Model-based study of oxidation processes in a jet engine fuel liquid phase
Автор: Orlovskaya N.F., Shupranov D.A., Bezborodov Yu. N., Nadeykin I.V.
Журнал: Сибирский аэрокосмический журнал @vestnik-sibsau
Рубрика: Технологические процессы и материалы
Статья в выпуске: 5 (26), 2009 года.
Бесплатный доступ
The process of oxidation in hexadecane liquid phase as a conventional model of oil hydrocarbons is investigated. The oxidation product structure is defined by means of Chromatography/Mass Spectrometry.
High-temperature oxidation, hexadecane, oxygen-containing organic compounds, jet fuel
Короткий адрес: https://sciup.org/148176063
IDR: 148176063
Текст научной статьи Model-based study of oxidation processes in a jet engine fuel liquid phase
Aviation kerosene is utilized in aircraft engines as a fuel and also as a coolant. Therefore, it should have the property of strong stability against high-temperature oxidation.
It would be of interest to investigate the processes flowing in high-temperature jet engine fuel oxidation liquid phase.
Hexadecane (HD) is a conventional model of oil hydrocarbons (fig. 1).
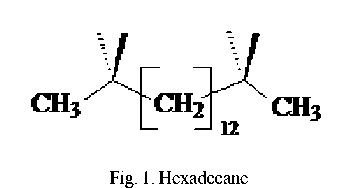
Hexadecane behavior in the process liquid phase oxidation was investigated by various authors and by different ways of reactor thermostatting [1].
The term “high-temperature” oxidation is usually applied to the processes flowing at the temperatures of 150 to 170 °C in case of hexadecane oxidation.
Previous research [1] has established that HD oxidation flowing at high temperature is an exothermal process.
At a certain moment, the so-called time-limited “thermal explosion” takes place in oxidation [1]. After the end of exothermal stage, the oxidation progresses at a lower speed.
Under the assumption [2] it occurs owing to formation of polar nanophase (inverted microemulsion, “water in oil” -type) on the basis of primary and secondary hydrocarbons oxidation products.
The nucleus of such reversed micellar aggregate under the assumption [2] contains a small amount of mono- and polycarboxylic acids and alcohols (polyols). The average sphere includes mainly fragments of ethers and esters. The external sphere consists mainly of long hydrocarbon chains providing stabilization of micelle in the non-polar hydrocarbon environment (fig. 2).
Changes of the oxidized hydrocarbon phase structure has been experimentally studied [2] indirectly, through a method for water-stain solubilization, for example, methyl-orange (MeOr).
Judging by changes in MeOr band position taking place with a rising hexadecane oxidation degree, the authors [2] have assumed that the localization of stain molecules in the oxidized hexadecane polar nanophase corresponds to a moderately polar oxidation product layer containing chemical bonds of type C-O-C, or similar ones.
Shift of MeOr absorption band in the process of increasing hexadecane oxidation degree has been obtained [2].
At the stage of deep oxidation the mechanism of reaction is especially complex. The prime oxidation products are generated. The physical and chemical properties of system are developed and they determine the system operational performance.
If the polar nanophase formation in oxidized hydrocarbons really occurs, the exploitation under heat can result in the formation of a complex colloid structure capable of impacting the flowing processes, on the base of hydrocarbon fuels.
This reasoning gave an impetus to thoroughly study the structure and the dynamics of high-temperature hexadecane oxidation products formation by air oxygen, in a liquid phase.
High-temperature (150 to 170 °C) hexadecane oxidation in a liquid phase by air oxygen in a bubbler reactor was carried out through flying products (condensate) selection and with an air bath. It allowed investigating the initial stages of deoxygenation change process in the reaction mixture and condensate following 2, 4, 5, 6, 7, 8 hours of oxidation.
The oxidation product structure in a condensate and reaction mixture was defined by means of gas chromatography with mass spectrometric detection (GC/MS).
Gas chromatograph-mass spectrometer: Agilent 7890A Gas Chromatograph and 5975C Gas Chromatograph/Mass Spectrometer.
Permanganatometry Method for Hexadecane Oxidation Determination. ThevalueofAO25 (deoxygenation/absorption of oxygen) corresponds to oxygen milligram quantity absorbed in 2 ml and conditionally counted on 100 ml of fuel at 25 °C and the reaction duration of 30 minutes [3].
25 ml of 0.1 N Potassium permanganate (KMnO4), 10 ml of 20 % sulfuric acid and 2 ml of fuel were placed into a 250-ml glass conical flask with a sealing plug.
The flask was closed up and put into water under the temperature of 25 ± 0,5 °C for 30 minutes, without stirring it up. Upon time being over, the oxidation reaction was terminated through injecting potassium iodide (2 g) with distilled water (100 ml) into the flask. The mixture was stirred up, and the isolated iodine was titrated with 0.1 N sodium thiosulfate Na2S2O3 atpresenceof1mlof0.5%starch solution (indicator). The quantity of sodium thiosulfate was equivalent to the quantity of potassium permanganate not reacted with fuel after 30 minute treatment:
AO.. 0.8i a+ b )-100/2, where 0.8 - oxygen milligrams isolated by 1 ml of 0.1 N KMnO4 in the acid medium and absorbed by fuel; 2 - ml of fuel introduced into the reaction; 100 - ml of fuel for which the value of value AO25 was conditionally recalculated; ci - 25 ml of 0.1 N KMnO4 solution introduced into the reaction; b - ml of 0.1 N Na2S2O3 solution utilized for titration of isolated iodine.
Dimension of AO25: mg 2/100mloffuel.
The metrological estimation of the method shows that the maximal deviation from the average parallel definition makes ± 2.0 % [3].
Then oxygen absorption was calculated from available data on sodium thiosulfate quantity utilized for titration.
All the data received during experiment are represented in tables 1-3 and the hexadecane mixture reaction and condensate oxidizability were demonstrated through diagrams (figures 3-5):
Hexadecane Oxidation Resistance. As follows from the diagram presented in figure 3, the amount of oxygen-containing compounds in the reactor eventually increases, and in the condensate the amount decreases. Probably it is explained by the fact that substances with greater molecular weight are formed during high-temperature oxidation.
Intheoxidationofparaffins, compoundswithamorecomplex chemical structure than simple acids (ketones, aldehydes, spirits orhydroperoxides), arealwaysfound. Occurrenceofmoreoxidized products (for example, lactones) is not necessarily consequence of repetitive oxygen attack upon the products already containing oxygen. Similaroxidizedproductsareformedattheminimaldepth of conversion, too. Lactones are internal complex esters of hydroxy acids. Hydroxy acids are easily dehydrated at higher temperatures. Among the oxidation products, formation both y -, and 5 -lactones could be observed.
High-temperature (150-170 °C) hexadecane oxidationwith air oxygen was investigated in a liquid phase as a model of hydrocarbon jet engine fuel, with sampling condensate and reaction mixture.
Values of AO25 parameter (absorption of oxygen) of reaction mixture and condensate received through hexadecane high-temperature oxidation were determined by permanganatometry.
The oxidation product structure was identified by means of a gas chromatography with mass spectrometric detection (GC/MS).
It was established that the amount of oxygen-containing compounds in the reactor eventually increases, and in condensate their amount decreases. Probably, substances with greater molecular weight are formed during high-temperature oxidation. Additional research are required to induce the law.
Among the hexadecane oxidation products, identified were: spirits, carbonyl compounds, carbon acids, esters of carbon acids and lactones (internal esters of -and -hydroxy carbon acids). Similar compounds can be a part of turned
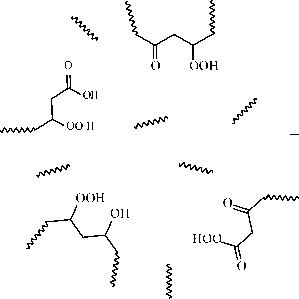
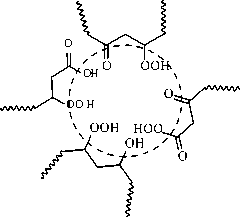
Fig. 2. Presumable structure of polar nanophase in the oxidized hydrocarbons
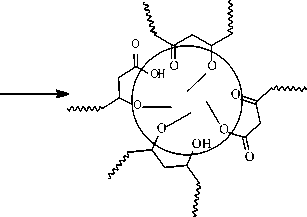
micellar aggregates, which impact upon physical and chemical properties and operational performance of jet engine fuels.


