keyingi hayot bosqichidir. Menopauza, shuningdek, boshqa tibbiy sabablarga ko'ra, tuxumdonlar olib tashlangan jarrohlik natijasida ham paydo bo'lishi mumkin. Qandli diabet va menopauza tanaga turli xil ta'sir ko'rsatishi mumkin, jumladan: qondagi yuqori shakar miqdori, infektsiyalarning rivojlanishi, uyquning buzilishi, vazn ortishi va jinsiy muammolar. Odatda, menopauza paytida tanadagi ba'zi o'zgarishlar 2-toifa diabet xavfini oshiradi: Metabolizm sekinlashadi va organizm kaloriyalarni unchalik samarali yoqmaydi, bu esa kilogramm ortishiga olib kelishi mumkin. Shunday qilib, ushbu maqolada biz qandli diabet va menopauza holatlarida organizmda kechadigan metabolik jarayonlar haqida muhokama qilamiz.
Kalit so'zlar: Menopauza, diabet, estrogen, tuxumdonlar, jarrohlik, shakar, infektsiya, kaloriyalar, metabolik jarayonlar.
METABOLIC PROCESSES THAT OCCUR DUE TO MENOPAUSE IN WOMEN WITH TYPE 2 DIABETES
Introduction: Diabetes and menopause may team up for varied effects on body, including: Changes in blood sugar level. The hormones estrogen and progesterone affect how cells respond to insulin. After menopause, changes in your hormone levels can trigger fluctuations in blood sugar level. Blood sugar level changes more than before, and goes up and down. If blood sugar gets out of control, you have a higher risk of diabetes complications. Weight gain. Patient might gain weight during the menopausal transition and after menopause. Weight gain may require an adjustment in her diabetes medication. Infections. Even before menopause, high blood sugar levels can contribute to urinary tract and vaginal infections. After menopause — when a drop in estrogen makes it easier for bacteria and yeast to thrive in the urinary tract and vagina — the risk is even higher. Sleep problems. After menopause, hot flashes and night sweats may keep patient up at night. In turn, the sleep deprivation can make it tougher to manage your blood sugar level. Sexual problems. Diabetes can damage the nerves of the cells that line the vagina. This can interfere with arousal and orgasm. Vaginal dryness, a common symptom of menopause, may worsen the issue by causing pain during sex.
American scientists began their scientific article in a remarkable way [1]: Type 2 diabetes has reached epidemic proportions in the United States. Large, randomized controlled trials suggest that menopausal hormone therapy (MHT) delays the onset of type 2 diabetes in women. However, the mechanisms and clinical implications of this association are still a matter of controversy. This review provides an up-to-date analysis and integration of epidemiological, clinical, and basic studies, and proposes a mechanistic explanation for the effect of menopause and MHT on type 2 diabetes development and prevention. We discuss the beneficial effects of endogenous estradiol with respect to insulin secretion, insulin sensitivity, and glucose effectiveness; we also discuss energy expenditure and adipose distribution, both of which are affected by menopause and improved by MHT, which thereby decreases the incidence of type 2 diabetes. We reconcile differences among studies that investigated the effect of menopause and MHT formulations on type 2 diabetes. We argue that discrepancies arise from physiological differences in methods used to assess glucose homeostasis, ranging from clinical indices of insulin sensitivity to steady-state methods to assess insulin action. We also discuss the influence of the route of estrogen administration and the addition of progestogens. We conclude that, although MHT is neither approved nor appropriate for the prevention of type 2 diabetes due to its complex balance of risks and benefits, it should not be withheld from women with increased risk of type 2 diabetes who seek treatment for menopausal symptoms.
Mauvais-Jarvis, Manson, Stevenson, Fonseca gave own conclusions in own article [1]: Although the underlying physiology remains unclear, RCTs suggest that MHT with estrogens may be effective in reducing the risk of type 2 diabetes in post-menopausal women. However, because of its complex balance of risks and benefits, and because the effect of MHT on diabetes prevention was not examined as a primary outcome in RCTs, MHT is neither appropriate nor FDA-approved for the prevention of type 2 diabetes in women. The task force for the treatment of symptoms of menopause concluded that there is inadequate evidence to make a firm recommendation regarding MHT and diabetes [2]. We agree but we also believe that the risks and benefits of MHT for management of menopausal symptoms in recently menopausal women have never been clearer. The depth of knowledge of estrogens’ clinical benefits and risks, accumulated from decades of studies in preclinical models and in women, is matched by few other FDA-approved drugs. In addition, the North American Menopause Society has established a decisionmaking algorithm for menopause management [3] that integrates the American College of Cardiology/American Heart Association atherosclerotic cardiovascular disease (ASCVD) risk prediction score [4]. Therefore, given the evidence available, our perspective regarding MHT and type 2 diabetes is the following: First, in young nondiabetic women (age 50 to 59 years and within 10 years after menopause) and with a hysterectomy, MHT with estrogens alone may be beneficial for the prevention of coronary disease and may also reduce the incidence of type 2 diabetes. Second, in young non-diabetic women without hysterectomy, MHT using estrogens with a progestogen has neutral or beneficial coronary effects [5, 6]. The available evidence argues that in these young women, MHT may reduce the incidence of type 2 diabetes. Oral or transdermal routes of estrogen delivery may be used if the 10-year risk of ASCVD in low (<5%) [2] but a non-oral route of estrogen delivery may be preferred if the 10-year risk of ASCVD is moderate (5 to 10%) or in obese women, due to an increased risk of venous thromboembolic events (VTE). Third, in young women with type 2 diabetes, MHT improves glycemic control, with oral estrogens providing a stronger improvement in insulin sensitivity than transdermal E2 at equivalent doses [7]. However, type 2 diabetes is considered a ASCVD risk factor in women [8]. Therefore, in normal weight diabetic women, an oral route of estrogen delivery may be used only if the 10-year ASCVD risk is low. Oral estrogens have beneficial effects on HDL and LDL cholesterol and may be prescribed in normal weight women with type 2 diabetes, because they produce a stronger improvement in insulin sensitivity [7]. In diabetic women with a moderate 10- year risk of ASCVD or/and in obese diabetic women, transdermal E2 will be preferred along with a progestogen that is neutral on coagulation and insulin sensitivity. Transdermal E2 has more beneficial effects on inflammatory markers and triglycerides than CE does and may not increase risk of VTE.
Assessment of age at menopause Menopausal status was evaluated using a subsection of the home interview questionnaire [9]. One set of questions was designed to obtain information on the timing of the last menstrual period, whether the respondent had experienced a natural menstrual period within the past 12 months and the age at last period for women who had not had a period for at least 12 months. Postmenopausal women were defined as women who reported an absence of menstrual periods for 12 months. For women who had experienced a natural menopause, age at menopause was defined as the selfreported age at the time of the last menstruation. For all women reporting menopause after gynaecological surgery or radiation therapy, and for those reporting any other operations before the age of 50 years that might have led to menopause, information on the exact date and type of operation was verified using general practitioner records, including correspondence from medical specialists.
As for as some group scientists go said [10] that In this large population-based study of postmenopausal women free of type 2 diabetes at baseline, we showed that early onset of natural menopause is associated with an increased risk of type 2 diabetes, independent of potential intermediate risk factors for type 2 diabetes (including BMI, glucose and insulin levels) and of levels of endogenous sex hormones and SHBG. We also showed that shared genetic factors could not explain the association between age at natural menopause and risk of type 2 diabetes. While most studies have investigated a link between age at menopause and cardiovascular outcomes, reporting an increased risk of CVD associated with early onset of menopause, few studies have examined a possible association between age at menopause with risk of type 2 diabetes [11]. Cross-sectional studies examining the association between age at menopause and type 2 diabetes have yielded contradictory results, showing either no association or an increased prevalence of type 2 diabetes in women who experience early onset of menopause [12-14]. Similar to our findings, Brand and colleagues, in a nested casecohort study, showed an increased risk of type 2 diabetes with early onset menopause, reporting similar size effects to those of the current investigation (HR 0.93 per 1 year older at menopause) [15]. However, we extended their findings and showed that this association was independent of potential mediators, including endogenous sex hormone levels. Early onset of natural menopause has been suggested to increase the risk of cardiometabolic diseases, including type 2 diabetes, due to early cessation of the protective effects of endogenous oestrogen [12]. Animal studies have shown that oestradiol decreases the amount of adipose tissue and has a protective role on glucose metabolism [16, 17]. Also, trials in postmenopausal women have linked oral oestrogen therapy with a lower risk of type 2 diabetes [18-20]. In contrast, observational data do not support a protective effect of oestrogen in cardiometabolic health. In postmenopausal women, higher endogenous oestradiol levels have been associated with higher levels of glucose and insulin, and an increase rather than decrease in diabetes risk [21-24]. Moreover, an early start to oestrogen exposure (i.e. an early age at menarche) and a high endogenous oestradiol status have been linked with insulin resistance and an increased risk of type 2 diabetes [25-27]. This evidence, which is also supported by our study, suggests that other menopause-related factors may explain the association between age at menopause and risk of type 2 diabetes. In the current study, we showed that neither SHBG levels nor androgen levels (both of which might be associated with menopause and with type 2 diabetes) could explain the association between early onset of natural menopause and risk of type 2 diabetes. A possible explanation for the observed association between age at natural menopause and risk of type 2 diabetes could be disruption of the hypothalamus-pituitary-ovarian axis, resulting in increased release of the gonadotropins and follicle-stimulating hormone by the pituitary gland. Our study did not include data on levels of follicle-stimulating hormone. However, observational studies have shown that low (rather than high) levels of follicle-stimulating hormone are associated with an increased risk of type 2 diabetes in postmenopausal women [28, 29]. Also, lifestyle factors such as smoking and alcohol consumption are closely linked to age at menopause; e.g. smokers reach menopause on average 2 years earlier than non-smokers [30]. Therefore, the relationship between age at menopause and type 2 diabetes is probably confounded by these factors. However, in our analysis, adjusting for both smoking and alcohol consumption and restricting the analysis to women who did not currently smoke had no impact on the results. Moreover, we found that age at natural menopause was associated with type 2 diabetes independent of glucose and insulin levels. Therefore, the mechanisms linking age at natural menopause with risk of type 2 diabetes remain unclear, and future studies are needed to explore which biological pathways are involved. Recent data show that an early natural menopause may be a marker of premature ageing and related to pathways linked to longevity. Furthermore, age at natural menopause is associated with DNA damage repair, which is also linked to risk of type 2 diabetes [31, 32]. Menopause, therefore, might be a marker of ageing of the somatic (non-reproductive) tissues. Owing to genetic variation, the soma of women equipped with less efficient DNA repair and maintenance might age faster compared with those with more efficient repair and maintenance. Hence, early menopause might be a consequence of accelerated ageing of the soma and might therefore be a very good predictor of general health in later life, including type 2 diabetes risk. However, when we adjusted for shared genetic factors, our results did not change. Nevertheless, genome-wide association studies previously identified approximately 56 SNPs across the human genome that account for only a small proportion of the interindividual variation in the age at menopause. Epigenetic modifications such as DNA methylation of cytosine residues in CpG dinucleotides and histone modification might constitute an additional pathway leading to menopause onset and type 2 diabetes [33]. Future studies should explore epigenetic modifications related to menopause onset and whether epigenetic signatures can explain the association between age at natural menopause and risk of type 2 diabetes. Strengths of our study include its prospective design, the long follow-up and adequate adjustment for a broad range of confounders and possible intermediate risk factors for type 2 diabetes. Moreover, incident diabetes was diagnosed via standardised blood glucose measurements at the repeated study centre visits and electronic linkage with pharmacy dispensing records in the study area. However, several limitations need to be taken into account. One limitation is reliance on retrospective self-reporting of age at natural menopause, which is subject to faulty memory and reporting bias, particularly in older women. However, the results did no differ when we stratified by age at enrolment. Also, because the outcome (type 2 diabetes incidence) was assessed prospectively, the subjective measure of age at natural menopause would probably lead to non-differential misclassification with respect to the outcome, and would therefore bias estimates toward the null. Furthermore, previous reports indicate that the validity and reproducibility of selfreported age at menopause is fairly good [34]. In addition, mean age at natural menopause in the current study is similar to the mean age at natural menopause reported by other studies in the Netherlands and United States [35, 36]. However, despite the prospective design, we cannot rule out the possibility that the observed associations may partly reflect unmeasured residual confounding or that diabetes can lead to early onset of menopause, as suggested recently [37]. Survival bias may also exist, since women included in our study may represent survivors of early menopause who did not develop type 2 diabetes or died prior to the enrolment date. There is also a time interval between the start of menopause and enrolment into the Rotterdam Study. However, when we stratified participants by age at enrolment, we did not find any difference in results. Furthermore, if survival bias were present, then the true point estimate for the relationship between early menopause and type 2 diabetes might be larger than we observed. Furthermore, all confounding factors and mediators considered in the current investigation were assessed years after menopause and not at the start of menopause, and oestradiol was measured using an immunoassay with a detection limit of 18.35 pmol/l, which is considered suboptimal particularly in postmenopausal women. Therefore, our results should be interpreted with caution. Similarly, the analysis on the role of endogenous sex hormones should be interpreted with caution since the levels of sex hormones were measured at a later time point, and not at menopause onset. The current evidence for an association between age at menopause and postmenopausal levels of endogenous sex hormones is not persuasive. Finally, the Rotterdam Study mainly includes individuals of European ancestry (98%). Thus, our findings may not extend to non-white ethnicities. Early onset of natural menopause is an independent marker of type 2 diabetes risk in postmenopausal women. Future studies are needed to examine the mechanisms behind this association and explore whether the timing of natural menopause can add value to diabetes prediction and prevention.
Endogenous insulin resistance and hyperinsulinaemia in type 2 diabetes lead to stimulation of ovarian granulosa cells, increasing recruitment and growth of small follicles. Here, enlarged ovaries with numerous small growing follicles are present and account for the increased prevalence of polycystic ovarian morphology in type 2 diabetes. [38,39] Insulin resistance can also affect ovulation by preventing the recruitment of a large dominant follicle, creating anovulatory states and menstrual disturbances. [40,41] In type 2 diabetes, endogenous hyperinsulinaemia drives hyperandrogenism (Figure 1).
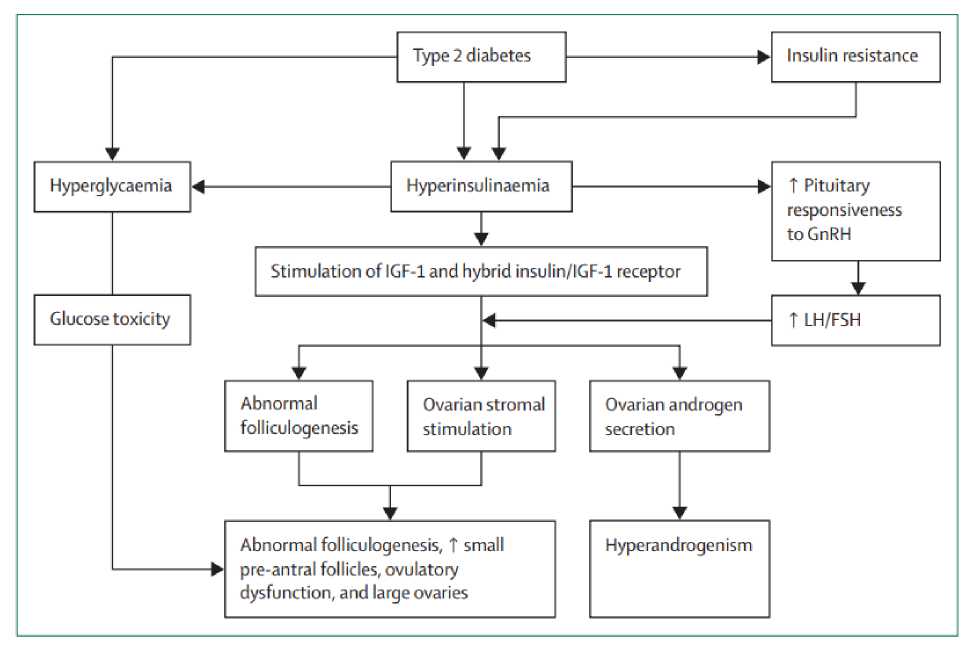
Figure 1. Mechanisms of interactions between type 2 diabetes and reproductive function GnRH=gonadotropin-releasing hormone. IGF-1=insulin-like growth factor 1. LH=luteinising hormone. FSH=follicle-stimulating hormone.
Deep down, metabolic disorders occurring in menopause, including dyslipidemia, disorders of carbohydrate metabolism (impaired glucose tolerance – IGT, type 2 diabetes mellitus – T2DM) or components of metabolic syndrome, constitute risk factors for cardiovascular disease in women. A key role could be played here by hyperinsulinemia, insulin resistance and visceral obesity, all contributing to dyslipidemia, oxidative stress, inflammation, alter coagulation and atherosclerosis observed during the menopausal period. Undiagnosed and untreated, metabolic disorders may adversely affect the length and quality of women’s life. Prevention and treatment preceded by early diagnosis should be the main goal for the physicians involved in menopausal care. This article represents a short review of the current knowledge concerning metabolic disorders (e.g. obesity, polycystic ovary syndrome or thyroid diseases) in menopause, including the role of a tailored menopausal hormone therapy (HT). According to current data, HT is not recommend as a preventive strategy for metabolic disorders in menopause. Nevertheless, as part of a comprehensive strategy to prevent chronic diseases after menopause, menopausal hormone therapy, particularly estrogen therapy may be considered (after balancing benefits/risks and excluding women with absolute contraindications to this therapy). Life-style modifications, with moderate physical activity and healthy diet at the forefront, should be still the first choice recommendation for all patients with menopausal metabolic abnormalities.
Conclusion: In conclusion, in this article we have discussed the metabolic processes that take place in the body in cases of diabetes and menopause. We have analyzed the opinions and conclusions of several scientists on this topic. We believe that this article can be an impetus for further in-depth research.
Список литературы Metabolic processes that occur due to menopause in women with type 2 diabetes
- Mauvais-Jarvis, F., Manson, J. E., Stevenson, J. C., & Fonseca, V. A. (2017). Menopausal Hormone Therapy and Type 2 Diabetes Prevention: Evidence, Mechanisms, and Clinical Implications. Endocrine Reviews, 38(3), 173–188. doi:10.1210/er.2016-1146
- Stuenkel CA, Davis SR, Gompel A, Lumsden MA, Murad MH, Pinkerton JV, Santen RJ 2015 Treatment of Symptoms of the Menopause: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 100:3975-4011
- Manson JE, Ames JM, Shapiro M, Gass ML, Shifren JL, Stuenkel CA, Pinkerton JV, Kaunitz AM, Pace DT, Kagan R, Schnatz PF, Kingsberg SA, Liu JH, Joffe H, Richard-Davis G, Goldstein SR, Schiff I, Utian WH 2015 Algorithm and mobile app for menopausal symptom management and hormonal/non-hormonal therapy decision making: a clinical decision-support tool from The North American Menopause Society. Menopause 22:247-253
- Goff DC, Jr., Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Jr., Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Tomaselli GF 2014 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 129:S49-73
- Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL, Anderson G, Howard BV, Thomson CA, LaCroix AZ, WactawskiWende J, Jackson RD, Limacher M, Margolis KL, Wassertheil-Smoller S, Beresford SA, Cauley JA, Eaton CB, Gass M, Hsia J, Johnson KC, Kooperberg C, Kuller LH, Lewis CE, Liu S, Martin LW, Ockene JK, O'Sullivan MJ, Powell LH, Simon MS, Van Horn L, Vitolins MZ, Wallace RB 2013 Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA 310:1353-1368
- Manson JE, Kaunitz AM 2016 Menopause Management--Getting Clinical Care Back on Track. N Engl J Med 374:803-806
- Salpeter SR, Walsh JM, Ormiston TM, Greyber E, Buckley NS, Salpeter EE 2006 Metaanalysis: effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes Metab 8:538-554
- Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, Newby LK, Pina IL, Roger VL, Shaw LJ, Zhao D, Beckie TM, Bushnell C, D'Armiento J, Kris-Etherton PM, Fang J, Ganiats TG, Gomes AS, Gracia CR, Haan CK, Jackson EA, Judelson DR, Kelepouris E, Lavie CJ, Moore A, Nussmeier NA, Ofili E, Oparil S, Ouyang P, Pinn VW, Sherif K, Smith SC, Jr., Sopko G, Chandra-Strobos N, Urbina EM, Vaccarino V, Wenger NK 2011 Effectiveness-based guidelines for the prevention of cardiovascular disease in women--2011 update: a guideline from the american heart association. Circulation 123:1243-1262
- Hofman A, Brusselle GG, Darwish Murad S et al (2015) The Rotterdam study: 2016 objectives and design update. Eur J Epidemiol 30:661–708
- Muka, T., Asllanaj, E., Avazverdi, N., Jaspers, L., Stringa, N., Milic, J., … Franco, O. H. (2017). Age at natural menopause and risk of type 2 diabetes: a prospective cohort study. Diabetologia, 60(10), 1951–1960. doi:10.1007/s00125-017-4346-8
- Ihara K, Fukano C, Ayabe T et al (2017) FUT2 non-secretor status is associated with type 1 diabetes susceptibility in Japanese children. Diabet Med 34:586–589
- Malacara JM, Huerta R, Rivera B, Esparza S, Fajardo ME (1997) Menopause in normal and uncomplicated NIDDM women: physical and emotional symptoms and hormone profile. Maturitas 28:35–45
- Di Donato P, Giulini NA, Bacchi Modena A et al (2005) Risk factors for type 2 diabetes in women attending menopause clinics in Italy: a cross-sectional study. Climacteric 8:287–293
- Luborsky JL, Meyer P, Sowers MF, Gold EB, Santoro N (2003) Premature menopause in a multi-ethnic population study of the menopause transition. Hum Reprod 18:199–206
- Brand JS, van der Schouw YT, Onland-Moret NC et al (2013) Age at menopause, reproductive life span, and type 2 diabetes risk: results from the EPIC-InterAct study. Diabetes Care 36:1012–1019
- Godsland IF (2005) Oestrogens and insulin secretion. Diabetologia 48:2213–2220 28.
- Stubbins RE, Najjar K, Holcomb VB, Hong J, Nunez NP (2012) Oestrogen alters adipocyte biology and protects female mice from adipocyte inflammation and insulin resistance. Diabetes Obes Metab 14:58–66
- Kanaya AM, Herrington D, Vittinghoff E et al (2003) Glycemic effects of postmenopausal hormone therapy: the Heart and Estrogen/progestin Replacement Study. A randomized, doubleblind, placebo-controlled trial. Ann Intern Med 138:1–9
- Margolis KL, Bonds DE, Rodabough RJ et al (2004) Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women’s Health Initiative Hormone Trial. Diabetologia 47:1175–1187
- Bonds DE, Lasser N, Qi L et al (2006) The effect of conjugated equine oestrogen on diabetes incidence: the Women’s Health Initiative randomised trial. Diabetologia 49:459–468
- Ding EL, Song Y, Manson JE, Rifai N, Buring JE, Liu S (2007) Plasma sex steroid hormones and risk of developing type 2 diabetes in women: a prospective study. Diabetologia 50:2076–2084
- Kalyani RR, Franco M, Dobs AS et al (2009) The association of endogenous sex hormones, adiposity, and insulin resistance with incident diabetes in postmenopausal women. J Clin Endocrinol Metab 94:4127–4135
- Golden SH, Dobs AS, Vaidya D et al (2007) Endogenous sex hormones and glucose tolerance status in postmenopausal women. J Clin Endocrinol Metab 92:1289–1295
- Goodman-Gruen D, Barrett-Connor E (2000) Sex differences in the association of endogenous sex hormone levels and glucose tolerance status in older men and women. Diabetes Care 23:912–918
- Livingstone C, Collison M (2002) Sex steroids and insulin resistance. Clin Sci (Lond) 102:151–166
- Lakshman R, Forouhi N, Luben R et al (2008) Association between age at menarche and risk of diabetes in adults: results from the EPIC-Norfolk cohort study. Diabetologia 51:781–786
- He C, Zhang C, Hunter DJ et al (2010) Age at menarche and risk of type 2 diabetes: results from 2 large prospective cohort studies. Am J Epidemiol 171:334–344
- Wang N, Kuang L, Han B et al (2016) Follicle-stimulating hormone associates with prediabetes and diabetes in postmenopausal women. Acta Diabetol 53:227–236
- Rocca WA, Shuster LT, Grossardt BR et al (2009) Long-term effects of bilateral oophorectomy on brain aging: unanswered questions from the Mayo Clinic Cohort Study of Oophorectomy and Aging. Womens Health (Lond) 5:39–48
- Taneri PE, Kiefte-de Jong JC, Bramer WM, Daan NM, Franco OH, Muka T (2016) Association of alcohol consumption with the onset of natural menopause: a systematic review and meta-analysis. Hum Reprod Update 22:516–528
- Jackson SP, Bartek J (2009) The DNA-damage response in human biology and disease. Nature 461:1071–1078
- Shimizu I, Yoshida Y, Suda M, Minamino T (2014) DNA damage response and metabolic disease. Cell Metab 20:967–977
- Muka T, Nano J, Voortman T et al (2016) The role of global and regional DNA methylation and histone modifications in glycemic traits and type 2 diabetes: a systematic review. Nutr Metab Cardiovasc Dis 26:553–566
- den Tonkelaar I (1997) Validity and reproducibility of self-reported age at menopause in women participating in the DOM-project. Maturitas 27:117–123
- Braem MG, Voorhuis M, van der Schouw YT et al (2013) Interactions between genetic variants in AMH and AMHR2 may modify age at natural menopause. PLoS One 8:e59819
- Hu FB, Grodstein F, Hennekens CH et al (1999) Age at natural menopause and risk of cardiovascular disease. Arch Intern Med 159:1061–1066
- Brand JS, Onland-Moret NC, Eijkemans MJ et al (2015) Diabetes and onset of natural menopause: results from the European Prospective Investigation into Cancer and Nutrition. Hum Reprod 30:1491–1498
- Willis D, Mason H, Gilling-Smith C, Franks S. Modulation by insulin of follicle-stimulating hormone and luteinizing hormone actions in human granulosa cells of normal and polycystic ovaries. J Clin Endocrinol Metab 1996; 81: 302–09
- Fulghesu AM, Villa P, Pavone V, et al. The impact of insulin secretion on the ovarian response to exogenous gonadotropins in polycystic ovary syndrome. J Clin Endocrinol Metab 1997; 82: 644–48
- Willis DS, Watson H, Mason HD, Galea R, Brincat M, Franks S. Premature response to luteinizing hormone of granulosa cells from anovulatory women with polycystic ovary syndrome: relevance to mechanism of anovulation. J Clin Endocrinol Metab 1998; 83: 3984–91
- Franks S, Mason H, White D, Willis D. Etiology of anovulation in polycystic ovary syndrome. Steroids 1998; 63: 306–07


