Метаболическая регуляция функций натуральных киллеров
Автор: Орлова Екатерина Григорьевна
Журнал: Вестник Пермского университета. Серия: Биология @vestnik-psu-bio
Рубрика: Иммунология
Статья в выпуске: 1, 2023 года.
Бесплатный доступ
Согласно современным представлениям, трансформация фенотипа и функций натуральных киллеров (NK-клеток) ассоциирована с метаболическим репрограммированием, а именно преимущественным использованием клеткой специфических метаболических путей для получения энергии. Ключевыми молекулами, участвующими в контроле метаболических программ NK-клеток, являются мишень для рапамицина в клетках млекопитающих (mTOR) и аденозинмонофосфат-активируемая протеинкиназа (AMPK). Модуляция активности mTOR и AMPK различными агентами определяет метаболическое репрограммирование, изменение фенотипа NK-клеток и функциональной активности. Особенности метаболической регуляции эффекторных функций NK-клеток зависят от их локализации, степени зрелости, продолжительности и специфичности активационных сигналов. Особое внимание уделяется изменению фенотипа, функциональной и метаболической активности NK-клеток при беременности, что ассоциировано с формированием периферической иммунной толерантности. Изучение метаболической регуляции функциональной активности NK-клеток необходимо для повышения эффективности NK-клеточной терапии.
Nk-клетки, метаболизм, децидуальный фенотип, иммунная толерантность, беременность
Короткий адрес: https://sciup.org/147240457
IDR: 147240457 | УДК: 632.939:612.112.94:612.63 | DOI: 10.17072/1994-9952-2023-1-83-94
Текст научной статьи Метаболическая регуляция функций натуральных киллеров
NK-клетки относятся к группе ILC (Innate Lymphoid Cells) и не имеют специфических антиген-распознающих рецепторов, гены которых подвергаются реаранжировке в процессе дифференцировки клеток, как Т- и В-лимфоциты (TCR и BCR). NK-клетки обладают широким спектром идентичных по структуре рецепторов, распознающих вирус-инфицированные, опухолевые, поврежденные клетки собственного организма и чужеродные клетки, и лизируют их путем контактного взаимодействия (Fas-Fas-L, PD-PD-L) и индукции апоптоза, но также и секретируя гранулы, содержащие литические продукты (перфорин, гранзимы) [Erlebacher et al., 2013]. Большая часть (> 90%) NK-клеток периферической крови имеют зрелый фенотип CD16+CD56dim и характеризуются высоким цитотоксическим потенциалом [Erlebacher et al., 2013]. Молекула CD56 (адгезивная молекула нервных клеток, NCAM) является одним из основных маркеров для NK-клеток и необходима для их миграции во вторичные лимфоидные органы [Erlebacher et al., 2013]. Молекула CD16 участвует в антителозависимой клеточной цитотоксичности, поскольку является рецептором для Fc-фрагмента IgG. NK-клетки распознают сенсибилизированные IgG клетки-мишени и осуществляют их цитолиз вышеописанными способами [Erlebacher et al., 2013].
Около 10% от общего числа NK-клеток периферической крови имеют менее зрелый регуляторный фенотип CD16dim/-CD56bright и характеризуются способностью к массивной продукции цитокинов в ответ на активацию. Эта субпопуляция преобладает во вторичных лимфоидных органах. CD16dim/-CD56bright NK-клетки экспрессируют большое количество рецепторов к цитокинам моноцитов/макрофагов и отвечают на стимуляцию продукцией интерферона-гамма (IFN-gamma), факторa некроза опухоли - альфа (TNF-alpha), гранулоцитарно-макрофагального колониестимулирующего фактора (GM-CSF), интерлейкина – 10 (IL-10), IL-13 [Erlebacher et al., 2013].
NK-клетки при беременности играют критическую роль в формировании иммунной толерантности организма матери к полуаллогенному плоду, а также выполняют фетотрофическую функцию [Saito, 2008; Shojaei, 2022]. При беременности количество цитотоксических CD16+CD56dim NK-клеток периферической крови снижается, а продукция IL-10 увеличивается более чем в 20 раз, что необходимо для снижения цитотоксического потенциала клеток периферической крови [Saito, 2008; Shojaei, 2022]. Повышение же количества цитотоксических CD16+CD56dim NK-клеток в периферической крови у женщин ассоциировано с привычным невынашиванием беременности [Erlebacher et al., 2013].
В период ранней беременности NK-клетки преобладают среди лимфоидных клеток децидуальной оболочки [Vacca et al., 2011; Erlebacher et al., 2013], и в течение первого триместра их количество увеличивается с 50 до 90%, главным образом, за счет миграции CD16dim/-CD56brightNK-клеток из периферической крови [van den Heuvel et al., 2005; Poli et al., 2009; Erlebacher et al., 2013; Montaldo et al., 2015; Bjorkstrom et al., 2016]. Однако существуют и другие источники пополнения пула децидуальных CD16dim/-CD56brightNK, такие как их формирование из лимфоидных CD34+предшественников в матке, а также пролиферации маточных NK-клеток in situ [Erlebacher et al., 2013].
Миграция CD16dim/-CD56bright NK из периферической крови в матку определяется ассоциированным с фазами менструального цикла или беременностью изменением гормонального фона и осуществляется посредством взаимодействия с хемокинами, продуцируемыми децидуальными клетками [van den Heuvel et al., 2005; Carlino et al., 2008; Poli, 2009; Erlebacher et al., 2013; Montaldo et al., 2015; Bjorkstrom et al., 2016]. Периферические (p) и децидуальные (d) CD16dim/-CD56brightNK характеризуются сходным паттерном хемокиновых рецепторов [Carlino et al., 2008]. Периферические CD16dim/-CD56brightNK имеют высокий уровень экспрессии хемокиновых рецепторов L-селектина (CD62L) и CCR7, что ассоциировано как с хомингом к лимфатическим узлам, так и с их повышенным присутствием в плаценте [Carlino et al., 2008].
Однако дальнейшая трансформация и приобретение толерогенного фенотипа CD56brightdNK-клеток находится под контролем факторов, продуцируемых децидуальным микроокружением. Так, сАМР (циклический аденозинмонофосфат), продуцируемый клетками эндометрия и присутствующий в большом количестве в зоне фето-плацентарного контакта, путем активации транскрипционного фактора FOXO1 (forkhead box protein O1) способствует увеличению экспрессии CD56 на pNK-клетках, мигрирующих в децидуа, снижает их цитотоксичность, но усиливает секрецию IFN-gamma и IL-10 [Jin et al., 2021]. Трансформирующий фактор роста бета (TGF-1beta), продуцируемый децидуальными макрофагами [Keskin et al., 2007; Allan et al., 2010], гипоксия [Cerdeira et al. 2013] и гликоделин А [Lee et al. 2019] участвуют в трансформации фенотипа и регуляции функциональной активности dNK. Так, продуцируемый децидуальными клетками гликоделин А взаимодействует с молекулами L-селектина на CD16-CD56brightNK-клетках, мигрировавших в матку, способствуя их дифференцировке [Chiossone et al., 2014; Melsen et al., 2016;]. Однако зрелые dNK лишаются экспрессии L-селектина, что свидетельствует об участии других ассоциированных с беременностью факторов дифференцировки dNK.
Децидуальные CD16-CD56brightNK отличаются от периферических CD16-CD56brightNK-клеток высокой экспрессией молекул Tim-3, CD9 и CD49a, продукцией цитокинов - IL-10, TGF-1beta, хемокинов (IL-8, LIF), факторов роста сосудов (VEGF), инсулино-подобного фактора роста (IGFBP-1), плацентарного фактора роста (PLGF), плейотропина [Koopman et al., 2003; Cerdeira et al., 2013; Fu et al., 2017; SongYan, 2022]. dNK-клетки обнаруживаются в непосредственной близости к спиральным артериям плаценты и, продуцируя факторы роста сосудов, моделируют рост спиральных артерий и инвазию трофобласта. При физиологически протекающей беременности IFN-gamma и TNF-alpha участвуют в ангиогенезе [Erlebacher et al., 2013]. Однако при спонтанных абортах также увеличивается доля dNK-клеток, продуцирующих IFN-gamma и TNF-alpha, что свидетельствует о том, что индукция синтеза этих цитокинов инициируется в ответ на различные факторы.
NK-клетки являются ведущими эффекторами формирования иммунной толерантности в зоне фето-плацентарного контакта [Saito, 2008; Shojaei, 2022]. dNK путем контактного взаимодействия и секреции толерогенных цитокинов (TGF-1beta, IL-10) индуцируют формирование адаптивных регуляторных Т-лимфоцитов с супрессорной активностью (iTreg) из наивных CD4+-T-лимфоцитов, препятствуя образованию регуляторных Т-лимфоцитов, продуцирующих IL-17 (iTh17), которые активируют провоспали-тельные клеточно-опосредованные реакции [Fu et al., 2010]. iTreg также подавляют цитотоксические реакции против трофобласта в зоне фетоплацентарного контакта [Koopman et al., 2003; Cerdeira et al., 2013; Fu et al., 2017; SongYan, 2022]. Продукция IFN-gamma CD56brightdNK-клетками препятствует трансформации наивных CD4+-T-лимфоцитов в iTh17 [SongYan, 2022]. Хотя в случае инфекции, CD56brightdNK сохраняют способность развивать цитотоксический ответ против патогенов и выполнять защитную функцию [Crespo et al., 2020].
«Сheck-point» молекула Tim-3 (T-cell Ig and mucin domain-containing protein 3) присутствует на большинстве лимфоидных клеток, однако NK-клетки обладают наибольшей экспрессией Tim-3, которая повышается при их трансформации в децидуальные [Sun et al., 2016; Орлова и др., 2022]. Экспрессия Tim-3 усиливается в ответ на активацию клеток и ограничивает продукцию провоспалительных цитокинов, дегрануляцию, цитотоксичность, повышает чувствительность к индуцированному апоптозу [Sun et al., 2016]. Лигандом для Tim-3 является галектин-9 (Gal-9), уровень которого в периферической крови нарастает при беременности [Sun et al., 2016], поскольку Gal-9 активно продуцируется клетками трофобласта [Sun et al., 2016].
В единичных исследованиях показано, что экспрессия молекулы CD49a, относящейся к семейству интегринов, коррелирует со снижением цитотоксичности и продукции INF-gamma NK-клетками, а также необходима для их миграции в матку [Li et al., 2019]. Увеличение экспрессии CD49a служит одним из маркеров трансформации периферических CD56brightCD16-NK в децидуальные [Martrus et al., 2017; Li et al., 2019], хотя присутствие CD49a описано и на периферических лимфоидных клетках [Орлова и др., 2022]. Молекула CD9 принадлежит к семейству тетраспонинов и регулирует адгезию и трансэндотелиальную миграцию лейкоцитов, а также экспрессию и активность других адгезионных молекул [Sánchez-Rodríguez, 2011]. Экспрессия CD9 изучена на децидуальных NK, но лишь в единичных исследованиях выявлена на лимфоцитах и NK периферической крови при беременности [Sánchez-Rodríguez, 2011; Орлова и др., 2022]. Лигандами для CD9, в том числе, являются продуцируемые клетками трофобласта ассоциированные с беременностью гликопротеины ("pregnancy specific glycoproteins», PSG), концентрация которых нарастает в периферической крови пропорционально сроку беременности [Sánchez-Rodríguez, 2011]. Взаимодействие CD9 с PSG регулирует продукцию цитокинов лейкоцитами в матке [Sánchez-Rodríguez, 2011].
dNK экспрессируют активационные (NKp46, NKp44, NKp30, NKG2D, CD94/NKG2C) и ингибиторные рецепторы (LILRB1, KIR2DL4, и CD94/NKG2A), имеют множество гранул, содержащих литические факторы (перфорин, гранулизин, гранзимы), однако характеризуются низкой цитотоксичностью в отношении клеток трофобласта [Moretta et al., 2000]. Баланс активирующих и ингибирующих рецепторов регулирует функции dNK [Moretta et al., 2000]. Так, взаимодействие ингибиторных рецепторов (LILRB1, KIR2DL4, CD94/NKG2A) dNK-клеток с неклассическими MHC, экспрессируемыми клетками трофобласта (HLA-G, HLA-E), угнетает цитотоксическую активность dNK в отношении клеток трофобласта [Yan et al., 2007].
Считается, что ингибирующий лектиноподобный рецептор С-типа NKG2A экспрессируется преимущественно на ранних стадиях дифференцировки NK-клеток [Béziat и др., 2010; Björkström и др., 2010]. Децидуальные CD56brightNK-клетки позитивны по NKG2A+ [Béziat и др., 2010]. Ряд авторов полагают, что экспрессия NKG2A является маркером созревания NK-клеток: от CD56brightNKG2A+ к CD56dimNKG2A+ и затем к CD56dimNKG2A- [Béziat и др., 2010]. CD56dimNKG2A- NK-клетки способны вновь экспрессировать NKG2A при стимуляции цитокинами IL-12 и IL-18, и присутствие NKG2A на них коррелирует с продукцией IFN-gamma [Béziat и др., 2010]. По сравнению с нормальной беременностью в группе женщин с рецидивирующими самопроизвольными абортами наблюдалась сниженная экспрессия NKG2A на dNK [Sotnikova et al., 2014]. Следует отметить, что все больше данных свидетельствует о том, что снижение уровня dNK-клеток и изменение их функциональной активности тесно связаны с неблагоприятным исходом беременности: преэклампсией и спонтанными абортами [Moretta et al., 2000; Koopman et al., 2003; Erlebacher et al., 2013]. Поэтому изучение факторов, регулирующих функциональную активность
NK-клеток, является значимой проблемой иммунологии и имеет важное научной и практическое значение.
Трансформация фенотипа и функциональной активности dNK-клеток также находится под контролем ассоциированных с беременностью факторов, присутствующих в периферической крови и децидуальном микроокружении (гормоны, цитокины и т.д) [Carlino et al., 2008]. Реализация регуляторных эффектов гормонов и цитокинов опосредована активацией внутриклеточных сигнальных путей при взаимодействии со специфическими клеточными рецепторами на NK-клетках [Carlino et al., 2008; Lee et al. 2019]. Внутриклеточные сигнальные молекулы являются эффективными индукторами метаболического репрограммирования, а именно, перехода иммунных клеток к преимущественному использованию специфических метаболических путей для получения энергии, что результируется в изменении фенотипа и функциональной активности клеток [Donnelly et al., 2014; Keating et al., 2016]. Особую значимость этот процесс приобретает при беременности, когда гормональный фон, основной обмен и иммунореактивность всего организма претерпевают существенные изменения. Целью данного обзора является систематизация данных литературы о роли метаболического репрограммирования в регуляции функциональной активности и фенотипа NK-клеток.
Взаимосвязь метаболических изменений с эффекторными функциями NK-клеток
В качестве основного источника энергии NK-клетки используют глюкозу, которая попадает в клетку с помощью белков-транспортеров (GLUT-1) [O’Brien, Finlay, 2019; Keating 2016]. Метаболизм глюкозы в клетке на первом этапе включает ее катаболизм до пирувата. Далее, при анаэробном гликолизе, пируват превращается в лактат под действием лактатдегидрогеназы (LDG). Основная часть пирувата переносится в митохондрии, где превращается в ацетил-КоА, вступает в цикл трикарбоновых кислот (TCA) и далее подвергается окислительному фосфорилированию [O’Brien, Finlay, 2019; Keating 2016]. Дополнительно ацетил-КоА образуется при окислении жирных кислот и также поступает в TCA [O’Brien, Finlay, 2019].
Незрелые NK-клетки обладают высокой метаболической активностью и в качестве основной метаболической программы используют аэробный гликолиз, поскольку преобладающим продуктом метаболизма глюкозы, даже в условиях нормоксии, становится лактат, что важно для реализации их пролиферативного потенциала [Chiossone et al., 2009; Marcais et al., 2014]. Гликолиз обеспечивает более быструю наработку ATP (аденозинтрифосфата), чем окислительное фосфорилирование, и дает промежуточные метаболиты, которые в дальнейшем можно использовать для процессов биосинтеза [Chiossone et al., 2009; Marcais et al., 2014].
По мере созревания NK-клеток поглощение глюкозы, экспрессия рецепторов-транспортеров питательных веществ (GLUT-1, CD71, CD98), ферментов гликолиза, пролиферативная активность снижаются, а окислительное фосфорилирование становится основной метаболической программой зрелых не стимулированных NK-клеток [Chiossone et al., 2009; Marcais et al., 2014].
При активации зрелых NK-клеток увеличивается потребление глюкозы, экспрессия рецепторов-транспортеров питательных веществ (GLUT-1, CD98 (транспортер нейтральных аминокислот), CD71 (рецептор к трансферрину), интенсивность гликолиза и окислительного фосфорилирования, возрастает митохондриальная масса и производство АТР [O’Brien, Finlay, 2019; Keating 2016]. Значимость гликолиза для реализаций эффекторных функций активированных NK-клеток подтверждается рядом экспериментальных данных. Так, угнетение гликолиза в процессе активации NK-клеток путем депривации глюкозы, либо добавлением ингибитора гексокиназы, либо лактата в культуры, снижает экспрессию активационных рецепторов NKp46, продукцию IFN-gamma, перфорина, гранзима B и цитотоксичность in vitro [van der Heiden et al., 2009; Husain et al., 2013; O’Brien, Finlay, 2019]. Ингибирование путей синтеза липидов оказывает минимальное влияние на эффекторные функции и пролиферацию NK-клеток [O’Brien, Finlay, 2019].
Согласно современным представлениям, ключевыми молекулами, участвующими в контроле метаболизма NK-клеток, являются мишень для рапамицина в клетках млекопитающих (mTOR) и AMP-активируемая протеинкиназа (AMPK). mTOR стимулирует анаболические процессы и рост клеток, а AMPK усиливает процессы катаболизма [O’Brien, Finlay, 2019].
mTOR обладает протеинкиназной активностью и образует два функционально различных комплекса: mTORC1 и mTORC2, отличных по чувствительности к ингибиторному эффекту рапамицина [Donnelly et al. 2014]. mTORC1 имеет решающее значение для дифференцировки NK-клеток, тогда как mTORC2 необходим для их терминального созревания, хотя функция последнего изучена недостаточно ввиду отсутствия специфических ингибиторов [Donnelly et al. 2014]. Мыши с делецией mTOR имеют меньшее количество зрелых NK-клеток в периферических органах, что свидетельствует о важности фермента для дифференцировки NK-клеток.
mTOR в клетке инициирует трансляцию белка. mTOR фосфорилирует рибосомальную протеинкиназу S6K1 (p70 рибосомальная S6 протеинкиназа) и эукариотический фактор инициации трансляции 4E-BP1 (4Е, связывающий белок 1), в результате чего образуется активный комплекс еIF4E, связывающий mRNA и рибосому [Donnelly et al. 2014].
По мере созревания NK-клеток экспрессия mTORС1 снижается [Chiossone et al., 2009; Marcais et al., 2014]. Базальный уровень активности mTORС1 незначителен. Активация mTORС1 в NK-клетках происходит в ответ на взаимодействие с различными факторами, главным образом, цитокинами, а также гормонами [Marcais et al., 2014; Donnelly et al., 2014]. Причем основные стимуляторы для NK-клеток - IL-2, IL-15, либо комбинации IL-2 и IL-12 или IL-12 и IL-18, повышают активность mTORС1 через PI3-K (фосфатидилинозитол-3-киназа) /Akt (протеинкиназа B) сигнальный путь [Nandagopal et al., 2014; Marcais et al., 2014; Donnelly et al., 2014]. Akt, основной активатор для mTOR, усиливает трафик GLUT1 к поверхности клетки, поглощение глюкозы и стимулирует гликолиз [Marcais et al., 2014; Donnelly et al., 2014]. Akt индуцирует экспрессию транспортных белков для аминокислот и может напрямую фосфорилировать ферменты гликолиза, способствуя увеличению его активности [Marcais et al., 2014; Donnelly et al., 2014].
Помимо основных активаторов, сигнальные молекулы других путей внутриклеточной трансдукции участвуют в активации mTORС1. Так, комплекс MEK/ERK (митогенактивируемая киназа/внеклеточная сигнальная киназа) активирует mTORС1 через TSC2 (туберозносклерозный белок 2) [Nandagopal et al., 2014; Marcais et al., 2014; Donnelly et al., 2014]. Фосфолипаза D катализирует гидролиз фосфатидилхоли-на с образованием фосфатидной кислоты, которая способна взаимодействовать с каталитической субъединицей mTORС1 и активировать ее [Nandagopal et al., 2014; Marcais et al., 2014; Donnelly et al., 2014].
IL-10 также стимулирует mTORС1 в NK-клетках, усиливая как гликолиз, так и окислительное фосфорилирование, что приводит к повышению цитотоксичности и продукции IFN-gamma [Wang et al., 2021]. Выявленные эффекты IL-10 отменялись рапамицином, что свидетельствует о важной роли mTORС1 в реализации эффектов цитокина на NK-клетки [Wang et al., 2021].
TGF-β1 угнетает пролиферацию и цитотоксичность NK-клеток, продукцию IFN-gamma как in vitro , так и in vivo [Viel, 2016], а также IL-2 и IL-15-индуцированную активацию гликолиза и окислительного фосфорилирования за счет ингибирования mTORС1 [Zaiatz-Bittencourt et al., 2018].
Аминокислоты, поступающие в организм с пищей или образующиеся в результате распада белков, участвуют в регуляции активности mTOR независимым от PI3/Akt путем [Donnelly et al., 2014]. Ведущая роль из аминокислот принадлежит лейцину, повышение концентрации которого с участием CD98 приводит к резкой активации mTOR [Donnelly et al., 2014].
Фермент mTOR регулирует метаболизм глюкозы в NK-клетках. mTORC1 присоединяется к внешней мембране митохондрий и регулирует поглощение кислорода и окислительную способность митохондрий [Donnelly et al., 2014]. mTOR-активированная рибосомальная протеинкиназа S6K1 оказывает прямые гликолитические эффекты. Дефицит S6K1 предотвращает усиление гликолиза в ответ на активацию NK-клеток [Donnelly et al., 2014]. Помимо этого, mTOR контролирует активность транскрипционных факторов c-MYC и SREBP (Sterol Regulatory Element Binding Protein), HIF-1alpha (hypoxia-inducible factor 1 alpha ), которые также являются важными метаболическими регуляторами [Donnelly et al., 2014]. c-MYC контролирует экспрессию генов, кодирующих факторы клеточного цикла, обеспечивая переход из G 0/1 -фазы в S-фазу, и подавляет экспрессию ингибиторов клеточного цикла [Donnelly et al., 2014]. c-MYC регулирует экспрессию всех генов, связанных с гликолизом, включая GLUT-1, LDH, а также переносчиков глутамина [Donnelly et al., 2014]. Глютамин поддерживает экспрессию c-MYC в NK-клетках. Ограничение поступления глютамина угнетает экспрессию c-MYC и нарушает эффекторные функции NK-клеток [Donnelly et al., 2014]. Транскрипционный фактор SREBP играет значимую роль в цитокин-индуцированном метаболическом репрограммировании NK-клеток. Установлено, что активность SREBP важна для усиления гликолиза и окислительного фосфорилирования [Assmann et al., 2017]. Предотвращение активации SREBP ингибировало выработку IFN-gamma и цитотоксичность NK-клеток [Assmann et al., 2017]. HIF-1alpha увеличивает экспрессию генов, вовлеченных в гликолиз: ключевых ферментов (LDH и др.), мембранных транспортеров глюкозы [Donnelly et al., 2014].
Фармакологическое ингибирование mTOR рапамицином в NK-клетках отменяет цитокин-индуцированную (IL-2/IL-12) стимуляцию поглощения глюкозы и гликолиза, экспрессию транспортера аминокислот CD98, снижает митохондриальную массу и мембранный потенциал и нарушает эффекторные функции, а именно секрецию гранзима B и IFN-gamma [Donnelly, 2014; Slattery, 2019]. Делеция mTOR или ингибирование SREBP2 ухудшают пролиферацию, дегрануляцию и цитотоксичность NK-клеток [Assmann et al., 2017]. Таким образом, метаболический статус NK-клеток напрямую взаимосвязан с реализацией их эффекторных функций. В незрелых или цитокин-активированных NK-клетках mTOR-зависмая активация гликолиза поддерживает пролиферацию, продукцию IFN-gamma, гранзима В, реализацию цитотоксической функции.
Цитокин-зависимая стимуляция PI3-K/Akt/mTOR отрицательно влияет на активность AMPK [Muller-Durovic et al., 2016; Chapman et al., 2017]. Тогда как AMPK, в свою очередь, также предотвращает активацию mTOR, напрямую фосфорилируя компоненты mTOR, такие как TSC2 и раптор (raptor), что ведет к инактивации mTOR-опосредованных клеточных процессов [Chapman et al., 2017]. Взаимодействие mTOR и AMPK может иметь решающее значение для перестройки метаболических путей в NK-клетках.
AMPK активируется в ответ на увеличение отношения AMP/АТP, а также рядом киназ, включая печеночную киназу B1 (LKB1) и кальций/кальмодулин-зависимую протеинкиназу II (CaMKKII), TGF-β-активированную киназу 1 (TAK1) [Muller-Durovic et al., 2016; Chapman et al., 2017]. CAMKK2 активи- рует АМРК вне зависимости от уровня АМР. Мишенью AMPK является, в том числе, ацетил-КоА-карбоксилаза, фосфорилирование которой подавляет синтез жирных кислот и способствует их окислению [Muller-Durovic et al., 2016; Chapman et al., 2017]. Показано, что активность AMPK при созревании NK-клеток меняется незначительно [Wang et al., 2016]. Однако терминально дифференцированные зрелые CD16+CD56dim NK клетки имеют высокий уровень экспрессии ингибиторного рецептора KLRG1 (Killer Cell Lectin-like Receptor G1) и AMPK [Muller-Durovic et al., 2016]. Лигандами для KLRG1 являются кадгерины Е, N (молекулы клеточной адгезии в эпителиальных тканях) [Tessmer et al., 2007]. Лигирование KLRG1 приводит к повышению активности AMPK в NK-клетках. Активация AMPK фармакологическими агентами подавляет пролиферацию и цитотоксичность NK-клеток, выработку гранзима B и IFN-gamma [Kim 2006; Muller-Durovic et al., 2016]. Активность KLRG1-AMPK сигнального пути в NK-клетках увеличивается с возрастом, что является одной из причин угнетения цитотоксической активности NK-клеток у пожилых людей [Muller-Durovic et al., 2016].
Суммируя все вышесказанное, можно заключить, что активация гликолиза и mTOR являются важным фактором в регуляции пролиферации, цитотоксической активности и продукции IFN-gamma NK-клетками. Активация AMPK, напротив, подавляет эти функции. Модуляция активности mTOR и AMPK определяет метаболическое репрограммирование фенотипа NK-клеток. Молекулярные механизмы метаболической регуляции функций NK-клеток, в зависимости от зрелости и активированности, отражены на рисунке.
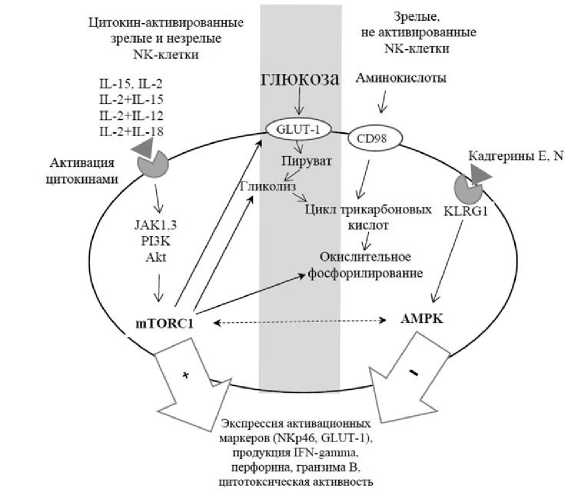
Молекулярные механизмы метаболической регуляции функций NK-клеток в зависимости от зрелости и активирующих стимулов
[Molecular mechanisms of metabolic regulation of NK cell functions depending on maturity and activation] Примечания: глюкоза попадает в клетку с помощью белков-транспортеров GLUT-1. Метаболизм глюкозы включает ее катаболизм до пирувата, далее при анаэробном гликолизе пируват превращается в лактат. Основная часть пирувата переносится в митохондрии, где вступает в цикл трикарбоновых кислот и далее подвергается окислительному фосфорилированию. Дополнительно используется окисление аминокислот и в меньшей степени липидов, поступающих в клетку с помощью белков-транспортеров (CD98). Незрелые или зрелые активированные цитокинами (IL-2, IL-15, либо их комбинации) NK-клетки обладают высокой метаболической активностью и в качестве основной метаболической программы используют аэробный гликолиз. Цитокин-зависимая активация повышает активность mTORС1 (мишень для рапамицина в клетках млекопитающих) через PI3-K (фосфатидилинозитол-3-киназа) /Akt (протеинкиназа B) сигнальный путь. mTORС1 стимулирует специфические транскрипционные факторы и активирует транскрипцию генов, кодирующих белки рецепторов-транспортеров питательных веществ (GLUT-1, CD98), ферментов гликолиза, усиливая потребление глюкозы, интенсивность гликолиза и окислительного фосфорилирования, что стимулирует пролиферацию, экспрессию активационных маркеров (NKp46, GLUT-1), продукцию IFN-gamma, перфорина, гран-зима B, цитотоксическую активность. Зрелые, не активированные NK-клетки характеризуются низкой метаболической активностью, а окислительное фосфорилирование становится основной метаболической программой. В зрелых терминально дифференцированных NK-клетках повышается экспрессия ингибиторного рецептора KLRG1 (Killer Cell Lectin-like Receptor G1) и AMPK (AMP-активируемая протеинкиназа). AMPK угнетает mTORC1, подавляет пролиферацию и цитотоксичность NK-клеток, выработку гранзима B и IFN-gamma. Сплошная стрелка – стимулирующий эффект. Пунктирная стрелка – угнетающий эффект.
Особенности метаболической регуляции основных субпопуляций NK-клеток
Известно, что CD16-/+CD56brightpNK являются менее зрелыми по отношению к CD16+CD56dimpNK-клеткам периферической крови и отличаются по интенсивности метаболических процессов [Keating et al., 2016]. При этом mTORC1 более активна в CD56brightpNK [Keating et al., 2016]. CD56brightpNK, в силу высокой экспрессии рецепторов цитокинов, демонстрируют более выраженные метаболические изменения, чем CD56dimNK-клетки в ответ на стимуляцию цитокинами, и более чувствительны к ингибированию метаболизма [Keating et al., 2016].
Так, в случае активации IL-2 или IL-12/IL-15 CD56brightNK-клетки преимущественно повышают экспрессию рецепторов-транспортеров питательных веществ CD71 и CD98 mTOR-зависимым образом, по сравнению с CD56dimрNK-клетками [Keating et al., 2016]. Кроме того, CD56brightpNK-клетки исходно обладают более высокой экспрессией GLUT-1, что позволяет им быстро поглощать глюкозу при активации. Ингибирование гликолиза значительно уменьшает продукцию IFN-gamma CD56brightNK-клетками, но оказывает минимальное влияние на дегрануляцию и секрецию гранзима B по сравнению с CD56dimtpNK [Keating, 2016]. В целом, CD56brightpNK-клетки более метаболически активны, чем CD56dimpNK, и это помогает CD56brightpNK-клеткам быстро вырабатывать большое количество IFN-gamma во время иммунных реакций. По данным этих же авторов, окислительное фосфорилирование, как и гликолиз, необходимы для поддержки продукции IFN-gamma в подгруппе CD56brightрNK-клеток периферической крови человека [Keating et al., 2016].
При этом даже среди CD56brightNK-клеток выявляются отличия в интенсивности метаболизма глюкозы в зависимости от локализации – периферическая кровь или вторичные лимфоидные органы [Salzberger et al., 2018]. Так, после стимуляции IL-12/IL-15 цитокинами CD56brightNK-клетки, резидентные в печени и селезенке, имеют более низкую экспрессию транспортера глюкозы GLUT-1, но более высокую экспрессию транспортера аминокислот CD98 по сравнению с CD56brightNK-клетками периферической крови [Salzberger, 2018].
В единичных исследованиях установлено, что CD56brightpNK-клетки периферической крови и CD56brightdNK-клетки, мигрировавшие в децидуа, находятся в разных условиях по обеспеченности глюкозой и кислородом, что обусловливает разный метаболический профиль и, как следствие, влияет на фенотип и функции [Yan et al., 2022]. В децидуальной оболочке клетки находятся в условиях ограниченного поступления глюкозы и кислорода [Yan et al., 2022].
Установлено, что децидуальные CD56brightdNK характеризуются более низкой интенсивностью гликолиза и более высокой активностью окислительного фосфорилирования по сравнению с CD56brightpNK периферической крови [Yan et al., 2022]. Причем кратковременное изменение метаболизма CD56brightdNK (гликолиза, окислительного фосфорилирования, окисления жирных кислот) не влияет на их цитотоксичность [Yan et al., 2022]. А угнетение гликолиза в CD56brightdNK-клетках усиливает выработку VEGF-A, но снижает продукцию IFN-gamma, TNF-alpha и пролиферацию [Yan et al., 2022].
Использование рапамицина для блокады mTORС1 снижает интенсивность как гликолиза, так и окислительного фосфорилирования, а также продукцию IFN-gamma и TNF-alpha, CD107a-зависимую дегрануляцию и пролиферацию, в то время как выработка VEGF-A не меняется [Yan et al., 2022]. Таким образом, mTORC1-зависимая активность гликолиза модулирует продукцию цитокинов (IFN-gamma, TNF-alpha), цитотоксическую реакцию и пролиферацию CD56brightdNK [Yan et al., 2022]. Но продукция VEGF-A зависит от активности гликолиза, но, по-видимому, регулируется mTORC1-независимыми механизмами. Наконец, этими же авторами было показано, что активность некоторых ферментов гликолиза и mTORC1 значительно снижены в CD56brightdNK женщин с привычным невынашиванием беременности, что открывает новые механизмы для понимания причин этого состояния [SongYan, 2022].
Функциональная и метаболическая активность CD56brightdNK находится под контролем факторов, продуцируемых микроокружением в децидуа. Так, TGF-1beta вырабатывается децидуальными стромальными клетками на границе раздела мать-плод и в сочетании с гипоксией эффективно способствует превращению CD56brightpNK в CD56brightdNK, которые секретируют высокие уровни VEGF-A, но обладают низкой цитотоксичностью [Sun et al., 2016]. TGF-1beta эффективно подавляет гликолиз и ослабляет гли-колиз-зависимые функции, как цитотоксичность и продукция IFN-gamma, TNF-alpha и пролиферацию [Sun et al., 2016]. Помимо этого, TGF-1beta повышает экспрессию ингибиторной «сheckpoint» молекулы Tim-3 на dNK [Sun et al., 2016]. Ингибирующий сигнал при взаимодействии Tim-3 с лигандом Gal-9 угнетает активность PI3K-AKT-mTOR сигнального пути, и, как следствие, подавляет цитотоксичность dNK-клеток в отношении клеток трофобласта, препятствуя дегрануляции, выбросу перфорина, гранзима B [Sun et al., 2016] Блокирование Tim-3 сигналинга усиливает цитотоксичность dNK к клеткам трофобласта. Tim-3+dNK экспрессируют больше маркеров зрелости и активации (CD94, CD69), чем TIM3-dNK. Сигналинг с Tim-3 регулирует продукцию цитокинов dNK IFN-gamma и TNF-alpha, необходимых для ремоделирования и роста спиральных артерий [Sun et al., 2016].
Молекулы гистосовместимости I класса HLA-E, продуцируемые в экстравезикулах клетками трофобласта, являются лигандами для активационных молекул CD94/NKG2A и CD94/NKG2C на dNK и играют важную роль в регуляции метаболизма NK. Так, взаимодействие молекулы CD94 на dNK с HLA-E трофобласта усиливает гликолиз и окислительное фосфорилирование в dNK, регулирует секрецию IFN-gamma и VEGF при беременности [Jiang et al., 2021].
IL-15 присутствует в децидуа и его выработка контролируется гормонами, особенно прогестероном [Marcais et al., 2014; Nandagopal et al., 2014]. IL-15 является критическим регулятором выживаемости и дифференцировки для NK и стимулирует в Akt/mTOR сигнальный путь NK-клетках, как упоминалось ранее [Marcais et al., 2014; Nandagopal et al., 2014]. В работах ряда авторов in vitro показано, что направленность эффектов IL-15 на функциональную активность NK-клеток находится в строгой зависимости от концентрации интерлейкина и продолжительности его действия [Marcais et al., 2014]. Сравнения метаболических и функциональных особенностей разных субпопуляций NK-клеток представлены в таблице.
Сравнение метаболических и функциональных особенностей субпопуляций NK-клеток [Comparison of metabolic and functional features of NK cell subpopulations]
|
Фенотип NK-клеток |
CD16+CD56dimрNK |
CD16-/negCD56bright pNK |
CD16-/negCD56bright dNK |
|
Локализация |
Периферическая кровь |
Периферическая кровь |
Децидуа |
|
Преобладающая метаболическая программа |
Окислительной фосфорилирование |
Аэробный гликолиз |
Окислительное фосфорилирование |
|
Ключевой регулятор |
AMPK |
mTORС1 |
? |
|
Доминирующая функция |
Высокая цитотоксическая активность |
Продукция цитокинов |
Продукция ангиогенных факторов, иммуносупрессорных цитокинов, индукция иммунной толерантности |
Примечания: р – периферические, d –децидуальные, AMPK – AMP-активируемая протеинкиназа, mTORС1 – мишень для рапамицина в клетках млекопитающих .
Заключение
В целом, можно заключить, что метаболический статус NK-клеток напрямую взаимосвязан с реализацией их эффекторных функций. Модуляция активности mTOR и AMPK различными агентами определяет метаболическое репрограммирование фенотипа NK-клеток и изменение их функциональной активности. Особенности метаболической регуляции эффекторных функций NK-клеток зависят от их степени зрелости, а также продолжительности и специфичности активационных сигналов. В стимулированных зрелых и незрелых NK-клетках mTOR-зависимое репрограммирование в сторону гликолиза /и окислительного фосфорилирования поддерживает пролиферацию, продукцию IFN-gamma, перфорина, гранзима В и реализацию цитотоксической функции в ответ на большинство активирующих стимулов. Значимость mTOR и гликолиза для реализации вышеупомянутых функций подтверждается экспериментами с использованием рапамицина, блокаторов и ингибиторов ферментов гликолиза. Увеличение активности KLRG1-AMPK сигнального пути в зрелых терминально дифференцированных NK-клетках угнетает mTOR, подавляет гликолиз-зависимые функции и способствует преобладанию окислительного фосфорилирования как основной метаболической программы. Функциональная и метаболическая активность децидуальных CD56brightdNK при беременности находится под контролем факторов, присутствующих в периферической крови и продуцируемых микроокружением в децидуа, но также определяется особенностями поступления кислорода и глюкозы. Изучение метаболической регуляции функциональной активности разных субпопуляций NK-клеток имеет большое значение для повышения эффективности NK-клеточной терапии.
Список литературы Метаболическая регуляция функций натуральных киллеров
- Орлова Е.Г. и др. Особенности экспрессии молекул Tim-3, CD9, CD49a лимфоцитами периферической крови при физиологической беременности // Вестник уральской медицинской академической науки. 2022. Т. 19, № 5. C. 461-473,
- Allan D.S. et al. TGF-ß affects development and differentiation of human natural killer cell subsets // Eur. J. Immunol. 2010. Vol. 40(8). P. 2289-2295.
- Assmann N. et al. Srebp-controlled glucose metabolism is essential for NK cell functional responses // Nat. Immunol. 2017. Vol. 18. P. 1197-1206.
- Beziat V. et al. NK cell terminal differentiation: correlated stepwise decrease of NKG2A and acquisition of KIRs // PLoS One. 2010. Vol. 5(8). e11966.
- Bjorkstrom N.K., Ljunggren H.G., Michaelsson J. Emerging insights into natural killer cells in human peripheral tissues // Nat. Rev. Immunol. 2016. Vol. 16(5). P. 310-320.
- Carlino C. et al. Recruitment of circulating NK cells through decidual tissues: a possible mechanism controlling NK cell accumulation in the uterus during early pregnancy // Blood. 2008. Vol. 111(6). P. 3108-3115.
- Cerdeira A.S. et al. Conversion of peripheral blood NK cells to a decidual NK-like phenotype by a cocktail of defined factors // J. of immunol. 2013. Vol. 190(8). P. 3939-3948.
- Chapman N.M., Shrestha S., Chi H. Metabolism in Immune Cell Differentiation and Function // Adv. Exp. Med. Biol. 2017. Vol. 1011. P. 1-85.
- Chiossone L. et al. Maturation of mouse NK cells is a 4-stage developmental program // Blood. 2009. Vol. 113(22). P. 5488-5496.
- Chiossone L. et al. In vivo generation of decidual natural killer cells from resident hematopoietic progenitors // Haematologica. 2014. Vol. 99(3). P. 448-457.
- Crespo A.C., et al. Decidual NK Cells Transfer Granulysin to Selectively Kill Bacteria in Trophoblasts // Cell. 2020. Vol. 182(5). P. 1125-1139.
- Donnelly R.P. et al. mTORC1-dependent metabolic reprogramming is a prerequisite for NK cell effector function // J. Immunol. 2014. Vol. 193. P. 4477-4484.
- Erlebacher A. Immunology of the maternal-fetal interface // Annu. Rev. Immunol. 2013. Vol. 31. P. 387411.
- Fu B. et al. Natural Killer Cells Promote Fetal Development through the Secretion of Growth-Promoting Factors // Immunity. 2017. Vol. 47(6), P. 1100-1113.
- Husain Z., Seth P., Sukhatme V.P. Tumor-derived lactate and myeloid-derived suppressor cells: Linking metabolism to cancer immunology // Oncoimmunology. 2013. Vol. 2(11). e26383.
- Jiang L. et al. Extracellular Vesicle-Mediated Secretion of HLA-E by Trophoblasts Maintains Pregnancy by Regulating the Metabolism of Decidual NK Cells // International journal of biological sciences. 2021. Vol. 17(15). P. 4377-4395.
- Jin X. et al. Decidualization-derived cAMP regulates phenotypic and functional conversion of decidual NK cells from CD56dimCD16- NK cells // Cell Mol. Immunol. 2021. Vol. 18(6). P. 1596-1598.
- Keating S.E. et al. Metabolic reprogramming supports IFN-y production by CD56bright NK cells // J. Immunol. 2016. Vol. 196(6). P. 2552-2560.
- Keskin D.B. et al. TGF beta promotes conversion of CD16+ peripheral blood NK cells into CD16- NK cells with similarities to decidual NK cells // Proc. Natl. Acad. Sci. USA. 2007. Vol. 104(9). P. 3378-3383.
- Kim K.Y. et al. Adiponectin is a negative regulator of NK cell cytotoxicity // J. Immunol. 2006. Vol. 176(10). P. 5958-5664.
- Koopman L.A. et al. Human decidual natural killer cells are a unique NK cell subset with immunomodu-latory potential // The J. of exp. medicine. 2003. Vol. 198(8). P. 1201-1212.
- Lee C.L. et al. Glycodelin-A stimulates the conversion of human peripheral blood CD16-CD56bright NK cell to a decidual NK cell-like phenotype // Hum. Reprod. 2019. Vol. 34(4). P. 689-701.
- Marcais A. et al. The metabolic checkpoint kinase mTOR is essential for IL-15 signaling during the development and activation of NK cells // Nat. Immunol. 2014. Vol. 15. P. 749-757.
- Martrus G. et al. Proliferative capacity exhibited by human liver-resident CD49a+CD25+NK cells // PloS One. 2017. Vol. 12(8), e0182532.
- Melsen J.E. et al. Human Circulating and Tissue-Resident CD56(bright) Natural Killer Cell Populations // Front. Immunol. 2016. Vol. 7. P. 262.
- Montaldo E. et al. Group 3 innate lymphoid cells (ILC3s): Origin, differentiation, and plasticity in humans and mice // Eur. J. Immunol. 2015. Vol. 45(8). P. 2171-2182.
- Moretta A. et al. Natural cytotoxicity receptors that trigger human NK-cell-mediated cytolysis // Immunol. Today. 2000. Vol. 21(5). P. 228-234.
- Muller-Durovic B. et al. Killer cell lectin-like receptor G1 inhibits NK cell function through activation of adenosine 5'-monophosphateactivated protein kinase // J. Immunol. 2016. Vol. 197(7). P. 2891-2899.
- Nandagopal N. et al. The Critical Role of IL-15-PI3K-mTOR Pathway in Natural Killer Cell Effector Functions // Front Immunol. 2014. Vol. 5. P. 187.
- O'Brien K.L., Finlay D.K. Immunometabolism and natural killer cell responses // Nat. Rev. Immunol. 2019. Vol. 19(5). P. 282-290.
- Poli A. et al. CD56bright natural killer (NK) cells: an important NK cell subset // Immunology. 2009. Vol. 126(4). P. 458-465.
- Saito S. et al. The balance between cytotoxic NK cells and regulatory NK cells in human pregnancy // J. of Reprod. Immunol. 2008. Vol. 77(1), P. 14-22.
- Salzberger W. et al. Tissue-resident NK cells differ in their expression profile of the nutrient transporters Glut1, CD98 and CD71 // PLoS One. 2018. Vol. 13. e0201170.
- Sánchez-Rodríguez E.N. et al. Persistence of decidual NK cells and KIR genotypes in healthy pregnant and preeclamptic women: a case-control study in the third trimester of gestation // Reprod. Boil. and endocrinol. 2011. Vol. 9. P. 8.
- Shojaei Z. et al. Functional prominence of natural killer cells and natural killer T cells in pregnancy and infertility: A comprehensive review and update // Pathol. Res. Pract. 2022. Vol. 238. P. 154062.
- Slattery K. et al. TGFß drives NK cell metabolic dysfunction in human metastatic breast cancer // J. Im-munother. Cancer. 2021. Vol. 9(2). e002044.
- Song Yan et al. The mTORC1 Signaling Support Cellular Metabolism to Dictate Decidual NK Cells Function in Early Pregnancy // Front Immunol. 2022. Vol. 13. P. 771732.
- Sotnikova N. et al. Interaction of decidual CD56+ NK with trophoblast cells during normal pregnancy and recurrent spontaneous abortion at early term of gestation // Scandinavian journal of immunology. 2014. Vol. 80(3), P. 198-208.
- Sun et al. Tim-3 is up regulated in NK cells during early pregnancy and inhibits NK cytotoxicity toward trophoblast in galectin-9 dependent pathway // PloS One. 2016. Vol. 11(1). e0147186.
- Tessmer M.S. et al. KLRG1 binds cadherins and preferentially associates with SHIP-1 // Int. Immunol. 2007. Vol. 19(4). P. 391-400.
- Vacca P. et al. Origin, phenotype and function of human natural killer cells in pregnancy // Trends Immunol. 2011. Vol. 32. P. 517-523.
- van den Heuvel M.J. et al. Trafficking of circulating pro-NK cells to the decidualizing uterus: regulatory mechanisms in the mouse and human // Immunol. Invest. 2005. Vol. 34(3). P. 273-293.
- Viel S. et al. TGF-ß inhibits the activation and functions of NK cells by repressing the mTOR pathway // Sci. Signal. 2016. Vol. 9(415). ra19.
- Wang Z. et al. (). IL-10 Enhances Human Natural Killer Cell Effector Functions via Metabolic Reprogramming Regulated by mTORC1 Signaling // Frontiers in immunology. 2021. Vol. 12. P. 619195.
- Yan S. et al. The mTORC1 Signaling Support Cellular Metabolism to Dictate Decidual NK Cells Function in Early Pregnancy // Frontiers in immunology.2022. Vol. 13. P. 771732.
- Yan W.H. et al. Possible roles of KIR2DL4 expression on uNK cells in human pregnancy // Am. J. Re-prod. Immunol. 2007. Vol. 57(4). P. 233-242.
- Zaiatz-Bittencourt V., Finlay D.K., Gardiner C.M. Canonical TGF-b signaling pathway represses human NK cell metabolism // J. Immunol. 2018. Vol. 200. P. 3934-3941.


