Metabolic profiling of bio-active components from Lantana camara, Parthenium hysterophorus, Ageratum conyzoidus and Ricinus communis by UV-VIS spectrum, FT-IR analysis and fluorescent spectroscopy
Автор: Dev Sharma Arun, Kaur Inderjeet
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 1 т.18, 2022 года.
Бесплатный доступ
Background: Lantana camara, Parthenium hysterophorus, Ageratum conyzoidus and Ricinus communis are underutilized plants. These plants are traditionally used in many health related areas. The context and purpose of the study: To investigate the photochemicals present in underutilized plants Lantana camara, Parthenium hysterophorus, Ageratum conyzoidus and Ricinus communis : Methods: Qualitative, quantitative screening, compound identification by UV-VIS method and Fluorescent spectroscopy and FT-IR based identification of functional groups of active chemical components were studied. Organic solvents leave extracts (ethanol, methanol, acetone) were tested for availability of secondary metabolites. Results, the main findings: UV-VIS spectrum showed different peaks with different absorption in all plants. The method of quantitative analysis in UV-VIS spectrum was proved in terms of linearity, LOD and LOQ AND various secondary metabolites were detected in the plant extracts. Florescent analysis confirmed the presence of secondary metabolites like alkaloids and phenolics. The FT-IR spectrum values indicated the presence of C-H stretching, O-H bending, C-C stretching and confirmed the presence of alcohols, phenols, alkanes and amines in acetone extracts. Conclusion: The results confirmed the fact that these plants poses important bioactive components useful for human mankind, so further investigation may needed.
Uv-vis, ftir, fluorescent, bioactive components
Короткий адрес: https://sciup.org/143178276
IDR: 143178276
Текст научной статьи Metabolic profiling of bio-active components from Lantana camara, Parthenium hysterophorus, Ageratum conyzoidus and Ricinus communis by UV-VIS spectrum, FT-IR analysis and fluorescent spectroscopy
Worldwide medicinal plants are most widely used as source of folk medicine. To cure various ailment plants as traditional medicine have been used all over the world (Newman, 016). Literature survey revealed that, despite great progress in synthetic chemistry and western medicine, plants are still backbone of primary healthcare. Worldwide underutilized plants are widely used as herbal medicine in villages (Moreira et al. , 014). So detailed investigation of these underutilized plants is need of the hour especially in developing and under developing counties, where primary healthcare strongly relay on traditional drugs. Aromatic or medicinal plants are most widely used due to nutrient and medicinal values and working as alternative to synthetic antioxidants or chemicals. Due to antioxidant properties the consumption of these plants is increasing day by day for development of novel and biodegradable effective drugs as alternative to contemporary medicine. Medicinal plants contribution to phytomedicine to the well being of world population, has attracted significant amount of interest from all disciplines. Plant based herbal formulations are healthier, safer and more reliable than synthetic medicines (Tlili et al , 010).
Medicinal plants have been used as important source of therapeutic drug molecules as they poses secondary metabolites which are potential source of drugs. The major bio-active components are secondary metabolites produced by these plants such as alkaloids, flavonoids, phenols, saponins, generally produced by plants as defense mechanism have been implicated as therapeutics drug molecules in medicinal plants (Gao et al 01 ). These components are reported to suppress redox reactions of free radicals in biological system. Herbal agents are mixture plant parts and their formulations are vital with respect to medicinal, nutrient and antioxidant values (Bajalan et al , 017).
Lantana camara a member of Verbenaceae family, native of tropical America, has been used as traditional herbal medicines for number of treatments like: cancer , skin itches, leprosy, rabies, chicken pox, measles, asthma and ulcers Sharma, 2 7) . Parthenium hysterophorus a species of flowering plant in the aster family, Asteraceae, is a noxious weed native to America,
Asia, Africa and Australia (Oudhia, 000). It confers many health benefits, viz remedy for skin inflammation, rheumatic pain, diarrhoea, urinary tract infections, dysentery, malaria and neuralgia. A. conyzoides (Asteraceae), commonly called Billygoat weed, Goat weed etc., native to tropical America, is small, aromatic, annual herb (Moreira et al , 014, Panda et al , 018). In alternative medicine, ageratum is for mnay ailments like epilepsy and wounds, including common colds, headaches, boils, eczema, bleeding wounds, and burns. R. communis is an annual or perennial member of family Euphorbiaceae Newman and Cragg, 016) It has been used as a therapeutic agent long many years used as an herbal medicine for treating many different diseases, disorders and also many infections. Although these plants are widely used as traditional medicine, few studies have been conducted of the pharmacological values of the plants. Hence, in the present study the bioactive constituents in underutilized plants Lantana camara, Parthenium hysterophorus, Ageratum conyzoidus and Ricinus communis evaluated by using UV-spectroscopy, Fluorescent spectroscopy and FT-IR analysis which may provide its use in traditional medicine.
MATERIALS AND METHODS
Sampling Site and site description
The samples of Lantana camara, Parthenium hysterophorus, Ageratum conyzoidus and Ricinus communis (Fig. 1) were collected growing under the natural conditions, Defence Colony, Jalandhar, located at 710 - 310 east latitude and 300 -330 north longitude. The city has a humid subtropical climate with cool winters and long, hot summers. The climate is dry on the whole. The experiments were conducted on February 0 0.
Preparation of plant extracts
Five gram of leaves of Lantana camara, Parthenium hysterophorus, Ageratum conyzoidus and Ricinus communis were homogenized in 5ml of each of each solvent like acetone, ethanol and methanol. The crude extracts were centrifuged at 10,000rpm for 15 min at room temperature. The supernatant was collected. The supernatants were removed through 0. µm (PALL Life
Sciences) syringe filters prior to analysis for UV, FT-IR and fluorescent techniques.
UV – fingerprint analysis
UV-spectrophotometric analysis was conducted on extracts of both plants using UV-VIS spectrophotometer (Labtronics) with slit width of nm, using a 10-mm cell at room temperature. The acetone, methanol and ethanol exacts were examined in the wavelength ranging from 00-400 nm. For UV profiling, the extract were centrifuged and filtered as explained previously and diluted 1: with same solvent. The peak values of the UV-VIS were recorded.
FT-IR analysis and Fluorescence spectroscopy analysis
FT-IR was used to identify functional groups. A small amount of extract was taken in the sample cup of a diffuse reflectance accessory. IR spectrum was obtained using FT-IR infrared spectrophotometer (Perkin Elmer, USA spectrophotometer). The sample was scanned from 4000 to 400 cm-1. The peak values of the FTIR were recorded. The fluorescence spectrum of each sample was measured on Perkin Elmer Spectrophotometer (FL6500). All experiments were done at room temperature (~30ºC).
Identification and Quantitation of fingerprints
The eight chemical standards - Rutin (in methanol), Ascorbic acid (in distilled water), Glutathione Reduced (in distilled water), Glutathione Oxidized (in distilled water), Tannic acid (in ethanol), Glycine Betaine (in ethanol), Proline (in ethanol) and Vanillic Acid (in methanol),were prepared in appropriate solvents with concentrations ranging from 5- 5 mg per ml. The calibration curve for each compound was established by plotting the peak area (y) versus the concentration (x) of each analyte and were fitted to a linear function of type y = ax + b. The limit of detection (LOD) and limit of quantification (LOQ) for each analyte were calculated with the corresponding standard solution on the basis of signal – to noise ratio (S/N) of 3 and 10, respectively. All the solutions were filtered through 0. µm filters prior to analysis. The UV-fingerprints showed abundant diversity of chemical constituents which were analysed by qualitation and quantitation in tissue extracted in acetone. So identification of eight different analytes viz. Rutin, Ascorbic acid, Glutathione Reduced, Glutathione Oxidized, Tannic Acid, Glycine Betaine, Proline and Vanillic acid was conducted manually by comparing their peaks with those of the extracts in acetone. Quantitation was established by preparing calibration curves with pure analytes. The concentrations of identified analytes in different samples were calculated according to the regression parameters derived from the standard curve (Table 1).
RESULTS AND DISCUSSION
Medicinal plants contain numerous bioactive photochemical constitutes which are responsible for diverse pharmacological activities Bajalan et al , 017). The pharmacological action of crude therapeutic agents are largely depend on the active bio-active constituents such as flavonoids, tannins, alkaloids several aromatic compounds and other secondary metabolites of plants which are primarily used as defense mechanism against many biotic and abiotic stresses (Pietra, 00 ). Due to safety concerns, some of these secondary metabolites of plants are important source of natural antioxidants that are preferred over synthetic drugs. The bio active metabolites have been shown to reduce the risk of many diseases by scavenging free radicals through various biological mechanisms. In the present study phytochemical analysis and chemical fingerprinting of underutilized plants Lantana camara, Parthenium hysterophorus, Ageratum conyzoidus and Ricinus communis was conducted by using UV-spectroscopy, Florescent spectroscopy and FT-IR techniques.
Fingerprint analysis
Spectroscopic techniques have become important analytic tools for qualitative and quantitative analysis of bio active components from plants. Secondary metabolites fingerprint characterization by spectroscopy provides valuable insights about qualitative and quantitative formulation of plant species. UV-VIS, fluorescent and FT-IR spectroscopy methods together or separate can be used in this sense as conventional methods (Hashimoto and Kameoka, 008). UV-spectroscopy is simple, cost effective and rapid tests for detecting phytocomponents. Therefore, in the present study, UV-VIS and FT-IR techniques are employed to evaluate the UV- and IR fingerprint in different solvents extracts of Lantana camara, Parthenium hysterophorus, Ageratum conyzoidus and Ricinus communis.
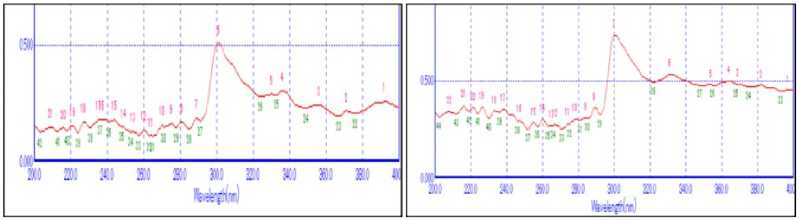
The UV- spectra was performed to indentify the Bioactive compounds containing π-bonds , σ-bonds and lone pair of electrons, chromrophores and aromatic rings. Success of any herbal drug largely depends on type of solvent used in the extraction. The present phytochemical study using UV-spectropscopy on Lantana camara, Parthenium hysterophorus, Ageratum conyzoidus and Ricinus communis using different solvent extracts revealed the presence of secondary metabolites. UV spectroscopy analysis of leaves of Lantana camara, Parthenium hysterophorus, Ageratum conyzoidus and Ricinus communis were studied in three different solvents: Acetone, Ethanol and Methanol. All the solvents showed the presence of secondary metabolites (Fig -4). Among all solvents maximum peaks (major and minor) were observed in acetone in all the plants (Table , Fig. ). Earlier studies have documented that solubility of secondary metabolites is governed by type of solvent used and nature of components and their interaction. Medini et al,.( 014) also documented that recovery of metabolites form plant materials is influenced by their solubility in the extraction solvent, the type of solvent (polarity) used, the degree of polymerization of metabolites and their interaction.
The qualitative UV Spectroscopy profile of acetone extracts of Lantana camara, Parthenium hysterophorus, Ageratum conyzoidus and Ricinus communis revealed different sharp peaks from 00-400 nm with absorbance of 0.8- .9, indicating the accumulation of secondary metabolites. In UV-profile, occurrence of peaks in the region from 00-400 nm is clear indication of the presence of unsaturated groups and heteroatoms such as S, N, O (Parekh and Chanda, 007). Occurrence of peaks at 00-400 nm reveals the presents of phenolic acid, flavonol and alkaloids in the Lantana camara, Parthenium hysterophorus, Ageratum conyzoidus and Ricinus communis extracts plant leaf. On comparison of the spectra of leaves with literature value, shows that the extract has some similar phenolics alkaloid, flavonoids, and flavonol compounds as reported in by Paulraj et al. , 011, Michalak, 006, , Niara et al , 016.
Further, it was observed that the pattern of fingerprints were different in all the plants. It was cited that genetic, environmental, age and geographical location often are the factors that influence the synthesis of secondary metabolites where the plants are grown and harvested thus causing variation in fingerprints. The spectrum of all the plants showed peak at about 300 nm. This confirms the presence of organic chromospheres in all the extracts acetone extracts. All plants showed some common peaks. It is quite possible, as secondary metabolites refer to generic term used for more than 00,000, different substances that are exclusively found in plants and spectral fingerprints are sum of spectra of every component present in the extracts (Talamond et al. , 015). Plant produce an array of metabolites in response to interaction with environment.
In addition to UV-VIS, florescent spectroscopy is more reliable and sensitive method for detection of bio molecules. Auto-fluorescent molecules are abundant in plant cells and their spectral images often used to analyze their spectra which give information about their accumulation and function under environmental conditions. Secondary metabolites and proteins are exclusively modulated in plants in response to interaction with environment (Mylle et al., 013). However, yielding information about their fluorescent based phytochemical analysis in Lantana camara, Parthenium hysterophorus, Ageratum conyzoidus and Ricinus communis is still not known. Since among all solvents maximum peaks were detected in acetone so acetone containing extracts were further examined by fluorescent and FT-IR spectroscopy methods. Acetone extracts having spectral signatures of Lantana camara, Parthenium hysterophorus, Ageratum conyzoidus and Ricinus communis are illustrated in Fig 5, indicating the extracts showed the presence of fluorescent metabolites. Different metabolites when excited with radiation of suitable wavelength, molecules widely distributed in tissue like proteins, coenzymes, flavonoids, phenolics, alkaloids, chlorophylls, cell wall component etc behave as endogenous flurophores. Lantana camara, Parthenium hysterophorus, Ageratum conyzoidus and Ricinus communis leaves extract when excited at wavelength 300 nm, a peculiar spectral fingerprints were detected. Both Major and minor peaks with different fluorescence quantum yields were detected in all the plants (Table 3) which was attributed to the presence of secondary metabolites like phenolics or alkaloids. The relative pattern of this fluorescence quantum yields is highly sensitive and species-specific and depends on environmental factors. For instance: when Lantana camara, Parthenium hysterophorus, Ageratum conyzoidus and Ricinus communis leave acetone and ethanol extracts were excited at wavelength e: 300, a peculiar spectral fingerprint was detected in far red fluorescent region (FRF) region. It was reported that candidates for FRF emission (lambda near 800 nm) are, anthocyanins, pterins, chorogenic acid, caffeic acid, coumarins ( aesculetin, scopoletin) and stilbenes (t-stilbene, rhaponticin) (Mylle et al., 013). Earlier studies documented that some alkaloids and anthocyanins emit fluorescence far-red region of visible spectra (Talamond et al, 015). However, in methanol extracts, fingerprints were detected in green florescent region (GFR) in all the plants, which may be attributed to the presence of flavonoids, terpenoids and flavins like FAD, FMN. In Lantana only, fingerprints were observed in Blue florescent region BFR), which may be due to accumulation of phenols and alkaloids. Cellular structures are believed to be protected from harmful effect of abiotic stresses by accumulating fluorescent secondary metabolites like terpenoids, carotenoids, phenolics which are important in pants for their normal growth and development (Lang et al., 1991) The fluorescence-emission spectrum in a particular plant can be considered as molecular signature that reflects environmental (biotic or abiotic) effects and can reveal the significance of secondary metabolites.
Fourier Transform Infrared Spectrophotometer (FT-IR) is perhaps most powerful, rapid, non destructive method to fingerprint plant extracts or powders and for detecting types of bonds and functional groups present in the extracts (Karpagasundari and Kulothungan, 016). By interpreting the IR absorption spectrum, the chemical bonds in a compound can be determined. FT-IR peak values and functional groups are tabulated in Table 4. The FT-IR profile of acetone extracts is illustrated in Fig 6. All extracts showed the presence of bio active compounds in all plants. In FTIR spectrum gave a broad peak at 3406 cm-1 which indicated the presence of O-H stretching due to alcohols. It showed peaks at about 1700 and 1600 cm-1 attributed to C-H bending and C-C stretching due to alkanes ands alkenes. The peak around 1400 and 1300 were due to aromatic and phenols. FT-IR spectrum confirmed the presence of aromatic compounds, alcohols, phenols, alkanes, alkynes, and amines in plant extracts (Liu et al.,
006). All these compounds belong to the secondary plant metabolites as per researcher explanations (Paulraj et al. , 011). The presence of above said secondary metabolites could be reason for its various medicinal properties of Lantana camara, Parthenium hysterophorus, Ageratum conyzoidus and Ricinus communis.
Identification and quantitative expressions of fingerprints
The UV-VIS spectroscopy fingerprints of all plants showed abundant diversity of bioactive constituents which were analyzed by qualitation and quantitation in leaves extracted in acetone. The method of quantitative analysis was proved in terms of linearity, LOD and LOQ. Linear regression analysis for each compound was performed by plotting the absorbance (y) against the concentrations(x, mg/ml) of the each analyte which was expressed by the equation given in the Table 5. Satisfactory calibration curve with good linearity were obtained with correlation coefficient more than 0.75 and sufficient sensitivity in terms of for all the analytes. The UV spectrum of acetone extracts showed prominent peaks including one major at 300 nm and various minor peaks, indicating the presence of major compounds. The small peaks may be attributed to the bio active compounds present in less concentration as well as disintegrated major compounds. In our work, the attempt to identification of eight standards like Rutin, Ascorbic acid, Glutathione Reduced, Glutathione Oxidized, Tannic Acid, Glycine Betaine, Proline and Vanillic acid was performed to confirm their prensce in studies acetone extracts of Lantana camara, Parthenium hysterophorus, Ageratum conyzoidus and Ricinus communis. The presence of all analytes were observed in Lantana camara and Ageratum conyzoidus. However in Parthenium hysterophorus and Ricinus communis 7 analytes were detected. Among all the analytes content of tannic acid was higher in all the plants followed by vanillic acid. Tannic acid and vanillic aid are polyphenolic compounds widely used in food industry as flavors, preservative and food additive properties. Polyphenolics are broadly distributed in the plant kingdom and are the most abundant secondary metabolites of plants (Hayat et al., 017). Due to potent antioxidant properties phenolics have drawn increasing attention due to their marked effects in the prevention of various oxidative stress associated diseases. Therefore, in the past few years, the identification of phenolic based derivatives from different plants extracts has become a major area of health- and medical-related research (Dai et al, 010). Plant phenolics are generally involved in defense against ultraviolet radiation or aggression by pathogens, parasites and predators, as well as contributing to plants colors. Tannic acids are polyphenolic compounds produced in plant parts like: leaves, barks, roots, fruits etc. From these basic structures, plants have the capability to synthesize many derivatives with diverse functions, including defense against herbivores and pathogens (Lin et al, 013). The accumulation of phenolics was considered as an adaptation mechanism to water or salinity stress. Hassan et al., 014 conducted a study on invasive plant Nicotiana glauca both qualitative and quantitative tests and revealed the presence of phenolic compounds. Vanillic acid is a benzoic acid derivative used as a flavoring agent. It is an oxidized form of vanillin produced during the conversion of vanillin to ferulic acid . Various studies documented the effectiveness of vanillic acid in the management of immune or inflammatory responses. For instance, vanillic acid enhanced the activity of human lymphocyte proliferation and secretion of interferon-gamma in human peripheral blood mononuclear cells (Chiang et al., 013). Another study has shown that vanillic acid has a hepatoprotective effect through its suppressive action on immune-mediated liver inflammation in concanavalin A-induced liver injury (Itoh et al., 009).
Table. 1. Linearity, LODs and LOQs for different analytes
Table 2. Total number of peaks detected three different solvents in UV Spectra
|
SNO. |
Plants |
TOTAL NUMBER OF DETECTED PEAKS |
||
|
Acetone |
Ethanol |
Methanol |
||
|
Leaves |
||||
|
1 |
LC |
4 |
14 |
3 |
|
PH |
44 |
8 |
1 |
|
|
3 |
AC |
37 |
7 |
1 |
|
4 |
RC |
53 |
11 |
11 |
Symbols used: A =Ageratum conyzoidus
RC = Ricinus Communis
LC: Lantana Camara
PH: Parthenium hysterosporus
Table 3. Fluorescent Spectroscopy analysis of leave extracts
|
S. No. |
Plant/extract |
λ emission |
Fluorescence Quantum Yields (intensity) |
||
|
λ Max |
λ Min |
Major |
Minor |
||
|
Acetone |
|||||
|
1. |
LC |
731 |
803 |
4484 |
50 |
|
. |
PH |
7 8 |
803 |
5788 |
155 |
|
3 |
AC |
7 8 |
800 |
700 |
150 |
|
4 |
RC |
7 8 |
779 793 |
177 |
84 13 |
LC: Lantana camara, PH: Parthenium hysteropsorus, AC: Ageratum conyzoidus RC: Ricinus communis
Table 4: WAVE NUMBER (cm-1) of dominant peak obtained from FT-IR spectra
|
Functional group |
Appearance (intensity) |
Compound |
Frequency Range |
Lantana |
Ricinus |
Parthenium |
Ageratum |
|
O-H stretch |
Strong, broad |
Alcohol |
3550-3 00 |
3416.37 |
3406.77 |
3415.99 |
3406.77 |
|
C-H stretch |
Medium |
Alkane |
3000- 840 |
||||
|
C-H bending |
Weak |
Aromatic compound |
000-1650 |
1705.08 |
170 .15 |
170 .6 |
170 .15 |
|
C=C stretch |
Strong |
Alkene |
1648-1638 |
||||
|
C=C stretch |
Medium |
Alkene |
166 -16 6 |
1635.05 |
1635.1 |
1635.05 |
|
|
C=C stretch |
Strong |
α, β ketone |
16 0-1610 |
||||
|
N-O asymmete |
Strong |
Nitro compound |
1550-1475 |
||||
|
C-C stretch |
Medium |
Aromatic |
1500-1475 |
14 1.40 |
14 1.40 |
||
|
O-H Bending |
Medium |
Phenol |
1390-1310 |
1361.97 |
1363. 8 |
136 .37 |
1363. 8 |
|
C-N stretch |
Medium |
Aliphatic amines |
1 50-10 0 |
1 46.61 1 4.36 |
1091.83 , 1 9.86 |
1 8.94 |
1 9.86 |
|
S=O stretch |
Strong |
Sulfoxide |
1070-1030 |
1091.83 |
|||
|
C-H Bending |
Strong |
1, ,3-tridistributed |
780± 0 |
783.51, 756.86, 716.44 |
778. |
779.54 714.51 |
90 .46 778. 714.38 |
|
C-Br stretch |
Strong |
Halo compound |
690-515 |
681.78 |
|||
|
C-I stretch |
Strong |
Halo compound |
600-500 |
Table 5: Content of analyzed compounds in different plants
|
SNo. |
Content of analysed compounds (mg/g DW) |
||||||||
|
Rutin |
Ascorbic acid |
Glutathione Reduced |
Glutathione Oxidized |
Tannic Acid |
GlycineB etaine |
Proline |
Vanillic acid |
||
|
1 |
L |
0.9 |
13.17 |
1 .80 |
11.9 |
29.33 |
10.01 |
6.36 |
0.0 |
|
P |
8.3 |
19.67 |
9.74 |
13.95 |
28.23 |
16.86 |
- |
1.7 |
|
|
3 |
A |
7. |
18.3 |
10.01 |
14. 1 |
28 .36 |
11.1 |
5. 3 |
. 3 |
|
4 |
R |
3. |
8. 3 |
9.3 |
8. 3 |
27.32 |
1 . 1 |
- |
3. 1 |
Symbols used – L = Lantana, P = Parthenium, A= Ageratum, R: Ricinus, (-) = Not detected

Lanatna camara
Parthenium hysterophorus

Age rat и m conyzoides
Ricinus communis
Figure 1 Pictorial representation of studied plants
A. LANTANA
В. PARTHENIUM
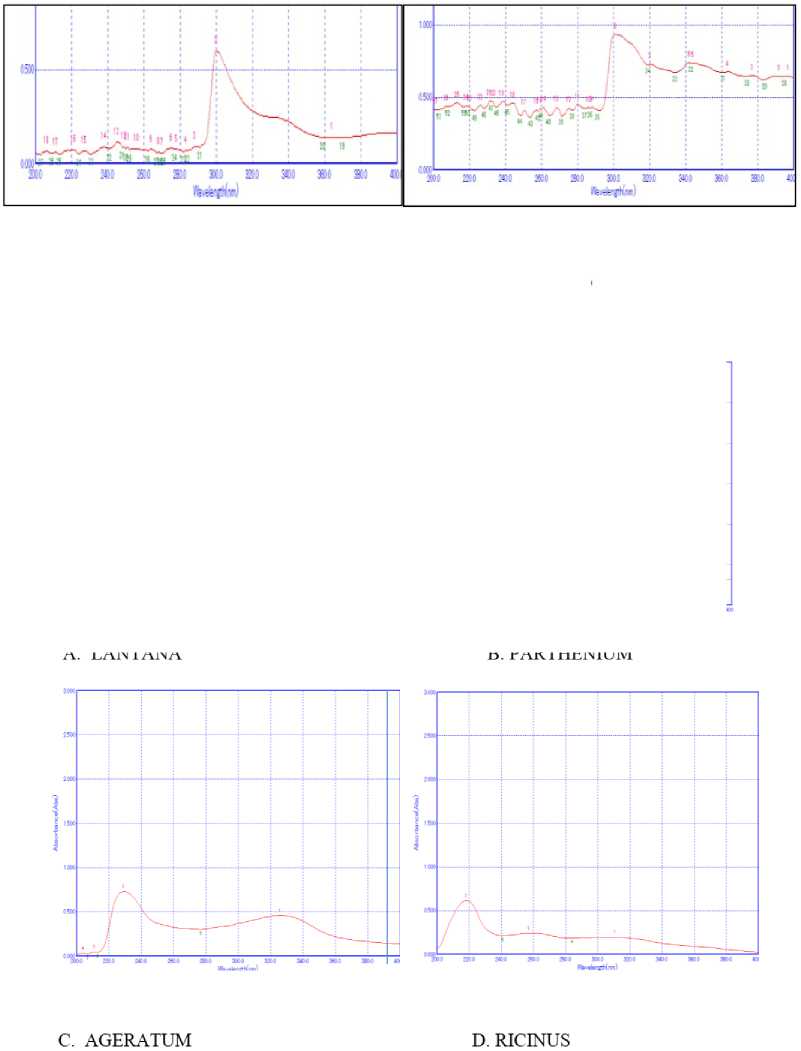
Figure 3 UV Fingerprinting Analysis of four leaves (A, B, C, D) extracted in ethanol
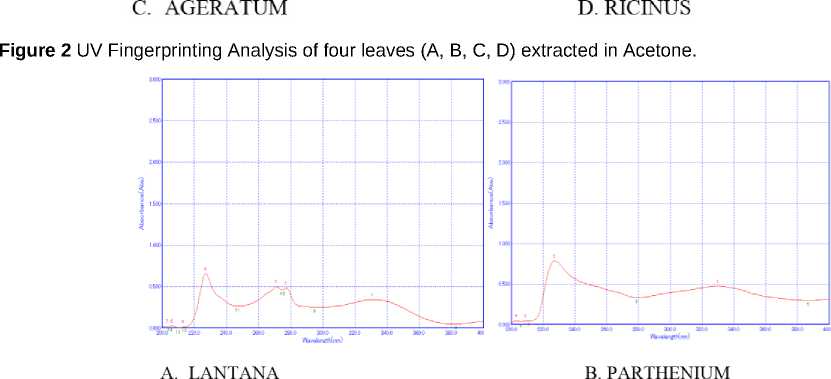
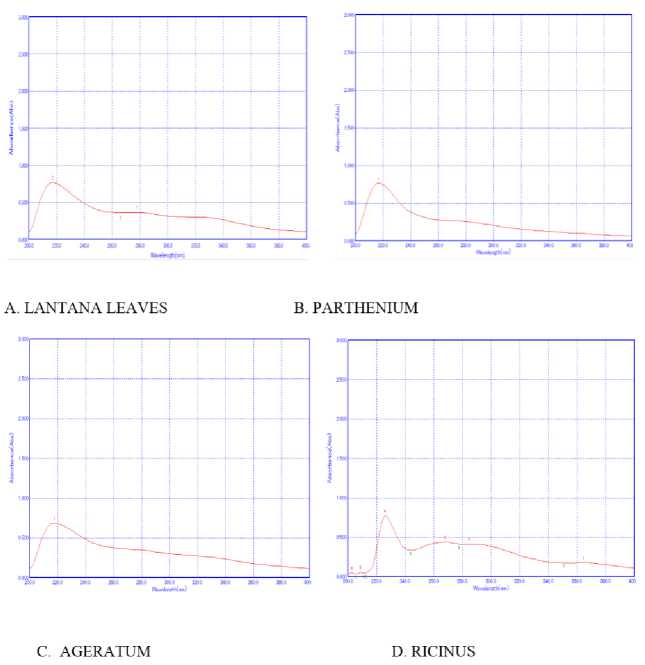
Figure 4 Fingerprinting Analysis of four leaves (A, B, C, D) extracted in methanol
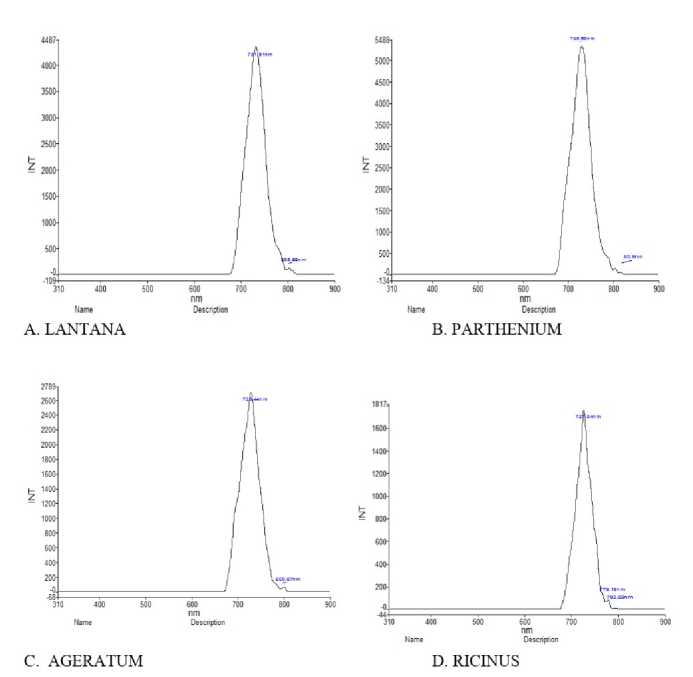
Figure 5 Fluorescence spectroscopy Analysis of plants extracted in acetone
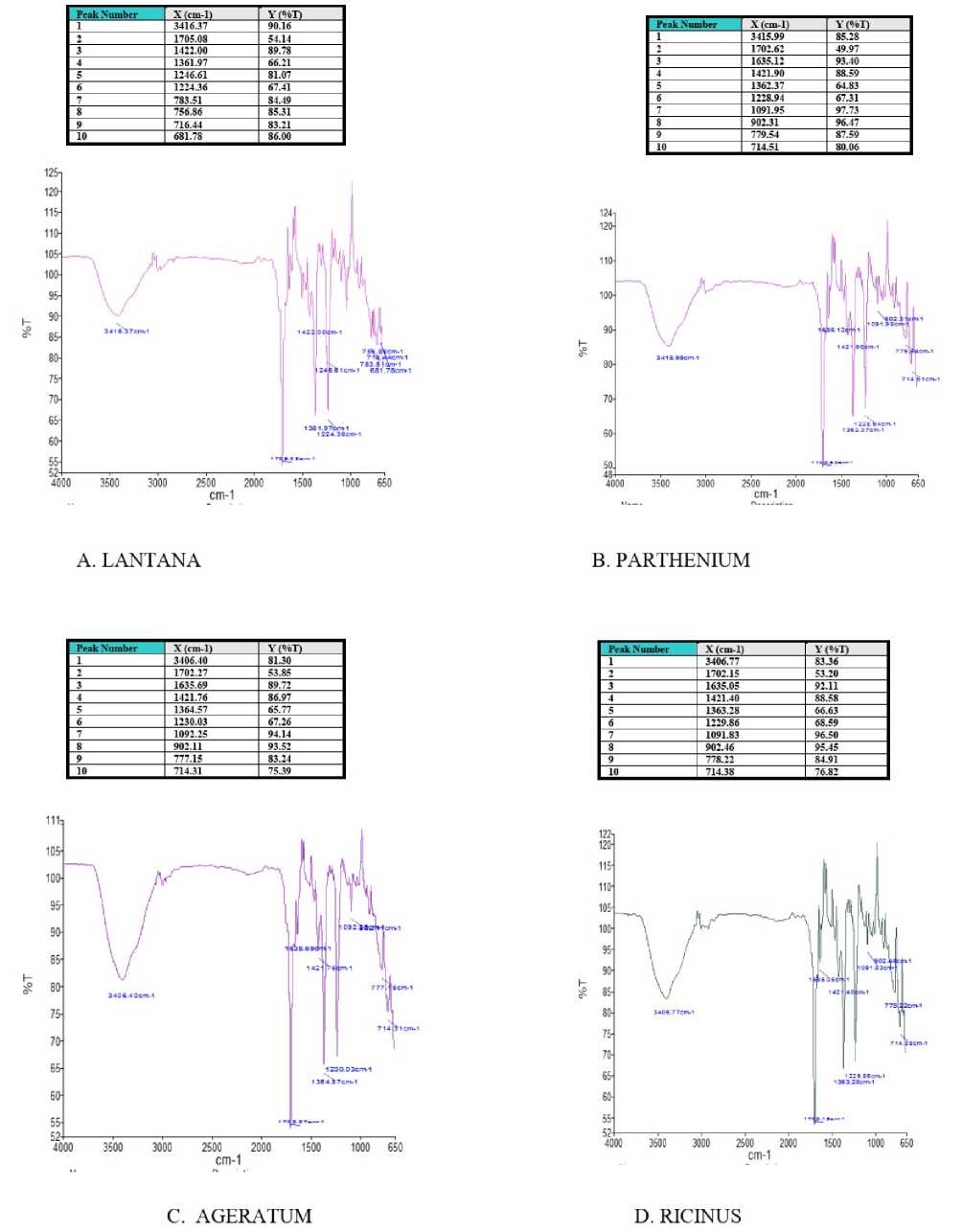
Figure 5 Fluorescence spectroscopy Analysis of plants extracted in acetone
CONCLUSION
In order to asses the bioactive constituents from underutilized plants Lantana camara, Parthenium hysterophorus, Ageratum conyzoidus and Ricinus communis , UV-VIS, FT-IR and Flurescent techniques to realize a detailed profile of the secondary metabolites. The spectrophotometric techniques showed the presence of various secondary metabolites like phenolics which can be isolated and further screened for different kind of biological activities as per their therapeutic use. The presence of bio active compounds in these plants lends credence to its use by the human community. It also hold for production of novel drug molecules, so recommended as a plants of phytophamceutical importance. This investigation has opened new avenues for possible use of these plants in drug development and treatment of many ailments. Hover, further studies may be undertaken to asses full bioactivity, toxicity profile and effect on ecosystem.
CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest.
Список литературы Metabolic profiling of bio-active components from Lantana camara, Parthenium hysterophorus, Ageratum conyzoidus and Ricinus communis by UV-VIS spectrum, FT-IR analysis and fluorescent spectroscopy
- Chiang L.C., Ng L.T., Chiang W., Chang M.Y., Lin C.C. (2003). Immunomodulatory activities of flavonoids, monoterpenoids, triterpenoids, iridoid glycosides and phenolic compounds of Plantago species. Planta Med. 69: 600-604
- Dai J, Mumper R.J. (2010). Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules, 15: 7313-7352
- Gao QH, Wu CS, Yu JG, Wang M, Ma YJ, Li CL. (2012). Textural characteristic, antioxidant activity, sugar, organic acid, and phenolic profiles of 10 promising jujube (Ziziphus jujuba Mill.) selections. J. Food Sci. 77: 1218–1225.
- Hashimoto A, Kameoka T. (2008). Applications of infrared spectroscopy to biochemical, food, and agricultural processes. Applied Spectroscopy Reviews, 43:416-51.
- Hayat M, Abbas M, Munir F, Hayat M Q, Keyani R, Amir R. (2017). Potential of plant flavonoids in pharmaceutical and nutraceutics. BiomolBiochem. 1: 12-17
- Iman Bajalan, Masoumeh Zand, Masoud Goodarzi, Mahin Darabi. (2017). Antioxidant activity and total phenolic and flavonoid content of the extract and chemical composition of the essential oil of Eremostachys laciniata collected from Zagros. Asian Pacific Journal of Tropical Biomedicine 7: 144-146
- Itoh A., Isoda K., Kondoh M., Kawase M., Kobayashi M., Tamesada M. Yagi K. (2009). Hepatoprotective effect of syringic acid and vanillic acid on concanavalin a-induced liver injury. Biol. Pharm. Bull. 32: 1215-1219
- Karpagasundari C, Kulothungan S. (2014). Analysis of bioactive compounds in physalis minima leaves using GC MS, HPLC, UV-VIS and FTIR techniques. Journal of Pharmacognosy and Phytochemistry. 3(2): 196e-201.
- Lang M, Stober F, Lichtenthaler HK. (1991). Fluorescence emission spectra of plant leaves and plant constituents Radiat. Environ. Biophys., 30: 333-47
- Lin C.K, Penesyan A, Hassan K.A, Loper J.E, Paulsen I.T (2013). Effect of Tannic Acid on the Transcriptome of the Soil Bacterium Pseudomonas protegens Pf-5. Applied & Environmental Microbiology 79: 3141-3145
- Liu H-X, Sun S-Q, LvG-H, Chan KKC. (2006). Study on Angelica and it’s a different extracts by Fourier Transform Infrared Spectroscopy and Two dimensional correlation. IR Spectroscopy. Spectrochimica acta. Part A, Molecular and Biomolecular Spectroscopy. 64(2): 321-e6.
- Medini F, Fellah H, Ksouri R, Abdelly C. (2008). Total phenolic, flavonoid and tannin contents and antioxidant and antimicrobial activities of organic extracts of shoots of the plant Limoniumdelicatulum. Journal of Taibah University for Science. 8: 216-224
- Michalak A. (2006). Phenolic Compounds and Their Antioxidant Activity in Plants Growing under Heavy Metal Stress. Polish J of Environ Stud 15: 523-530.
- Moreira DL, Teixeira SS, Monteiro MHD, De-Oliveira ACAX, Paumgartten FJR. (2014). Traditional use and safety of herbal medicines. Rev Bras Farmacogn . 24(2): 248-257.
- Mylle E, Codreanu MC, Boruc J, Russinova E. (2013), Emission spectra profiling of fluorescent proteins in living plant cells. Plant Methods. 9:1–8.
- Niara Moura P, Lima de Barrosb Y, Ionaldo Diniz Basílio JL, Maria de Fátima A. (2016), Microscopic and UV/Vis spectrophotometric characterization of Cissampelos pareira of Brazil and Africa. Revista Brasileira de Farmacognosia. 26: 135-146.
- Newman DJ, Cragg GM. (2016). Natural products as sources of new drugs from 1981 to 2014. J Nat Prod 79(3): 629-661
- Oudhia, P. (2000). Allelopathic effects of Parthenium hysterophorus and Ageratum conyzoides on wheat var.Sujata". Crop Research. 20 (3): 563–566.
- Panda SK, Luyten W. (2018), Antiparasitic activity in Asteraceae with special attention to ethnobotanical use by the tribes of Odisha, India. Parasite. 25: 10.
- Parekh J, Chanda V. (2007); In vitro Antimicrobial activity and Phytochemical analysis of some Indian medicinal plants. Turkish Journal of Biology. 31: 53‐58.
- Paulraj K, Irudayaraj V, Johnson M, Patric D. (2011). Phytochemical and anti-bacterial activity of epidermal glands extract of Christella parasitica (L.) H. Lev. Asian Pac. J. Trop Biomed. 1: 8-11.
- Pietra F. (2002). Biodiversity and natural product diversity (The role of biotechnology). Tetra. Org. Chem. Ser. 21: 205–213
- Sharma O. P. (2007). A review of the hepatotoxic plant Lantana camara". Critical Reviews in Toxicology. 37 (4): 313–352.
- Talamond, P. Verdeil JL, Conejero G. (2015). Secondary metabolite localization by auto-fluorescence in living plant cells. Molecules, 20, 5024-5037.
- Tlili N, Khaldi A, Triki S, Munné-Bosch S. (2010). Phenolic compounds and vitamin antioxidants of caper (Capparis spinosa). Plant Foods Hum. Nutr. 65: 260–265.


