Modification with amino groups of composite SiO2-TiO2 and pure TiO2 spheres prepared via the peroxo route
Автор: Morozov R.S., Avdin V.V., Krivtsov I.V., Gorshkov A.A., Urzhumova A.V., Osinskaya A.V., Yuzhalkin D.S.
Журнал: Вестник Южно-Уральского государственного университета. Серия: Химия @vestnik-susu-chemistry
Рубрика: Физическая химия
Статья в выпуске: 3 т.10, 2018 года.
Бесплатный доступ
A series of porous composite SiO2-TiO2 and pure TiO2 spherical particles were prepared via the peroxo route and subsequently used as the support for catalyst. Aminopropyltrimethoxysilane (APTMS) was grafted to the surface of support in the strictly unhydrous media leading to bonding of free amino groups to the support surface covalently. Procedure of APTMS grafting is easy to perform and may be spread for grafting other different functional groups to the inorganic surfaces of the catalyst support. The support samples were calcined at various temperatures in order to optimize the preparation conditions and boost the density of surface amino groups. It has been found that the quantity of grafted APTMS varies insignificantly for the different support samples. Grafted amino groups would be applied as active catalytic sites in different reactions of organic chemistry such as acylation of amines and alcohols, polymerization of lactones with hydroxyl groups, isomerization of unsaturated compounds, aldol condensation, Diels-Alder, Michael, Knoevenagel reactions. The mechanisms of reaction activation by amino groups are the transfer of electron density to a reacting molecule and formation of an intermediate complex. While widely applied catalysts are liquid amines, it is desirable to transform these substances into the heterogeneous form for better regeneration and purification of reaction products from the initial reagents. Prepared catalysts exhibit high amino groups load equal to 1 mmol/g, taking into account the localization of amino groups on the surface of the catalyst.
Peroxo method, sio2, tio2, aptms, amino groups, base catalysis
Короткий адрес: https://sciup.org/147233099
IDR: 147233099 | УДК: 546.865+547.53.024+548.312.5 | DOI: 10.14529/chem180303
Текст научной статьи Modification with amino groups of composite SiO2-TiO2 and pure TiO2 spheres prepared via the peroxo route
Catalysts are inherent part of modern organic chemistry. Catalysts simplify proceeding of chemical reactions, make it faster, more selective, allowing to lower the temperatures of processes. Wide groups of synthetic reactions proceed with homogeneous Lewis basic catalysts – these are amines, quaternary ammonium salts, nitrogen-containing heterocycles, phosphines, etc. The catalytic mechanism of Lewis basic sites origins from the interaction between a base site and an electrophilic compound, the activation of reagents proceeds through the transfer of electron density towards the reacting molecule. The intermediate compound is usually a highly reactive hypervalent [1] complex. Lewis base catalysts involve electrophilic, nucleophilic and ambiphilic reagents [2] to the reaction. This group of reaction includes: acylation of amines and alcohols [3], polymerization of lactones with hydroxyl groups [4], isomerization of unsaturated compounds [5], aldol condensation [6], Diels-Alder [7], Michael [8], Knoevenagel [9] reactions, etc. While the widely applied base catalysts are homogeneous, the desired requirement is to transform the homogeneous catalysis to the heterogeneous form. This improvement allows getting rid of catalyst traces in products and regenerating the catalyst itself. To do this the homogeneous catalyst may be anchored on the solid support – TiO2, SiO2 are widely used. These oxides are non-toxic, inert, cheap and can even improve reactivity of the anchored catalyst because of the enhanced adsorption of reagents on the surface. They exhibit optimal distribution of catalyst active sites, high surface area [10, 11]. Nevertheless that deposition of the thin layer of a catalyst on the surface of a support allows avoiding the agglomeration of catalyst particles. The mechanism of reaction rate enhancement on the heterogeneous catalysts in comparison to the homogeneous ones includes the adsorption of reacting species on the catalyst surface – this increases concentration of reagents near the active sites [12]. Varying the catalyst support it is possible to precisely tune such properties as specific surface area, point of zero charge (affects the reagents adsorption), size of pores (allows molecule size selectivity). The shape of support particles is not the least important parameter – spherical particles are most desired for the flow processes because they arrange the laminar flow of reaction solution providing more stable conditions.
The composite SiO 2 –TiO 2 oxide has higher stability of porous structure in conditions of high temperature and moisture than the individual oxides. In the present study both pure TiO 2 and composite SiO 2 –TiO 2 materials have been used as the catalyst support. Pure TiO 2 and composite SiO 2 –TiO 2 materials for the catalyst support can be prepared via different synthesis routes: sol–gel, hydrothermal, high temperature oxidation, etc. Here we propose the recently developed peroxo route for preparation of composite oxide materials. This route allows the preparation of spherical particles with high surface area and desired size [13, 14] without using toxic, unstable, expensive titanium alkoxides. The method allows for porous structure without the use of templates, via the simple reflux procedure.
Subsequent procedure of aminopropyltrimethoxysilane grafting results in the inorganic support modified with amino groups. Grafted amino groups are thermally stable up to 250 °C. Their quantity reaches 1.31 mmols/g, taking into account their surface localization, the density of amino groups is very high. Basicity of amino groups is on the medium level for catalysis of such reactions as Knoevenegel and Michael condensation, the solid support is stable at reaction conditions, the spherical shape is suitable for the flow process.
Experimental
Chemicals and characterization Titanium oxysulfate hydrate (TiOSO 4 ·H 2 O) containing 17 % of H 2 SO 4 was used as titania source, it was provided by Alfa Aesar. TEOS (tetraethylorthosilicate), aqueous ammonia (NH 3 ^H 2 O) solution of 25 wt %, n- propanol, and H2O2 were of analytical grade and were produced by Reachim. Aminopropyltrimethoxysilane (APTMS) of 98 % purity was provided by Sigma Aldrich.
Morphological investigation of the prepared materials was performed by a JEOL JSM 7001F field emission scanning electron microscope (SEM). Specific surface area, and pore size distribution of mesopores were probed by N 2 adsorption at 77 K using an ASAP 2020 Micromeritics analyzer. All aminomodified samples were degassed at 100 ºC for 2 h prior to analysis. Thermoanalytical studies were performed using a simultaneous TG-DSC thermal analyzer Netzsch 449 F1 in the temperature range from 25 to 1000 ºC in argon flow at the heating rate of 10 K min–1. FTIR spectra were recorded using a Shimadzu IR Affinity spectrometer. Calcination of materials was performed in a NABERTHERM oven in the atmosphere of air.
Preparation of porous TiO 2 and SiO 2 –TiO 2 spheres via the peroxo route Preparation of support materials proceeded according to the protocol, described in the articles [13, 14]. Peroxo route was used for the preparation of both pure TiO 2 and composite SiO 2 –TiO 2 spheres. For the surface area the enhancement reflux procedure was applied [14].
Preparation proceeded in the following way: 10 mmol of TiOSO 4 was dissolved in distilled water, after this 10 mL of aqueous ammonia were added. The colloid precipitate of titanium dioxide formed immediately. Precipitate was washed with deionized water several times, put on the ice bath, and under the vigorous stirring 10 mL of hydrogen peroxide were added dropwise. Then the pH value was adjusted to 9.5 with diluted aqueous ammonia, volume adjusted to 100 mL. The transparent green solution of peroxotitanate complex was formed. Separately solution of 10 mmol (2.2 mL) TEOS in 97.8 mL of propanol was prepared and poured into the aqueous peroxotitanate solution. The solution immediately turned turbid due to formation of colloid particles of mixed oxide SiO 2 –TiO 2 . The mixture was left for stirring for 24 hours and then washed and dried. In the case of pure TiO 2 particles preparation solution of TEOS in propanol was changed to pure propanol.
Procedure of surface area enhancement was held via the reflux procedure; dried particles were put into the water–ethanol solution with 1:1 ratio and 60 mL volume, the pH value of solution adjusted to 5 by diluted HCl. Then the solution was subjected to reflux procedure during 24 hours. Afterwards the precipitate was washed, dried and calcined at 400 °C and 700 °C during 1 hour. A piece of both pure TiO 2 and composite SiO 2 –TiO 2 was left uncalcined and was subjected to washing in the Soxlet apparatus with CHCl 3 during 6 hours in order to check the performance of uncalcined support for the catalyst.
Grafting of amino group to substrates For grafting of APTMS to the inorganic substrate the literature sources offer different conditions: gas phase, liquid, supercritical conditions, various solvents, temperatures, catalysts, various ways of substrate preparations. The most widely used solvent for this reaction is dry toluene – because it is nonpolar enough to exclude the interaction with the surface hydroxyls, it dissolves APTMS pretty well and it boils at rather high temperature. Other solvents may be used: hexane, methanol, ethanol or toluene with the water addition. If the reaction media contains water the grafting consists of 2 parallel reactions: hydrolysis of alkoxy group and condensation of the emerging hydroxyl groups of APTMS with the surface hydroxyl groups of the substrate or with the hydroxyl groups of the neighbouring APTMS. In this case a multilayer cover of the substrate with APTMS forms. Otherwise, when the solution does not contain water, alkoxy groups react directly with the surface hydroxyls with the alcohol elimination. The absence of water leads to the monolayer of grafted silane. Steric factors are also studied in the literature. As we have three functional alkoxy groups per a reacting molecule we would expect 1, 2 or 3 bonds of each silane formed with the surface, but in the case of steric factors 3 bonded silanes were not detected – only 1 or 2 – bonded, which was verified by the means of NMR [15], etc. Other bonds are formed with the neighboring silane. Condensation of the neighbouring alkoxy groups of the already grafted silanes also takes place – this forms firm and solid Si–O–Si bonds. Catalytic effect is related to amino groups [16]. Scheme of APTMS grafting is on the Fig. 1
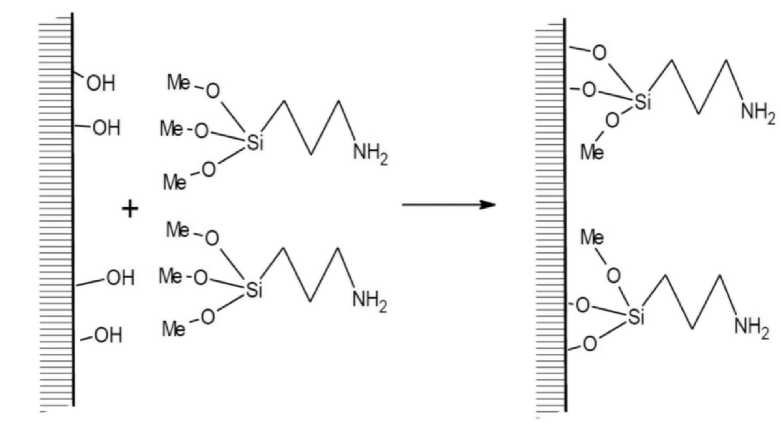
Fig. 1. Scheme of APTMS grafting to surface hydroxyl groups of substrates
Theoretical quantity of APTMS needed for the reaction may be calculated from the surface density of hydroxyl groups. The data of hydroxyl concentration on thermally treated silicas are accessible. The surface density of the silanol groups on silica is known to be not firmly dependent on the source of silica, the initial density of fully hydroxylated silica is known to be 4.2 – 5.7 nm–2, after treatment in vacuum at 200 °C all silanol groups are kept intact , after treatment at 400 °C the surface density of silanols is about 2.5 units per nm–2 and after treatment at 700 °C isolated hydroxyls are still present and the density is about 1.1 nm–2 [17]. We suppose that the density of silanol groups on the surface of composite SiO 2 –TiO 2 and pure TiO 2 is approximately the same. Excessive calculated quantity of APTMS has been accepted to be 50 % (w/w) to the weight of the substrate . Eight substrates were considered for the grafting procedure. Specifications are presented in the table 1.
Table 1
Specification of substrates
|
Substrate name |
Specfication of the substrate |
Sample name after APTMS grafting |
|
T |
Pure TiO 2 , non-porous, calcined 400 ° C |
T Amino |
|
T Sox |
Pure TiO 2 , porous, washed in Soxlet |
T Sox Amino |
|
T400 |
Pure TiO 2 , porous, calcined 400 °C |
T 400 Amino |
|
T700 |
Pure TiO 2 , porous, calcined 700 ° C |
T 700 Amino |
|
ST |
Composite SiO 2 -TiO 2 , non-porous, calcined 400 ° C |
ST Amino |
|
STSox |
Composite SiO 2 -TiO 2 , porous, washed in Soxlet |
ST Sox Amino |
|
ST400 |
Composite SiO 2 -TiO 2 , porous, calcined 400 ° C |
ST 400 Amino |
|
ST700 |
Composite SiO 2 -TiO 2 , porous, calcined 700 ° C |
ST 700 Amino |
Before the grafting procedure samples should be properly treated to remove all hindering compounds from the surface of the samples. As it was already mentioned, we used three different pretreatment ways for porous samples before APTMS grafting: washing on the Soxlet apparatus with CHCl 3 for 6 hours, calcination at 400 °C for 1 hour, and calcination at 700 °C for 1 hour. Non-porous samples were also subjected to APTMS grafting after calcinations at 400 °C during 1 hour. After this step all samples were degassed in high vacuum for 1 hour at 100 °C to remove the hindering molecules from the surface .
APTMS grafting itself proceeded through the following way: 200 mg of each substrate was put into 30 mL of dry toluene, under stirring 100 ul of APTMS (50 %w/w) was poured into the reaction media and installed under reflux condenser for 24 hours. After reaction ending the solution was decanted, the sample dried and washed in the Soxlet apparatus for 6 hours with the CHCl 3 .
Results and Discussion
Scanning Electron Microscopy Scanning electron microscope observation shows spherical morphology of both pure TiO 2 and composite SiO 2 –TiO 2 oxide (Fig. 2).
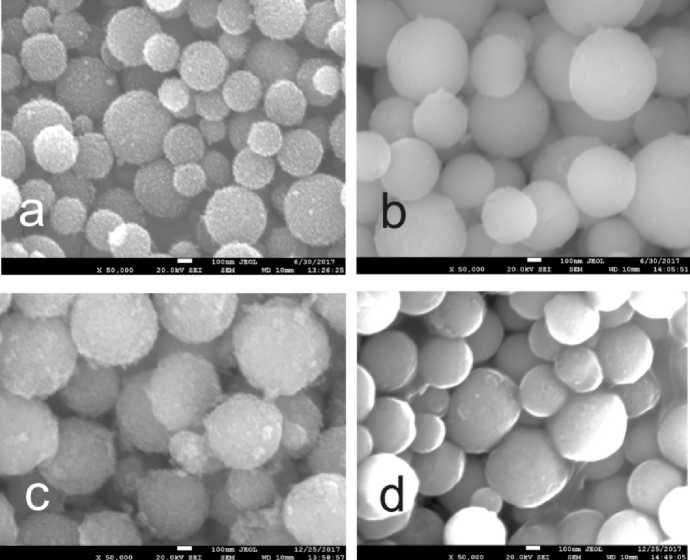
Fig. 2. Microphotographs of a) porous TiO 2 spheres, calcined at 400 °C; b) porous composite SiO 2 –TiO 2 spheres, calcined at 400 °C; c) porous TiO 2 spheres, calcined at 400 °C, grafted with APTMS;
d) porous composite SiO 2 –TiO 2 spheres, calcined at 400 °C, grafted with APTMS
The obvious difference between ungrafted pure TiO 2 and composite SiO 2 –TiO 2 is rougher surface of the former. The rough surface of TiO 2 spheres consists of smaller crystalline structures with sizes about several nanometers, which may be detected by the means of X-ray diffractometry [13, 14]. Voids between crystal particles were studied by the means of low-temperature nitrogen adsorption. It has been stated that these are mesopores [14]. On the contrary, the surface of composite oxide looks even at such magnification. For this material only micropores are detected by the means of low-temperature nitrogen adsorption [14]. This is in good correlation with low-temperature nitrogen adsorption data, where mesopores were only detected in inpure TiO 2 after APTMS grafting. Microphotographs after APTMS grafting demonstrate capping of TiO 2 pores. The surface of this material becomes smoother, while the surface of composite oxide does not change a lot and reveals the initial even character.
FTIR Investigation The FTIR spectra were recorded with KBr as a support for the sample. Resolution of spectra was set at 4 cm–1. The absorption peaks at wavelengths 2930 and 2864 cm–1 emerging after APTMS grafting are attributed to stretching vibration of C-H [18, 19] bonds. Quantitative estimation of grafted APTMS by calculation of the peak areas was presented in the literature [18], but in this case it is restricted due to the wide absorption band of O-H bonds at 2800–3700 cm–1, which overlap with the C-H bond absorption band. As pure TiO 2 has weak absorption at 1000–1200 cm–1, we can see the emerging peaks at 1032 cm–1 and 1129 cm–1, which correspond to absorption of the Si–O bonds of grafted APTMS [20]. As the SiO 2 –TiO 2 samples already have the Si-O bonds, the region of 10001200 cm–1 does not change after grafting of APTMS. The band at about 1650 cm–1 is attributed to the O–H bonds of inner structure [21], which does not disappear even after calcinations at 400 °C. The weak adsorption band at 1565 cm–1 corresponds to the bending vibration of the N–H [22–24] bonds. The N–H bond also has weak absorption band at about 3250 cm–1, which corresponds to its stretching vibrations [20]. Low-intensity band at 1217 cm–1 is caused by absorption of the C–N bond [19] (Fig. 3). The FTIR spectra clearly show the presence of grafted aminopropyl groups on the substrate surface.
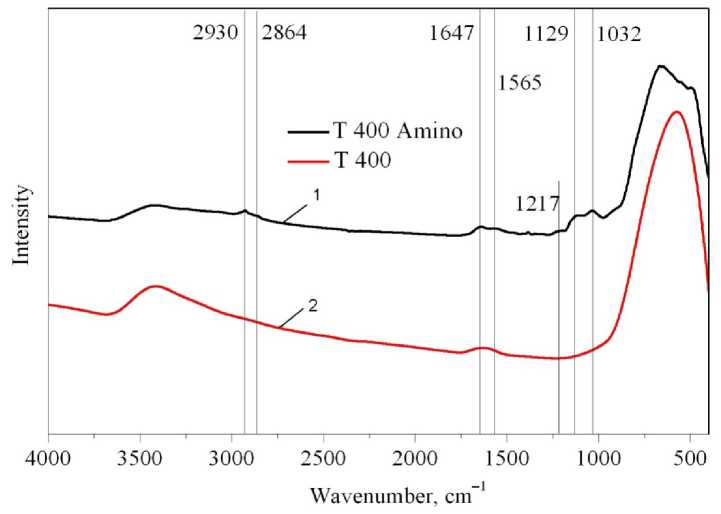
Fig. 3. FTIR spectra of pure porous TiO 2 sample before and after APTMS grafting: 1 – T 400 Amino; 2 – T 400
The FTIR spectra of the composite material reveal additional peaks at 1045 cm–1, 458 cm–1, which are initially present because of the Si–O bond absorption [14]. The peak at 952 cm–1 corresponds to the Si–O–Ti bonds of the substrate matrix [13]. High optical density of the composite substrate in region 1000–1200 cm–1 does not allow capturing the Si–O and C–N bonds of APTMS, while the appearance of the C–H and N–H bonds absorption can easily be detected. Wavelengths of the O–H and N–H bonds are slightly shifted to lower wavelengths, namely 1627 and 1519 cm–1, respectively (Fig. 4).
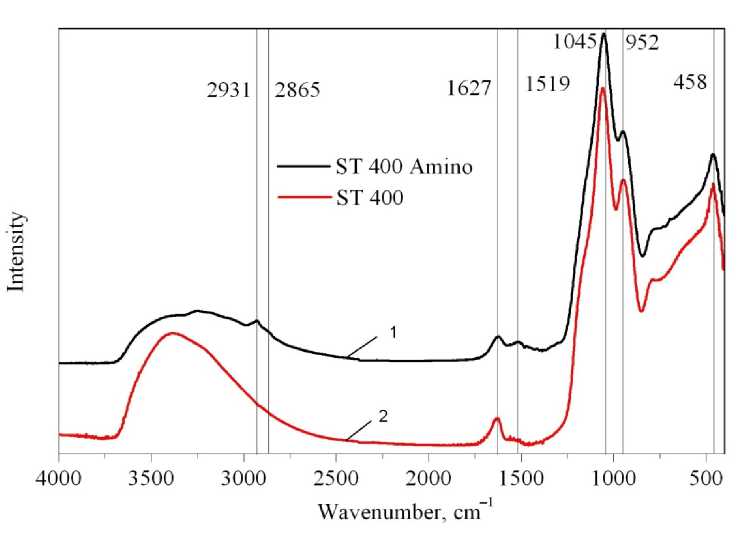
Fig. 4. FTIR spectra of pure porous composite SiO 2 –TiO 2 sample before and after APTMS grafting:
1 – ST 400 Amino; 2 – ST 400
Thermal Analysis Thermogravimetry is the most widely used way to evaluate the quantity of grafted organic moieties because it is simple to perform and gives accurate results [25–28]. Thermal analysis proceeded in the inert atmosphere (Ar) for better separation at different stages of weight loss. Heating rate was set at 10 K/min, heating was held up to 1000 °C.
Typical graphs of thermal analysis for TiO 2 with grafted APTMS are presented in Fig. 5.
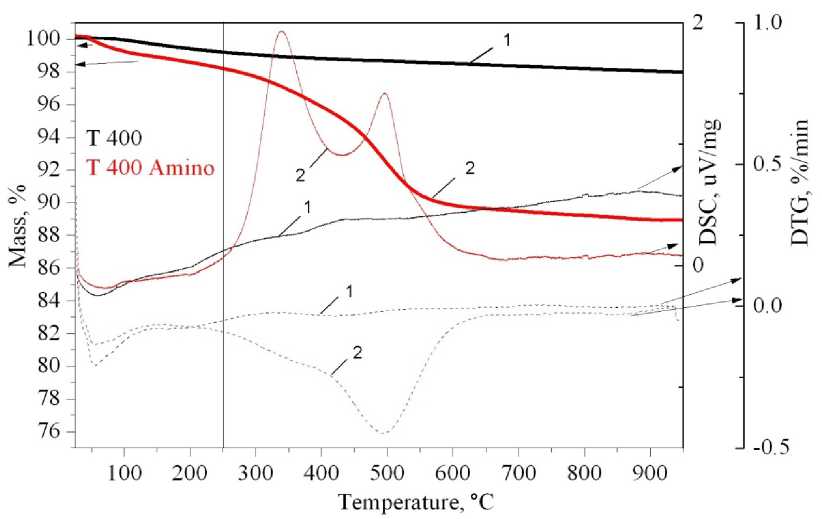
Fig. 5. Thermal analysis of pure TiO 2 , calcined at 400 °C before and after APTMS grafting.
The solid heavy line is the TG curve, the solid light line is the DSC curve, the dash line is the DTG curve: 1 – T 400; 2 – T 400 Amino
Samples with grafted APTMS pass through two stages of weight loss under heating. The first stage lasts from room temperature up to 250 °C and corresponds to the loss of physisorbed water and other compounds. The second stage – from 250 to 1000 °C – corresponds to the chemisorbed organic moieties degradation. Degradation of organic groups proceeds through two stages (DSC curve, DTG curve). The first stage lasts from 250 to 400 °C and corresponds to organic chain degradation and rupture of the C–H bonds, the amino groups also degrade through this process. The second stage – from 400 to 1000 °C – corresponds to loss of carbon, which appears through organic moieties degradation.
For composite SiO 2 –TiO 2 material the weight loss shifts to higher temperatures, and the exothermic peaks are twin. Positive thermal effect for composite SiO 2 –TiO 2 material at 670 °C corresponds to the TiO 2 crystallization. For pure TiO 2 this peak is absent because of its initial crystallinity (Fig. 6).
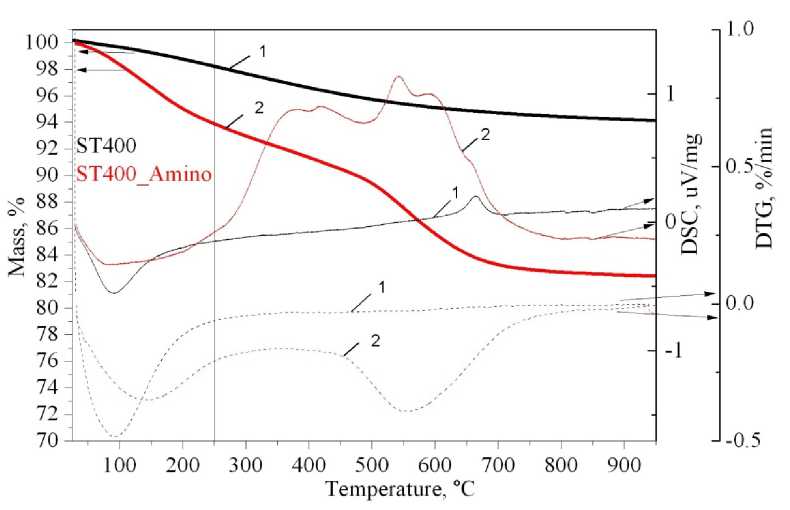
Fig. 6. Thermal analysis of composite SiO 2 –TiO 2 , calcined at 400 °C before and after APTMS grafting.
The solid heavy line is the TG curve, the solid light line is the DSC curve, the dash line is the DTG curve: 1 – ST 400; 2 – ST 400 Amino
For aminomodified material the stage of weight loss from to 250 to 1000 °C corresponds to grafted aminopropyl moieties loss. While for the non-modifed one it corresponds to degradation of hydroxyl groups and, in a lesser degree, of the remaining solvent or precursor. Therefore the difference of weight loss from to 250 to 1000 °C between these two samples is assumed to be the content of aminopropyl groups. The results are presented lower. As expected, grafting capacities of non porous materials are lower than those of the porous one. For the composite substrate this difference is approximately 4-fold, while for TiO 2 this difference is much less. Treatment procedure has minor effect on the amount of aminopropyl groups content. Values are similar for all 3 types of pretreatment. Both composite SiO 2 -TiO 2 and pure TiO 2 materials have similar values of aminopropyl groups after grafting (Table 2).
Table 2
Content of grafted aminopropyl groups subject to pretreatment conditions, %
|
Sample name |
Aminopropyl groups content, % (mmol/g) |
|
T Amino |
4.7 / (0.81) |
|
T Sox Amino |
7.6 / (1.31) |
|
T 400 Amino |
7.3 / (1.26) |
|
T700Amino |
5.8 / (1.00) |
|
ST Amino |
1.6/ (0.28) |
|
ST Sox Amino |
6.9/ (1.19) |
|
ST 400 Amino |
6.1/ (1.05) |
|
ST 700 Amino |
6.5/ (1.12) |
Another conclusion is that the quantity of grafted APTMS depends non- linearly on the specific surface area of the substrate. For example, when the surface area of porous and non porous substrates differs by 10–20 times, the quantity of grafted APTMS differs only by 1.5–4 times. Quantities of grafted aminopropyl groups are presented in Fig. 7.
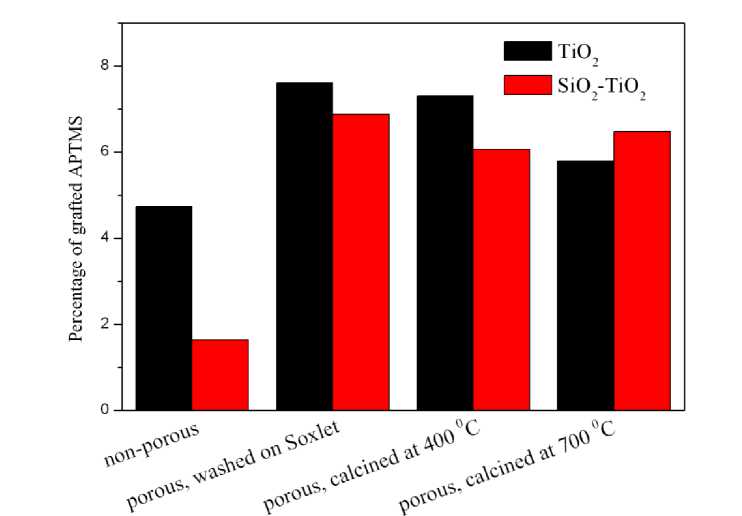
Fig. 7. Content of grafted aminopropyl groups subject to pretreatment conditions, %
Thermal Stability of Organic Groups In order to investigate thermal stability of organic functional groups, sample T 400 Amino was calcined at 250 °C and 400 °C in air atmosphere during 1 hour – according to stages of weight loss on thermal analysis. Equal masses (2 mg) of sample T 400 Amino before calcination, after calcination at 250 °C in air, after calcination at 400 °C in air were mixed with 20 mg of KBr to get the spectra (Fig. 8).
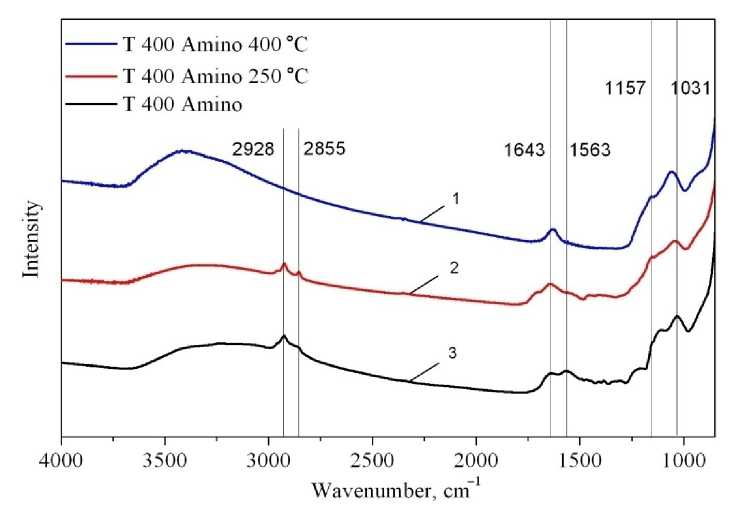
Fig. 8. Thermal stability of aminopropyl groups:
1 – T 400 Amino 400 °С; 2 – T 400 Amino 250 °С; 3 – 2 – T 400 Amino
After calcination at 250 °C the FTIR pattern is completely similar to the non-calcined sample: the characteristic peaks at 2928, 2855, 1563 cm–1 have the same intensities – meaning that aminopropyl groups are stable up to 250 °C. After calcination at 400 °C the adsorption bands at 2928, 2855 cm–1, and 1563 cm–1 , corresponding to C-H and N-H absorption, respectively, disappear – relating to degradation of the organic chain, with the Si-O bonds at wavelengths 1000–1200 cm–1 still present. This says that the degradation of organic moieties proceeds from 250 °C to 1000 °C through 2 stages: the 250–400 °C degradation of organic chain, the 400–1000 °C carbon residues loss.
Surface Area Characteristics of Materials after APTMS Grafting After APTMS grafting all samples have negligible microporosity. Taking into account the most abundant size of micropores equaling 0.5 nm [14] and the length of APTMS molecule equaling 0.7 nm, we suppose that APTMS does not penetrate into the micropores but grafts to the external surface and completely blocks the orifice of micropores even for the small molecules of N 2 with 0.3 nm size. Values are presented in Table 3.
Table 3
Specific surface area of materials after APTMS grafting
|
Sample name |
BET Surface area m2/g |
|
T_Sox_Amino |
24.1 |
|
T_400_Amino |
68.1 |
|
T_700_Amino |
41.5 |
|
ST_Sox_Amino |
25.0 |
|
ST_400_Amino |
20.2 |
|
ST_700_Amino |
14.6 |
Despite the micropore blocking, 2 samples still have mesopores even after APTMS grafting. These are T 400 Amino and T 700 Amino. Medium pore sizes of mesopores after APTMS grafting are: 4 nm for T400 Amino and 10 nm for T 700 Amino (Fig. 9). The preparation procedure allows fine tuning for the pore sizes of resulting material, as well as for the specific surface area. These properties are very important in such fields as catalysis and sorption.
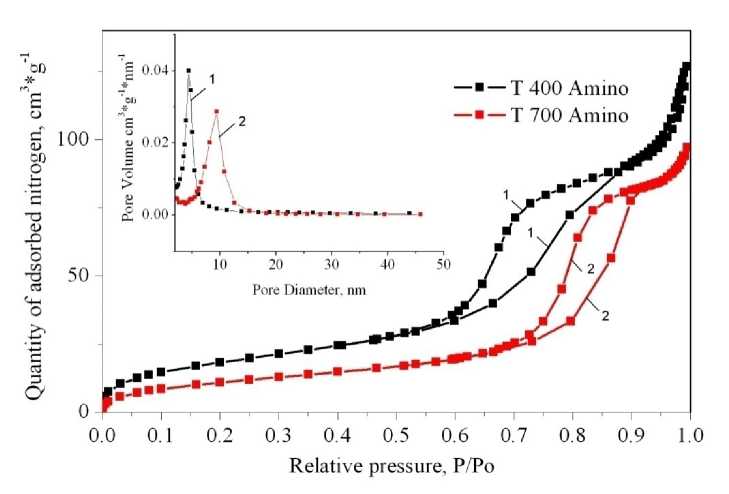
Fig. 9. Isotherms of nitrogen adsorption on the T400 Amino (1) and T700 Amino (2) materials
Conclusions
The peroxo method was used for the preparation of spherical nanoparticles of TiO2 and composite SiO2–TiO2 oxides which were subsequently thermally treated at 400 °C and 700 °C or just left to dry without calcination. These substrates were modified with aminopropyl groups, grafted to the surface hydroxyl groups of substrates. Grafting of amino groups was verified by the means of infrared spectroscopy. The quantities of grafted aminopropyl groups were measured by the thermal analysis and did not change significantly with the temperature of thermal pretreatment. The values are 6–8 % m/m, which is approximately equal to 1 mmol of amino groups per gram of the catalyst. Despite the low specific surface area of materials after APTMS grafting two of them still have mesopores. These are the TiO2 spheres, calcined at 400 °C and 700 °C prior to APTMS grafting. Pore size distribution in these materials is pretty narrow and allows us to apply the obtained catalysts for the selective reactions between reagents with stated sizes.
Acknowledgements
South Ural State University is grateful for financial support of the Ministry of Education and Science of the Russian Federation (grant No 4.9722.2017/8.9).
The article is completed with the financial support of the Russian Federation government (Government Regulation № 211 16.03.2013), agreement № 02.A03.21.0011.
Список литературы Modification with amino groups of composite SiO2-TiO2 and pure TiO2 spheres prepared via the peroxo route
- Nelson S.G., Zhu C., Shen X. Catalytic Asymmetric Acyl Halide-Aldehyde Cyclocondensation Reactions of Substituted Ketenes. Journal of American Chemical Society, 2004, vol. 126, pp. 14-15. DOI: 10.1021/ja0391208
- Denmark S.E., Beutner G.L. Lewis Base Catalysis in Organic Synthesis. Angewandte Chemie - International Edition, 2008, vol. 47, no. 9, pp. 1560-1638. DOI: 10.1002/anie.200604943
- Cameron L.L., Wang S.C., Kluger R. Biomimetic Monoacylation of Diols in Water. Lanthanide-Promoted Reactions of Methyl Benzoyl Phosphate. Journal of the American Chemical Society, 2004 vol. 126, № 34, pp. 10721-10726. DOI: 10.1021/ja049538l
- Coulembier O., Mespouille L., Hedrick J.L., Waymouth R.M., Dubois P. Metal-free Catalyzed Ring-Opening Polymerization of β-Lactones: Synthesis of Amphiphilic Triblock Copolymers Based on Poly(DimethylmalicAcid). Macromolecules, 2006, vol. 39, no. 12, pp. 4001-4008. DOI: 10.1021/ma060552n
- Trost B. M., Kazmaier U. Internal Redox Catalyzed by Triphenylphosphine. Journal of Americal Chemical Society,1992, vol. 114, № 20, pp 7933-7935. DOI: 10.1021/ja00046a062
- Nakagawa T., Fujisawa H., Nagata Y., Mukaiyama T. Lithium Acetate-Catalyzed Aldol Reaction between Aldehyde and Trimethylsilyl Enolate in Anhydrous or Water-Containing N,N-Dimethylformamide. Bulletin of the Chemical Society of Japan, 2004, vol. 77, pp. 1555-1557.
- DOI: 10.1246/bcsj.77.1555
- Suttibut C., Kohari Y., Igarashi K., Nakano H., Hirama M., Seki C., Matsuyama H., Uwai K., Takano N., Okuyama Y., Osone K., Takeshita M., Kwon E. A Highly Enantioselective Diels-Alder Reaction of 1,2-Dihydropyridine Using a Simple β-Amino Alcohol Organocatalyst for a Practical Synthetic Methodology of Oseltamivir Intermediate. Tetrahedron Letters, 2011, vol. 52, № 37, pp. 4745-4748.
- DOI: 10.1016/j.tetlet.2011.06.109
- Knudsen K.R., Mitchell C.E.T., Ley S. V. Asymmetric OrganocatalyticConjugate Addition of Malonates to EnonesUsing a Proline Tetrazole Catalyst. Chemical Communications, 2006, pp. 66-68.
- DOI: 10.1039/b514636d
- Ramachary D.B., Anebouselvy K., Chowdari N.S., Barbas C.F. III Direct Organocatalytic Asymmetric Heterodomino Reactions: The Knoevenagel/Diels-Alder/Epimerization Sequence for the Highly Diastereoselective Synthesis of Symmetrical and Nonsymmetrical Synthons of Benzoannelated Centropolyquinanes. Journal of Organic Chemistry, 2004, vol. 69, pp. 5838-5849.
- DOI: 10.1021/jo049581r
- Bagheri S., MuhdJulkapli N., Bee Abd Hamid S. Titanium Dioxide as a Catalyst Support in Heterogeneous Catalysis. The Scientific World Journal, 2014, Volume 2014, Article ID 727496.
- DOI: 10.1155/2014/727496
- Sahoo S., Bordoloi A., Halligudi S.B. Ordered Mesoporous Silica as Supports in the Heterogeneous Asymmetric Catalysts. Catalysis Surveys from Asia, 2011, vol. 15, № 3, pp. 200-214.
- DOI: 10.1007/s10563-011-9122-z
- Schlögl R. Heterogeneous Catalysis. Angewandte Chemie - International Edition, 2015, vol. 54, № 11, pp. 3465-3520.
- DOI: 10.1002/anie.201410738
- Morozov R., Krivtsov I., Avdin V., Amghouz Z., Khainakov S.A., García J.R. PeroxoMethod for Preparation of Composite Silica-Titania Spheres. Journal of Non-Crystalline Solids, 2016, vol. 435, pp. 8-16.
- DOI: 10.1016/j.jnoncrysol.2015.12.024
- Morozov R., Krivtsov I., Avdin V., Amghouz Z., Gorshkov A., Pushkova E., Bol'shakov O., Bulanova A., Ilkaeva M. Microporous Composite SiO2-TiO2 Spheres Prepared via the Peroxo Route: Lead(II) Removal in Aqueous Media. Journal of Non-Crystalline Solids, 2017, article in press.
- DOI: 10.1016/j.jnoncrysol.2017.11.031
- Valkenberg M.H., Hölderich W.F. Preparationand Use of Hybrid Organic-Inorganic Catalysts. Catalysis Reviews - Science and Engineering, 2002, vol. 44, № 2, pp. 321-374.
- DOI: 10.1081/CR-120003497
- Etienne M., Walcarius A. Analytical Investigation of the Chemical Reactivity and Stability of Aminopropyl-Grafted Silica in Aqueous Medium. Talanta, 2003, vol. 59, № 6, pp. 1173-1188.
- DOI: 10.1016/S0039-9140(03)00024-9
- Zhuravlev L.T. Concentration of Hydroxyl Groups on the Surface of Amorphous Silicas. Langmuir, 1987, vol. 3, № 3, 316-318.
- DOI: 10.1021/la00075a004
- Bukleski M., Ivanovski V., Hey-Hawkins E.A Direct Method of Quantification of Maximal Chemisorption of 3-Aminopropylsilyl Groups on Silica Gel Using DRIFT Spectroscopy. SpectrochimicaActa - Part A: Molecular and Biomolecular Spectroscopy, 2015, vol. 149, pp. 69-74.
- DOI: 10.1016/j.saa.2015.04.026
- Palimi M.J., Rostami M., Mahdavian M., Ramezanzadeh B. Surface Modification of Cr2O3Nanoparticles with 3-Amino propyl Trimethoxysilane (APTMS). Part 1: Studying the Mechanical Properties of Polyurethane/Cr2O3 Nanocomposites. Progress in Organic Coatings, 2014, vol. 77, pp. 1663-1673.
- DOI: 10.1016/j.porgcoat.2014.05.010
- Zhao Q., Bai C., Zhang W., Li Y., Zhang G., Zhang F., Fan X. Catalytic Epoxidation of Olefins with Graphene Oxide Supported Copper (Salen) Complex. Industrial and Engineering Chemistry Research, 2014, vol. 53, № 11, pp. 4232-4238.
- DOI: 10.1021/ie500017z
- Wang Y.M., Liu S.W., Xiu, Z., Jiao X.B., Cui X.P., Pan J. Preparation and Photocatalytic Properties of Silica Gel-Supported TiO2. Materials Letters, 2006, vol. 60. pp. 974-978.
- DOI: 10.1016/j.matlet.2005.10.061
- Fellenz N., Martin P., Marchetti S., Bengoa F. Aminopropyl-Modified Mesoporous Silica Nanospheres for the Adsorption of Cr(VI) from Water. Journal of Porous Materials, 2015, vol. 22, № 3, pp. 729-738.
- DOI: 10.1007/s10934-015-9946-4
- Gianotti E., Dellarocca V., Marchese L., Martra G., Coluccia S., Maschmeyer T. NH3 Adsorption on MCM-41 and Ti-grafted MCM-41. FTIR, DR UV-Vis-NIR and Photoluminescence Studies. Physical Chemistry Chemical Physics, 2002, vol. 4, № 24, pp. 6109-6115.
- DOI: 10.1039/b207231a
- Jain A., Hirata G.A., Farías M.H., Castillón F.F. Synthesis and Characterization of (3-Aminopropyl)trimethoxy-silane (APTMS) Functionalized Gd2O3: Eu3 Red Phosphor with Enhanced Quantum Yield. Nanotechnology, 2016, vol. 27, № 6, Article ID 065601.
- DOI: 10.1088/0957-4484/27/6/065601
- Lim M.H., Stein A. Comparative Studies of Grafting and Direct Syntheses of Inorganic-Organic Hybrid Mesoporous Materials. Chemistry of Materials, 1999, vol. 11, № 11, pp. 3285-3295.
- DOI: 10.1021/cm990369r
- Bereznitski Y., Jaroniec M., Kruk M., Buszewski B. Adsorption Characterization of Octyl Bonded Phases for High Performance Liquid Chromatography. Journal of Liquid Chromatography and Related Technologies, 1996, vol. 19, № 17-18, pp. 2767-2784.
- DOI: 10.1080/10826079608015109
- Jaroniec C.P., Gilpin R.K., Jaroniec M. Adsorption and Thermogravimetric Studies of Silica-Based Amide Bonded Phases. Journal of Physical Chemistry B, 1997, vol. 101, № 35, pp. 6861-6866.
- DOI: 10.1021/jp964002a
- López-Aranguren P., Fraile J., Vega L.F., Domingo C. Regenerable Solid CO2 Sorbents Prepared by Supercritical Grafting of Aminoalkoxysilane into Low-Cost Mesoporous Silica. The Journal of Supercritical Fluids, 2014, vol. 85, pp. 68-80.
- DOI: 10.1016/j.supflu.2013.10.020


