MgH2 insights used for hydrogen storage and catalysis: preparation, characterization and mechanism
Автор: Chen Pengzhou, Levtsev Aleksei, Shu Yugang
Журнал: Бюллетень науки и практики @bulletennauki
Рубрика: Технические науки
Статья в выпуске: 6 т.9, 2023 года.
Бесплатный доступ
Magnesium is a rich element on earth, with high reversible, cycle stability and antitoxicity, low cost, and high-volume hydrogen capacity, its theoretical hydrogen storage capacity up to 7.6 wt %. Magnesium-based hydrogen storage materials are considered as one of the most promising hydrogen storage media. However, due to its high thermodynamic stability and slow hydrogen adsorption kinetics, the high adsorption temperature and long adsorption time limit its practical application. In order to overcome the thermodynamic and kinetic obstacles of magnesium-based hydrogen storage materials in practical application and improve the hydrogen storage performance of MgH2, catalytic modification is a very effective method at present. This review summarizes the preparation methods, characterization methods and reaction basis of various catalysts modified by MgH2. Finally, we analyze the development trend of various catalysts in the future, as well as the application prospects.
Hydrogen storage material, mgh2, catalyst
Короткий адрес: https://sciup.org/14128000
IDR: 14128000 | УДК: 621.355:546.46 | DOI: 10.33619/2414-2948/91/50
Текст научной статьи MgH2 insights used for hydrogen storage and catalysis: preparation, characterization and mechanism
Бюллетень науки и практики / Bulletin of Science and Practice
UDC 621.355:546.46
Today, the large-scale use of fossil fuels brings serious environmental pollution, as well as energy security problems. In this context, there is an urgent need to transform the energy mix and develop and utilize clean and renewable energy sources to alleviate environmental problems and energy security issues. Among many clean energy sources, hydrogen energy has a very high energy density compared with other energy sources, environmental protection and clean, as the most promising clean energy appears in people's vision. However, in the utilization of hydrogen energy, the problems related to hydrogen production, hydrogen storage and hydrogen use need to be solved. The biggest obstacle is the hydrogen storage technology. The development of hydrogen storage technology plays a crucial role in the large-scale utilization of hydrogen energy. In order to realize the hydrogen energy society, we must find a low-cost and high-density hydrogen storage method [1, 2].
At present, hydrogen storage methods mainly include: high pressure gas storage (at room temperature, 350-700 bar), cryogenic liquid storage (-2530С, 5-10 bar) and solid state storage. Compressed hydrogen storage, the disadvantage of relatively low volume density and high cost limits its wide application. However, compared with compressed gas and low temperature liquid, solid hydrogen storage has low working pressure, safe operating temperature and pressure parameters, and high volume density, which has been widely studied as a promising hydrogen storage mode. Among solid hydrogen storage materials, magnesium-based materials have the advantage of high volume and weight hydrogen storage capacity, and are considered to be one of the most promising hydrogen storage materials, which has been widely studied in the past few decades [3].
For magnesium-based materials, unfavorable thermodynamic and kinetic barriers hinder their practical application. In order to enable magnesium-based materials to be manufactured and used on a large scale, researchers have improved the properties of magnesium hydrogen through various ways, including: nanostructure, alloying, catalysis, modified surface properties, formation of composite materials, etc. Among them, the addition of catalyst is one of the most effective and easy methods to improve the performance of MgH 2 . The addition of catalyst not only improves the hydrogen storage kinetics, but also suppresses the merger and growth of powder or crystallization during the long-term cycle [4].
With the rapid development of society today, the existence of human beings is the increasingly intensified conflict between resources, environment and environment, and people gradually realize the relationship between environment and resources. China is a developing country with a large population and relatively poor per capita resources. The lack of energy supply has become one of the bottleneck factors restricting the sustainable development of the national economy. So as to develop a clean, efficient, safe, saving renewable new energy is urgent. As a renewable resource, wind energy is widely used in the field of power generation. At present, through the development and utilization of wind energy, primary energy, such as tidal energy and solar energy, the amount of non-renewable energy can be reduced within a certain range. Hydrogen fuel cell is a new type of environmentally friendly secondary energy. Using hydrogen as fuel can effectively relieve the environmental pressure brought by fossil energy. However, due to the influence of the region, equipment and time limitations, it cannot be widely applied in a large area, which has certain limitations. Due to its high energy density, environmental friendliness, hydrogen energy is gradually becoming an ideal secondary energy, which has been paid attention to by more and more researchers in the world. And compared with wind, tidal and solar, primary energy, which is limited by geography, hydrogen energy is clean, pollution-free, and the nature of storage and transportation. Therefore, studying hydrogen production technology has great significance to alleviate energy crisis and environmental pollution. Hydrogen preparation routes are diversified, usually prepared by solar energy and other primary energy sources. The preparation process is environmentally friendly and pollution-free. In addition, due to the small density of hydrogen, it can be used to deliver hydrogen directly to the user without requiring a complex separation and purification device. Hydrogen gas can be stored in closed containers and transported everywhere for use. In addition, hydrogen can also be used as a feedstock for fuel cells or other energy conversion devices, thus reducing the consumption of fossil fuels. So hydrogen energy is seen as a potential energy source that can effectively cope with the future energy crisis [5-9].
The preparation and utilization of hydrogen are divided into three parts, namely preparation, storage and utilization. At present, the production and application of hydrogen energy in China are mainly concentrated in the production and storage link. Among them, the development of preparation and use is relatively mature, and there are still great technical difficulties in the storage of hydrogen, which hinders the large-scale use of hydrogen energy. At present, China mainly adopts the traditional water lysis method, adsorption method and other hydrogen storage methods to realize hydrogen storage, but these two methods have certain disadvantages. Therefore, the research and development of safe and reliable hydrogen storage technology is of great significance for the development of hydrogen energy [10].
From the state of hydrogen existence, hydrogen storage mode can be divided into three categories, first, compressed gas storage, one is liquid hydrogen storage, the storage at low temperature, the other is solid hydrogen storage, with adsorption or reaction storage, gaseous hydrogen storage is simple, need less conditions, development is mature, but the hydrogen storage to withstand high pressure cylinder, expensive, and through the cylinder storage hydrogen leakage risk, cause its safety is poor. Therefore, it is of great significance to study and develop low-cost liquid hydrogen storage. Liquid hydrogen storage is to reduce the temperature and realize the liquefaction of hydrogen gas. Because its temperature requirements are always at low temperature, its application range is relatively single, mainly used in aviation. Compared with gaseous hydrogen storage, solid hydrogen storage has the advantages of low cost and safe storage, so it has become the focus of current research. There are two methods for solid hydrogen storage to store hydrogen in solid, one is the physical adsorption of carbon materials; the other is chemical adsorption, including metal oxides, carbon-based composites and graphene. The other is the generation of MgH 2 by chemical reactions. Due to the complex process, high cost and poor storage stability, solid hydrogen storage has not been used. Due to the low use cost, solid hydrogen storage has good safety performance, and has become the focus of research in this era [11].
Chemical hydrogen storage materials are mainly formed by hydrogen binding to other elements in the form of atoms or ions, including: metal hydrides and coordination hydrides. Metal hydride, as a kind of solid hydrogen storage material, has been studied earlier. It is a hydride formed by hydrogen and metal elements combined in the form of atoms or ions. In nature, there are a large number of metal hydrides, including a variety of naturally generated and synthetic metal hydrides. Metal hydrides are usually highly stable, causing their hydrogen release reaction to meet certain conditions. Therefore, in practical application, people usually have metal hydride used as a special type of solid hydrogen storage material. It is divided according to the energy and hydrogen reaction metal, can be divided into rare earth (RE), titanium (Ti) and wrong (Zr) three categories, magnesium (Mg) is a hydrogen storage alloy, but also vanadium (V) based solid solution [12].
Coordination hydride is not easy to decompose, resulting in increased hydrogen release temperature. Therefore, it is of great significance to study and develop low-cost liquid hydrogen storage. The theoretical hydrogen storage capacity of coordination hydride is usually large, and it is divided according to the different ligands. It can be divided into aluminum hydride, nitrogen hydride and borohydride. Among them, aluminum hydride and nitrogen hydride have a large hydrogen absorption amount, and can reach saturation adsorption at room temperature, so they have been widely concerned. Metal aluminum hydride is represented by NaAlH 4 , which has a large hydrogen storage capacity (7.4 wt %), but because NaAlH 4 has good stability, its hydrogen discharge temperature is high, and its hydrogen absorption reaction conditions are relatively strict, resulting in its hydrogen absorption reaction. Therefore, fluorine-containing compounds are usually used in industry to prepare metal nitride or hydrogen. In terms of metal nitrogen-hydrogen system, Li-Mg-N-H system has been studied deeply. The dynamic performance of the system is poor, and it usually needs to be improved by adding a catalyst. At present, the commonly used metal silicon nitride, aluminum nitride and metal borohydride can not meet the above requirements. Zhang et al. doped Mg (BH 4 ) 2 in the 2 LiNH-MgH system, and found that the initial hydrogen discharge temperature of the sample was reduced from 150C to 130°C, and the hydrogen discharge amount of the sample could reach 4.2 wt% within 20 min at 200 C. Because metal borohydride is prone to hydrolysis at room temperature, this system is mainly used in high temperature and high hydrogen release gas pressure environment. The metal borohydride has a large theoretical hydrogen storage capacity, but after hydrogen release, it produces boron with very low chemical activity, which results in the conditions of hydrogen absorption is relatively harsh and poor reversibility. Therefore, how to improve the metal borohydride can quickly and effectively convert into chemical bonded hydrogen in the hydrogen atmosphere has become one of the current research hotspots. For example, Mg (BHz) fully release hydrogen after producing Mg, B, etc., and its subsequent hydrogen release reaction basically depends on Mg to achieve [10-15].
Because the theoretical hydrogen storage quantity of MgH 2 hydrogen storage material is large (7.6 wt%), which has good reversibility and high natural abundance, it has been widely studied. However, the Mg–H bond has high thermal stability, resulting in high dehydrogenation temperature and slow kinetics. Magnesium-based hydrides are considered as one of the most promising hydrogen storage materials because of their good stability. MgH 2 At 300°C or higher before hydrogen release, its hydrogen absorption kinetics is quite slow. Therefore, there are great limitations in practical applications. In order to improve the hydrogen storage performance of magnesium-based hydride, the addition of catalyst is one of the most effective ways to change the catalyst performance and the most easy to achieve success. The addition of catalyst can provide more activation sites for hydrogen dissociation and diffusion, so as to improve their performance. In addition, the activity and stability can be enhanced by increasing the metal element content in the alloy or by using a composite catalyst. The effectiveness of various catalysts in improving the MgH 2 performance has been validated. Among them, the precious metal palladium is the most important one, but it has some shortcomings, such as being expensive and difficult to recycle. These include transition metals, metal oxides, and some other metal-based compounds. These catalysts all have relatively high activity and stability. In today's continuous in-depth research, compared with single-phase catalysts, hybrid catalysts generally show strong catalytic properties because of their special synergistic effect [13].
The catalyst preparation method also affects the catalytic performance. Different methods are used to prepare catalysts, which also have different catalytic effects, here, we will introduce a variety of catalyst preparation methods [14].
There are many kinds of catalyst preparation methods, usually used catalyst preparation methods are: ball grinding method, calcination synthesis method, solid hydride combustion synthesis method, hydrothermal synthesis and chemical synthesis, melt spinning, sol gel and etching method. These methods all have their own characteristics and advantages, but they also have some defects. At present, the relevant researchers are still seeking some new ways to prepare related catalysts. One of the most common and widely studied applications is the combination of two or more methods to prepare catalysts, such as mixed acid hydrolysis and metal ion doping. And the catalyst in the preparation process rarely only a single method for preparation, often a number of comprehensive use. The efficient alloy catalyst was successfully prepared by using — ball grinding — wet chemical ball grinding process. This preparation technique can improve the reaction rate and avoid problems such as metal loss. Graphene and CeF 3 were prepared by wet ball grinding and hydrogenation, and almost all catalyst preparation processes were prepared by ball grinding [15].
Ball grinding method is also called solid powder processing technology, practice has proved that this is a kind of innovative significance and potential alloy forming method, prepare many different kinds of substances, including amorphous alloy powder, nanocrystalline powder, metal compounds, composite and nanocomposite powder and nano materials, through the metal ball and catalyst, the catalyst rolling shear. In this process, grains of various morphology and sizes can be produced, some of which have high mechanical strength. Such as the amorphous preparation of cast Mg2Ni, there are new elements between the catalyst. The ball grinding is not only conducive to the production of a large number of defects, but also conducive to the production of grain boundary, and makes the Mg 2 Ni hydride formed in situ have a good distribution, thus obtaining the obvious hydrogen absorption performance. The ball grinding treatment finizes the particles, and the catalyst dispersion, micro-cracks will appear, which are conducive to the diffusion of H atoms in MgH 2 desorption / absorption [16].
The Mg 2 NiHx nanoparticles obtained from the Mg and Ni reaction catalyzed MgH 2 well and play a crucial role in improving the dehydrogenation properties of large-size MgH 2 . In terms of dynamics, the Mg 2 NiHx produced in situ is superior to the externally doped Mg 2 NiHx at both the high-density interface and the catalytic active site, and thus it is concluded that Mg 2 NiHx exhibits excellent catalytic activity in large-size MgH 2 .
Li et al et al. used nitrate citrate self-combustion method, with 99.99% purity and particle size of 20 nm [7, 9]. After doping with MgH 2 , mgH 2 grains became smaller, which increased the density and grain boundary of surface defects, and significantly improved the dehydrogenation performance of MgH 2 . We studied the influence of different conditions on the catalyst activity and the selectivity of the catalytic cracking gasoline. The starting desorption temperature of 7 mol% MnFe 2 O 4 -doped MgH 2 was 300°C, with a decrease of 140°C compared to undoped MgH 2 [3, 4]. With solid hydride combustion synthesis (HCS) process, by MgH2 and Mg2NiHx heterogeneous hydride (Mg 2 NiH0 3 and Mg 2 NiH 4 ) local assembly of hydride by hydrogenation combustion synthesis, synthesis can be observed on the microscopic map multiphase hydride atoms effective mixing, on the Mg / MgH 2 matrix formed rich grain boundary and phase boundary interface, used as a diffusion channel, is beneficial to improve the hydrogen diffusion rate. Play a crucial role in improving the dehydrogenation properties of large-size MgH 2 . From a kinetic perspective, the Mg 2 NiHx generated in situ is superior to the out-doped Mg 2 NiHx at both the high-density interface and the catalytic active site [16].
XRD is called X-ray diffraction (X-RayDiffraction). Through the diffraction in the crystal, the X-ray signal characteristics are obtained, and the diffraction pattern is obtained after processing.
The hydrogen storage alloys prepared by the composite system have the characteristics of large specific surface area and uniform pore distribution. Through the spectrum information, we can realize the conventional microscope phase measurement, and the "perspective eye" to see whether there are defects (dislocation) and lattice defects [17].
XRD and XRD results of MgH 2 samples after adding different amounts of catalyst for the same period of time. Generally speaking, a small amount of catalysts can not significantly improve the catalytic performance and maximize the catalytic performance. However, adding a large number of catalysts will make the hydrogen storage amount of MgH 2 greatly reduced. The XRD spectrum of the catalyst was used to determine the phase of the substance in the preparation of the catalyst, and the composition of the catalyst was compared with the PDF card and the original design. Comparing the change of diffraction peak to judge the change of the substance reaction, whether the reaction or whether a new substance is produced. For example, the XRD results of the resulting MgH 2 samples were analyzed by adding different amounts of the samples for grinding for 20 hours of KNbO 3 (Figure 1, 2).
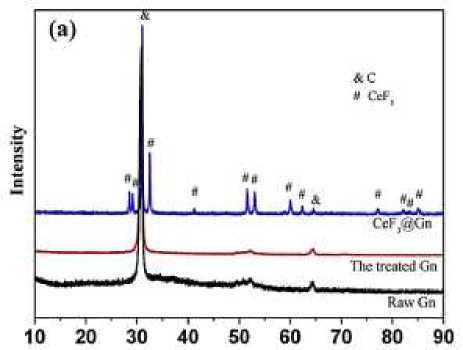
Figure 1. The XRD of the MgH 2 sample
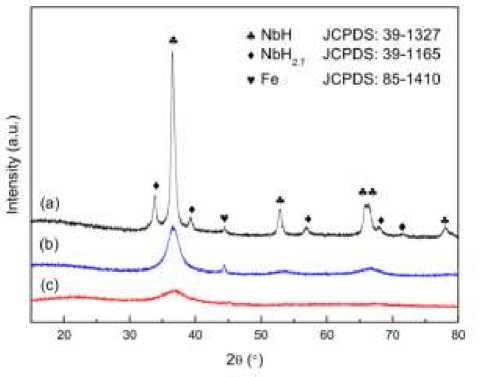
Figure 2. The XRD results of the MgH 2 samples
A large number of Ni peaks were not found in the experiment, indicating that all dispersed Ni particles reacted with Mg in situ to form binary intermetal compounds (Mg 2 Ni). As shown in Figure Figure (A), it is clearly seen that the diffraction peaks in the XRD spectrum are consistent with the standard peaks of C and CeF 3. This indicates the successful acquisition of pure additives.
By conducting XRD test of MgH 2 in the hydrogen absorption state of doped catalyst and MgH 2 in the hydrogenation state of doped catalyst, XRD can reveal the phase transition of the catalyst during dehydrogenation and rehydrogenation. This indicates that TiF3 is stable as a catalyst and no chemical reaction occurs during HEBM [17].
The SEM (Scanning electron microscopy) is a scanning electron microscope. Can characterize the distribution of catalyst in the composite material, by observing the hydrogen absorption process and hydrogen process, has many times after the process of hydrogen absorption composite material, the characteristics of microscopic morphology, to react the composite material in the process of microscopic morphological changes, such as after many hydrogen absorption process, reunion became obvious. For example, SEM observed that carbon catalyst (CNTs) can effectively control the growth of particles [3].
The SEM images of the samples before and after the catalyst are compared to see if the catalyst can form a large number of nucleation sites on the surface of the MgH2 matrix, resulting in surface activation and specific surface area of MgH2 particles.
After ball grinding of doped MgH 2 , the grain boundary increases due to the reduced particle size and increased surface defect density. Moreover, high concentrations of nanocatalyst particles form a large number of nucleation sites on the surface of the MgH 2 matrix, resulting in surface activation and specific surface area of MgH 2 particles.
The distribution of CNTs in the composite was characterized by SEM, and the loading amount of the prepared catalyst was quantified by differential scanning calorimetry combined with the thermogravimetric method (TG-DSC, STA449 F3, Netzsch). The structure and morphology of the synthetic materials were characterized by powder x-ray diffraction scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The X-ray emitted by the x-ray tube radiates to the surface of the sample to be tested, driving out the electrons in the inner layer of the sample to be tested and forming holes. The whole atomic system is conducted in a non-stable excited state. We can accurately analyze the structure, composition and performance of the strength and relative position of the spectral line and the correspondence between it and the sample components. In addition, the outer electrons spontaneously return to the inner layer and fill the holes with the radiation transition to generate the characteristic X-rays. Its energy is not affected by the incident radiation, which is the difference of energy between the two energy levels. This phenomenon is called the silicon-lithium infiltration effect. When the characteristic X-ray photon enters into the silicon-permeable lithium detector, the silicon atoms are ionized, generating multiple electron-hole pairs, which is proportional to the photon energy. According to the corresponding number of photoelectron energy and the number of electron holes of different species, the absorption coefficient or transmission rate corresponding to a specific wavelength range can be calculated. The pairing of these electron holes was collected by bias, converted after a series of converters to voltage pulses, supplied to the multi-pulse height analyzer, and counting the number of pulses in each band of the energy spectrum [15].
The morphology and nanostructures obtained from the samples were measured by transmission electron microscope (TEM, Tecnai G2 F30 S-TWIN) and scanning electron microscope (SEM, Hitachi SU-70), and the distribution of NiFe 2 O 4 on nanoscale MgH2 matrix was analyzed by TEM In order to explore the distribution of elements in the composite material composed of composite catalyst and MgH 2 , we can conduct EDS mapping analysis to obtain the elements are evenly distributed in MgH 2 . An EDX detector (Oxford Microanalysis 6767) is equipped to observe the morphology of the sample. Argon protective gas was used during the measurement to prevent h 2 O and O 2 contamination. The XPS spectrum directly shows that the doped NbHx has good stability, and the desorption activation energy of the sample by differential scanning calorimetry and Kissinger results. According to the apparent activation energy (Ea) at different heating rates, comparing the size of the activation energy can show how stable the composite system is compared with the pure MgH 2 . The thermal stability of the fully amorphous Mg 2 Ni alloy prepared by HEBM was studied by constant heating rate differential scanning calorimetry (DSC).
Based on the microstructure analysis and the excellent hydrogen storage properties, the catalytic mechanism is proposed. The mechanism of action of the catalyst additives is very important. The significant improvement in the kinetics of catalytic Mg rehydrogenation is attributed to the catalyst-doped Mg surface, which ultimately reduces the activation energy barrier for molecular hydrogen dissociation. The catalyst is evenly distributed on the surface of Mg, which produces the polycore site of the hydride (MgH2) phase during the hydrogenation process, which prevents the grain from growing up, and finally improves the cycle performance [18].
First-principles calculations show that Ni and Cu doped MgH 2 can effectively increase the interaction between H and substituted atoms, thereby weakening the stability of MgH 2 , reducing the bonding strength of MgeH, and improving the hydrogen storage properties of MgH 2 .
The action basis of the catalyst additive is very important, which provides a theoretical basis for the future research and design of the catalysts. At present, researchers have deduced that the role of different catalysts in improving MgH2 is different, and the main bases can be summarized as follows: hydrogen overflow (hydrogen flow effect), channel effect, channel effect, electron transfer between different valence states, and elongation of Mg-H bond.
According to the hydrogen overflow mechanism, the catalyst has a good porous structure and a high specific surface area, and the catalytic mechanism conducive to hydrogen dissociation of Ni 50% Cu solid solution can be summarized as the "hydrogen overflow" effect of Mg 2 Ni (Cu). It can promote the dissociation and recombination of hydrogen molecules, which can significantly enhance the catalytic effect of MgH 2 hydrogen storage properties [19].
Electrocatalytic hydrogen evolution reaction (hydrogen evolution reaction, HER) is an effective means to produce high purity hydrogen, which is expected to solve the gas energy and environmental problems caused by the large application of fossil energy. The atomization process cannot be realized without providing enough energy to achieve resonance or excitation energy. The key to this response is high efficiency and low cost, long-life electrocatalyst. At present, the best performance is mainly based on the precious metal platinum (Pt) electrocatalyst, but the expensive and rare reserves restrict its widespread use. Therefore, the development of new non-precious metal electrocatalysts with high activity, high stability and low cost is very popular. Researchers have made many efforts to develop non-Pt electrocatalyst, but its performance is still unable to meet the needs of large-scale use. Therefore, it is important to study the new non-silver nanoparticle / polymer composite system to achieve high activity, high stability and long service life electrocatalytic reactor. The interface electronic structure of HSBB catalyst is determined by the difference between the work function between the metal and the carrier Δφ, which then affects the interface hydrogen overflow process. By changing the surface atomic distribution of the material, the △ ф can be regulated, which means optimizing the electrode potential and improving the catalytic activity. The interface charge density will be diluted and distributed when Δφ is relatively small, resulting in the weakening of proton adsorption at the interface and the strengthening of proton adsorption on the metal surface. Therefore, catalysts with larger Δφ have better stability and activity. At this time, the energy barrier for hydrogen to diffuse across the interface is significantly reduced, and the excellent HER catalytic performance is finally obtained [19].
The decrease of the grain size of Ea compared with MgH 2 calculated by the Kissinger method can shorten the hydrogen diffusion channel, which is beneficial to improve the kinetic performance. After the ball grinding, Gn is disordered and irregularly dispersed, which is conducive to reducing the particle size and playing the role of "hydrogen diffusion channel".
The thermodynamic catalysis is mainly due to the electron exchange reaction with hydrogen molecules during the dissociation-absorption or recombination-desorption process. Like the addition of a Ti-based catalyst, an electron transfer occurs.
Further studies on MgH 2 Mg in the future are mainly mechanistic studies. The diffusion and bonding processes of hydrogen in MgH 2 need to be studied at the atomic and molecular scales. The precise interaction between the MgH 2 and the additives needs to be explained. Further theoretical calculations contribute to understanding the hydrodehydrogenation process of MgH 2 . A basic understanding of the MgH 2 is essential for further research [2].
The synergistic effects of stress strain and catalysts are needed to achieve simultaneous enhancement of MgH2 thermodynamics and kinetics. Further research results on these issues will provide important guidance for the development of high-performance magnesium and other metal hydride hydrogen storage. PCT measurements showed improved kinetics and enthalpy, enthalpy and entropy for pure MgH 2 . However, the heavily doped sample hinders the diffusion path of the hydrogen. In conclusion, the catalyst-enhanced MgH 2 overcomes many of the difficulties of bulk materials in activation energy and surface interaction dynamics.
Список литературы MgH2 insights used for hydrogen storage and catalysis: preparation, characterization and mechanism
- Xu, N., Yuan, Z., Ma, Z., Guo, X., Zhu, Y., Zou, Y., & Zhang, Y. (2023). Effects of highly dispersed Ni nanoparticles on the hydrogen storage performance of MgH2. International Journal of Minerals, Metallurgy and Materials, 30(1), 54-62. https://doi.org/10.1007/s12613-022-2510-8
- Zhang, J., Zhang, B., Xie, X., Ni, C., Hou, C., Sun, X., ... & Du, W. (2023). Recent advances in the nanoconfinement of Mg-related hydrogen storage materials: A minor review. International Journal of Minerals, Metallurgy and Materials, 30(1), 14-24. https://doi.org/10.1007/s12613-022-2519-z
- Lu, Z., He, J., Song, M., Zhang, Y., Wu, F., Zheng, J., ... & Chen, L. (2023). Bullet-like vanadium-based MOFs as a highly active catalyst for promoting the hydrogen storage property in MgH2. International Journal of Minerals, Metallurgy and Materials, 30(1), 44-53. https://doi.org/10.1007/s12613-021-2372-5
- Zhang, H., Xu, P., Chen, Z., Zhang, H., Shao, W., Li, Y., ... & Huang, Z. (2023). Dualfunctional electrostatic self-assembly nanoparticles enable suppressed defects and improved charge transport in perovskite optoelectronic devices. Chemical Engineering Journal, 459, 141559. https://doi.org/10.1016/j.cej.2023.141559
- Santos, C., Attah-Baah, J. M., Junior, R. S. S., Mâcedo, M. A., Rezende, M. V., Matos, R. S., ... & Ferreira, N. S. (2023). Insights into the Fe3+ Doping Effects on the Structure and Electron Distribution of Cr2O3 Nanoparticles. Nanomaterials, 13(6), 980. https://doi.org/10.3390/nano13060980
- Ji, P., Yu, R., Wang, P., Pan, X., Jin, H., Zheng, D., ... & Mu, S. (2022). Ultra‐fast and indepth reconstruction of transition metal fluorides in electrocatalytic hydrogen evolution processes. Advanced Science, 9(3), 2103567. https://doi.org/10.1002/advs.202103567
- Li, Ying (2022). Technical scheme of hydrogenation station based on solid state transportation of magnesium hydroxide (MgH2). Gas and Heat, 42(08), 20-23.
- Nyahuma, F. M., Zhang, L., Song, M., Lu, X., Xiao, B., Zheng, J., & Wu, F. (2022). Significantly improved hydrogen storage behaviors in MgH2 with Nb nanocatalyst. International Journal of Minerals, Metallurgy and Materials, 29(9), 1788-1797. https://doi.org/10.1007/s12613-021-2303-5
- Zhang, Jianjun (2022). Atomic-scale design of gas adsorption and catalytic properties of two-dimensional MgH2 systems based on first principles. Shandong University.
- Meng, Yuqin (2022). Study on the hydrogen storage properties of Ni-doped nano-MgH2. Northwest Normal University.
- Huang. Qiang, Huang. Pengru, Xu. Fen, & Sun, Lixian (2022). (Fe+Co) Study on the thermodynamic stability and bonding mechanism of co-doped MgH2 hydrogen storage materials. Journal of Guilin University of Electronic Technology, 42(03), 245-251.
- Jian, Ni, Liu, Yongfeng, Gao, Mingxia, & Pan, Hongge (2022). Catalytic modification and mechanism of MgH2 by graphene-loaded Ti-Ni bimetals. Journal of Materials Science and Engineering, 40(03), 381-389.
- Song, M., Zhang, L., Zheng, J., Yu, Z., & Wang, S. (2022). Constructing graphene nanosheet-supported FeOOH nanodots for hydrogen storage of MgH2. International Journal of Minerals, Metallurgy and Materials, 29(7), 1464-1473. https://doi.org/10.1007/s12613-021-2393-0
- Fu, Hong (2022). Preparation of carbon-based transition metal oxide composite catalyst and regulation of MgH2. Guangxi University.
- 赵思魏, 付钢, 甄文清, & 杨丽. (2022). Nb 2 C 及 Ni 功能化材料光催化性能的第-性原理 研究. Journal of Petrochemical Universities/Shiyou Huagong Gaodeng Xuexiao Xuebao, 35(5).
- Dong, Zizheng (2022). Study on hydrogen storage properties and synergistic mechanism of MgH2-NiF2-HSPC. Shandong University.
- Yu, Dunxi, Liu, Ying, Yu, Xin, Wu, Jianqun, & Han, Jingkun (2022). Progress on hydrogen production by hydrolysis of hydrogen storage medium MgH2. Journal of Huazhong University of Science and Technology (Natural Science Edition), 50(07), 110-120.
- Chen, Rumeng (2022). The exogenous hydrogen donor MgH2 regulates the occurrence of PCD in the rice aleurone layer through H2O2. Hainan University.
- Tian, Zhihui (2022). The influence of carbide on the hydrogen storage properties of MgH2 and its catalytic mechanism. Hebei University of Technology.


