Modulation of Cell Components and Specific Isoforms of Antioxidant Enzymes in Safflower Under Water Stress and Recovery
Автор: Thippeswamy M., Rajasreelatha V., Haleshi C., Chinta Sudhakar
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 1 т.17, 2021 года.
Бесплатный доступ
Drought tolerance represents a growing threat to crop productivity. Safflower is one of the major oilseed crop enriched with various nourishing elements. In order to unravel the drought responses and recovery, a changes in cell components and specific isoforms of antioxidative enzymes in safflower (Carthamus tinctorius L. cultivar A1). Water stress (0.0%, 75%, 50% and 25% of soil moisture levels) was induced to safflower plants after 21 days of vegetative growth. After 8 days of stress imposition, plants were harvested and analysed for various parameters. A drastic decrease in the relative water content was observed during the stress and resumed the normal level after recovery. The extent of membrane damage was high under higher stress levels. These plants also showed increased levels of lipid peroxidation as evidenced from the increased malondialdehyde content, coupled with the increase in activities of antioxidant enzymes and their isoforms whereas the values in stress-recovered plants observed to be near to their respective controls. On activity gels, 12 distinct isoforms of SOD were detected; among these 10, 11 and 12 isoforms were specific under moderate and severe water stresses. Three isoforms of CAT were identified of which 2 were specific under severe water stress. In the case of POD, isoforms 2, 4, 6 and 7 were common for moderate and severe stresses whereas, POD 3 and 5 were observed only under severe stress and POD1 remained as unchanged. On recovery, the cell components and specific isoforms of antioxidative enzyme levels appeared to be that of the normal controls could be considered and used as selection criteria for improving safflower drought tolerance.
Antioxidative enzymes, Carthamus tinctorius, isozymes, safflower, stress-recovery, water stress
Короткий адрес: https://sciup.org/143173879
IDR: 143173879
Текст научной статьи Modulation of Cell Components and Specific Isoforms of Antioxidant Enzymes in Safflower Under Water Stress and Recovery
Water deficiency is one of the most important environmental constraints that regulate plant growth, development and productivity. Plants developed different adaptive mechanisms to withstand the stress by altering their cellular metabolism and thereby invoking various defense mechanisms (Bartels and Sunkar 2005). Exposure to abiotic and biotic stresses such as drought stress, heat stress, cold stress, salt stress and plant diseases often leads to trigger new metabolic pathways and the resultant increased metabolic rate lead to the evolution of highly reactive free radicals in mitochondria, chloroplasts and peroxisomes (Foyer and Noctor 2003; Cheong et al., 2003). These toxic reactive oxygen species (ROS) cause extensive lipid peroxidation thereby bringing about loss of cell membrane integrity leading to electrolyte leakage and can react with proteins, lipids and DNA and this can inactivate an antioxidant defense system (Galle and Feller 2007; Gilca et al., 2007; Gill and Tuteja 2010; El-Shabrawi et al., 2010; Dada et al ., 2020). ROS-mediated membrane damage is reported to be one of the major impacts of water stress in several crops like common bean, Fagus sylvatica, cowpea, tomato, safflower, sunflower and lettuce (Turkan et al., 2005; Galle and Feller 2007; Manivannan et al., 2007; Oh et al., 2008; Amirkhiz et al., 2015; Biradar et al., 2019)
ROS are considered harmful because they react with proteins, lipids, DNA, and other biomolecules, thus altering their structure and function. Some of the more common enzymatic reactions in which ROS are produced include the mitochondrial electron transport chain, nonmitochondrial Fenton reaction, nitric oxide synthase (NOS), microsomal cytochrome P450 enzymes, peroxisomal b-oxidation, and the respiratory burst of phagocytic cells (Gilca et al., 2007).
One common link among the different stresses is that they all produce an oxidative burst (Mittler 2002). The cytotoxic effects of ROS explain the evolution of complex arrays of non-enzymatic and enzymatic detoxification mechanisms in plants (Apel and Hirt 2004). The non-enzymatic constituents include α-tocopherol, ascorbate and reduced glutathione which remove, neutralize and scavenge the ROS (Hossain et al., 2010). Multiple antioxidant enzyme systems are involved in the enzymatic scavenging of ROS. Superoxide dismutase (SOD) reacts with the superoxide radical to produce H2O2 and further scavenging is done by catalases (CAT) and peroxidases (POD). CAT has been found predominantly in leaf peroxisomes where it functions chiefly to remove H2O2 formed in photorespiration or in β-oxidation of fatty acids in the glyoxysomes (Aghaei et al., 2009). Among peroxidases, ascorbate peroxidase (APX, EC 1.11.1.11) and glutathione peroxidase (GPX, EC 1.11.1.9) uses ascorbate and glutathione as electron donors respectively, are well known for their role in H2O2 detoxification in plants. This pathway functions in chloroplast as well as in the cytosol (Smironff and Colombe 1998; Mittler 2002; Sekmen et al., 2007). Increased cellular glutathione and glutathione reductase have been reported in plants which are tolerant to abiotic stresses (Apel and Hirt 2004; Singh et al., 2008). Under prolonged harsh environmental conditions, most plants that are homoiohydric (desiccation intolerant) get irreversibly damaged, which leads to early senescence and sudden death. Resurrection plants survive by maintaining the metabolic functions in an almost completely dehydrated state, and recover their activity readily upon re-hydration (Veljovic-Jovanovic et al., 2006; Galle and Feller 2007). The capacity to recover is especially strong and extremely rapid in poikilohydric (desiccation-tolerant) plants. Investigation of this unique adaptation mechanism to drought stress may give new insights into the process of water stress in plants or even help to improve drought tolerance in crops (Gill and Tuteja 2010; Sajedi et al., 2012).
Ostentatious research activities establish the importance of free-radical scavenging systems in drought and desiccation tolerance (Oh et al., 2008; Karuppanapandian et al., 2011). Although few reports on the expression and regulation of isoforms SOD, CAT and POD are available, their roles on plant growth under water deficit are yet to be investigated (Parida et al., 2004; Sajedi et al., 2012). In this context, we studied the changes in cell components such as relative water content (RWC), malondialdehyde (MDA), cell membrane integrity (CMI) and induction of specific isoforms of antioxidative enzymes to assess the tolerance potentials of safflower, a relatively drought tolerant crop.
MATERIALS AND METHODS
Plant material, growth and stress treatments
Seeds of Carthamus tinctorius L. cultivar A-1 (procured from Agricultural Department, Davanagere, India) were surface sterilized with 0.1 % (w/v) sodium hypochlorite solution for 5 min, thoroughly rinsed with distilled water and germinated in plastic pots containing soil and sand (2:1) mixture allowed to grow for twenty one days. The pots were maintained under 12 h natural photoperiod, (28ºC ± 4ºC) and watering daily. Three-week-old plants were separated into four sets. One of the sets maintained at 100 % soil moisture level (SML) served as control. Remaining three sets of the plants were subjected to stress by with holding the supply of water at SMLs 75, 50 and 25 % (represented as mild, moderate and severe stress) for eight days. Leaf samples were collected from the plants and then rewatered to reach the soil moisture 100 % thereby allowing the plants to recover. After 48 h, leaf samples were collected from these recovered plants. Leaf samples were flash frozen and stored at -80 ºC for further analysis.
Relative water content (RWC)
RWC of safflower leaves under the control and experimental conditions were determined. Fully expanded leaves were collected and fresh weight (FW) was immediately recorded. The leaves were then soaked for 4 h in double distilled water at room temperature under constant light and the turgid weight (TW) was recorded. After drying for 24 h at 80 ºC, total dry weights (DW) were recorded and the RWC was calculated according to Smart and Bingham (1974).
Cell membrane integrity
Cell membrane integrity was assessed by determining the extent of membrane leakage. Leaf discs of 1 cm diameter were excised using cork borer and incubated in 10 ml of water for 2 h. The solution was filtered and OD was read at 273 nm (Initial OD). Subsequently leaf discs were boiled in the same solution for 30 min, cooled, filtered and OD was measured at 273 nm (Final OD). Percentage of leakage was calculated according to Leopold et al., (1981).
Lipid peroxidation
Lipid peroxidation was determined by measuring the amount of MDA formed after the thiobarbituric acid reaction (Heath and Packer 1968). Crude extract was mixed with the same volume of a 0.5 % thiobarbituric acid solution containing 20 % tricholoroacetic acid and heated at 95°C for 30 min followed by chilling on ice. The mixture was then centrifuged at 3000 ×g for 10 min and absorbance of the supernatant was monitored at 532 and 600 nm. After subtracting the non-specific absorbance (600 nm), the MDA concentration was determined using its molar extinction coefficient (155 mM-1 cm-1).
Enzyme extraction
Plant samples were homogenized in a mortar and pestle with ice-cold 100 mM potassium phosphate buffer, pH 7.0 containing 0.1 mM EDTA. The homogenate was filtered through muslin cloth, centrifuged at 16 000 ×g for 15 min. and the supernatant was used for assaying SOD, CAT and POD enzyme activities.
Enzyme assays
Total SOD (EC 1.15.1.1) activity was determined by measuring the ability to inhibit the photochemical reduction of nitroblue tetrazolium (NBT) chloride (Giannopolitis and Ries 1977). The reaction mixture (1.5 ml) contained 50 mM phosphate buffer (pH 7.8), 0.1 μM EDTA, 13 mM methionine, 75 μM NBT, 2 μM riboflavin and 50 μl enzyme extract. Riboflavin was added last and the tubes were shaken and kept under 40-W fluorescent tube light for 15 min. Reaction was stopped by switching off the light and the absorbance was immediately recorded at 560 nm. One micro mole of SOD activity was defined as the amount of enzyme required to cause 50 % inhibition of the NBT photoreduction.
Total CAT (EC 1.11.1.6) activity was determined according to Beers and Sizer (Beers and Sizer 1952) with minor modifications. The reaction mixture (1.5 ml) consisted of 100 mM phosphate buffer (pH 7.0), 0.1 μM EDTA, 20 mM H2O2 and 50 μl enzyme extract. Reaction was started by the addition of enzyme extract and the utilization of H2O2 was monitored by absorbance measurement at 240 nm and quantified using its molar extinction coefficient (36 M-1 cm-1).
Total POD (EC 1.11.1.7) activity in the 1.5 ml reaction mixture containing 100 mM phosphate buffer (pH 7.0), 0.1 μM EDTA, 5 mmol guaiacol, 15 mM H 2 O 2 and 50 μl enzyme extract was determined according to Urbanek et al., (1991). Addition of enzyme extract started the reaction and the increase in absorbance was recorded at 470 nm for 1 min. Enzyme activity was quantified by measuring the amount of tetraguaiacol formed and the molar extinction coefficient (26.6 mM-1 cm-1) was used for calculations.
Native PAGE and activity staining
Leaf samples were homogenized in buffer containing 0.5 M Tris-HCl (pH 6.8), 20 % glycerol, 50 μl of β-mercaptoethanol and 0.2 % PVP. The homogenate was centrifuged at 10000 ×g for 15 min at 4°C. Total protein content of the supernatant determined (Lowry et al., 1951) and the samples were subjected to discontinuous poly acrylamide gel electrophoresis (PAGE) under nondenaturing conditions keeping the total protein content as equal. Discontinuous system with 10 % separating gel was used for SOD and POD; and in the case of CAT, 8 % separating gel was used (Laemmli 1970). Electrophoresis was carried out at 4 ºC in cold room. After running, the gels for SOD staining were incubated in 100 ml of the solution containing 0.05 M Tris-HCl (pH 8.2), 3 mg riboflavin, 75 mg EDTA and 10 mg NBT for 30 min at room temperature in dark and then exposed to bright light. SOD isoforms were visualized as achromatic bands and the reaction was stopped immediately by washing gel with water, 7 % acetic acid, followed by water and finally fixed in 50 % ethanol (Beauchamp and Fridovich 1971). For CAT staining, the gels were incubated in 100 ml of doubledistilled water containing 20 ml of 50 % H 2 O 2 for 25 min at room temperature, briefly rinsed with water and then keeping inside the solution containing 1 % ferric chloride and 1 % potassium ferricyanide (Woodbury et al., 1971). Color development was continued for 4 min and the reaction was stopped with a brief wash in doubledistilled water. POD isoforms were visualized according to the method of Schraumen (1966). 100 mg benzidine-diHCl was added 0.2 M sodium acetate buffer (pH 5)
and dissolved in it by boiling for 5 min., filtered and brought to room temperature. 2 ml H 2 O 2 was then added and the gel was then transferred to the solution and shaken continuously. Brownish bands appeared within 10 min and the reaction was stopped by washing in distilled water.
RESULTS
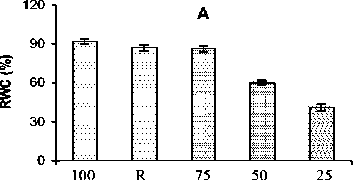
Effect on Relative water content
RWC in the leaves of safflower was found to be reduced by 6, 35 and 55% under mild, moderate and severe stress respectively (Fig. 1A). However, RWC was increased near to the control values (94%) after 48 h of recovery.
Effect on Cell membrane injury
As the intensity of water stress increase, the extent of membrane damage seems to be increasing (Fig. 1B). But the injury level was found to be reduced after the recovery from drought.
Effect on Lipid peroxidation
The MDA content gradually increased with increase in stress intensity from mild to severe stress (Fig. 1C) whereas the level reduced significantly in stress-recovered plants after 48 h.
Antioxidative enzyme activities and isoforms
The specific activity of SOD increased in plants under stress and it decreased after 48 h in re-hydrated plants, but the response patterns were different under various stress regimes. For mild, moderate and severe stresses, the SOD activity increased over the control by about 28, 102 and 134 % respectively, on day-8 post induction and reduced gradually to the control values (118 %) in stress-recovered plants after 48 h (Fig. 2A). Increase in SOD activity was also coincided with a difference in the SOD isoform expression also. Native PAGE identified twelve SOD isoforms in stress samples and among them six (SOD 3, 4, 6, 7, 8 and 9) are commonly appeared under all stress levels; but SOD 1 and SOD 2 were specific under mild water stress (Fig. 3A). Isoforms of SOD 5, 10, 11 and 12 were expressed during moderate and severe stress conditions. The isozyme pattern observed in the controls and stress-recovered plants were similar.
Activity of CAT was found to be increased due to water stress and the percentage of increase was 29, 58 and 76 % in mild, moderate and severe stress conditions (Fig. 2B). But after 48 h of recovery, the CAT activity was about 20 % higher than the control value indicating a possible role in membrane repair/synthesis during the stress recovery. Among the three isoforms detected on native PAGE, CAT 1 was significantly expressed under all levels of stress and the intensity decreased significantly on re-hydration as evident from the band intensity. However CAT 3 was expressed during moderate and severe stresses whereas CAT 2 was specifically expressed under severe stressed conditions. CAT 2 and 3 isoforms were not found in stress-recovered plants (Fig. 3B).
The percentage of increase in activity in the case of POD was 44, 88 and 112 % in mild, moderate and severe stress conditions respectively. But the activity decreased and reached near to control values after 48 h on recovery (Fig. 2C). Seven peroxidase isoforms, of which POD 1, found to be unchanged as observed on the native gel. POD isoforms 2, 4, 6 and 7 were showed differential responses due to stress. POD 3 and 5 were expressed during moderate and severe stress (Fig. 3C), but totally disappeared after 48 h on recovery.
Soil moisture levels (%)
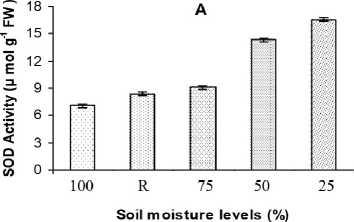
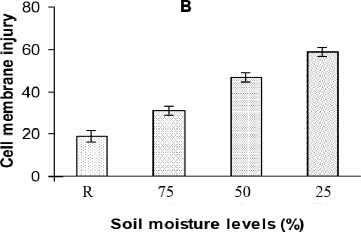
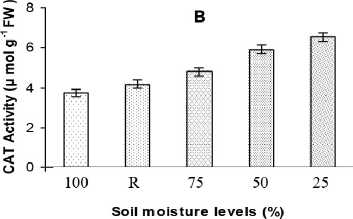
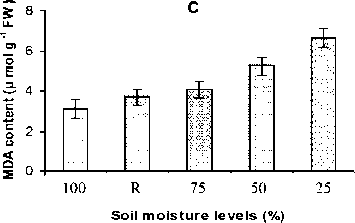
Figure 1. Effect of water stress on relative water content (A), cell membrane stability (B) and lipid peroxidation (C) All the values are mean triplicates ± S.D. Soil moisture level 100% = Control; R = stress-recovered; 75% = mild stress; 50% = moderate stress; 25% = severe stress
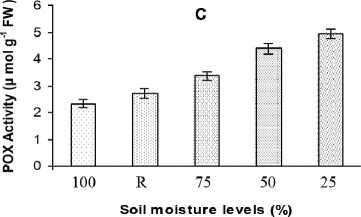
Figure 2. Antioxidant enzyme activities (1unit = 1 μmol min-1) under different SMLs; SOD activity (A), CAT activity (B) and POD activity (C) in leaves of safflower. All the values are mean triplicates ± S.D. Soil moisture level 100% = Control; R = stress-recovered; 75% = mild stress; 50% = moderate stress; 25% = severe stress
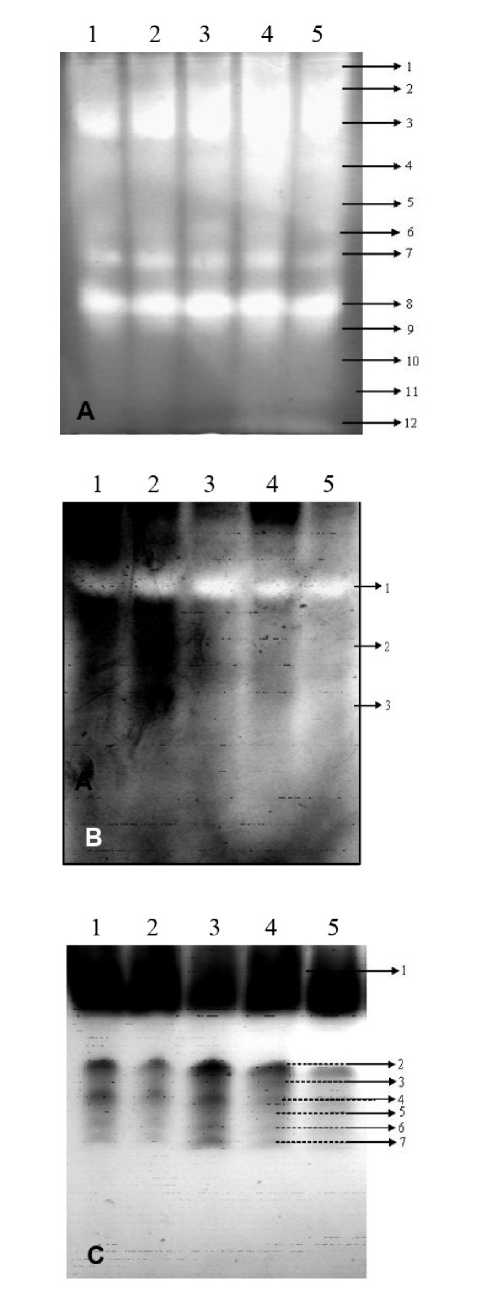
Figure 3. Native gels showing the isoforms of SOD (A), CAT (B) and OD (C) isolated from the leaves of safflower. Lane 1-control; 2-stress-recovered; 3-mild stress; 4-moderate stress and 5-severe stress. Arrows indicate the different isoforms.
DISCUSSION
Plants overwhelmed various abiotic stresses through several morphological, physiological and biochemical mechanisms. When plants are subjected to different stresses there was a progressive reduction in RWC in safflower leaves with the increase in stress severity. This was consistent and the percentage was significantly higher than the values reported earlier in wheat genotypes (Sairam and Rao 2002). Tissue relative water content (RWC) is reported to be a reliable and less error prone measurement of plant water status and has been used as one of the indices to determine drought tolerance. Accordingly, a reduction in RWC has been observed in plants subjected to severe drought stress (Ranganayakulu et al., 2012; Thippeswamy et al., 2013). Even a reduction even up to 44.81 % is also not surprising in plants under the stress, since the stress lowers water potential significantly, as observed in the leaves of ground nut (Ranganayakulu et al., 2012), Phillyrea angustifolia (Munne-Bosch and Penuelas 2003) and common bean (Turkan et al., 2005).
Cell membrane stability has often been used to assess drought and salinity tolerance in different crops (Premachandra et al., 1992; Matsuo et al., 2006; Eraslan et al., 2008; Thippeswamy et al., 2013). CMS exhibit a positive correlation with the osmotic potential, K+ concentration, osmotic adjustment, relative water content and with the parameters that are equally affected by water and salinity stresses (Farooq and Azam 2006; Singh et al., 2008; Vijayalakshmi et al., 2016). However, depletion of water is considered as one of the major causes of increased cell membrane permeability in plants growing under osmotic stress (Sudhakar et al., 2001; Matsuo et al., 2006). The membrane repair observed in the plants recovered from the stress indicates the tolerate capacity.
Water stress is known to be inducing extensive lipid peroxidation, which has been used as indicator of stress induced oxidative damage in membranes (Baisak et al., 1994; Munne-Bosch and Penuelas 2003). MDA is produced when polyunsaturated fatty acids in the membrane undergo peroxidation (Meloni et al., 2003; Negre-Salvayre et al., 2008). An increase in the level of
MDA during draught stress has been reported in foxtail millet (Sreenivasulu et al., 1999), mulberry (Sudhakar et al., 2001), Bruguiera parviflora (Parida et al., 2004), maize (Azevedo Neto et al., 2005), common bean (Turkan et al., 2005) spikes of gladiolus (Singh et al., 2008) spinach (Eraslan et al., 2008) safflower (Sajedi et al., 2012) and rice (Dada et al ., 2020). In the case of safflower the drought stress and recovery was observed the same kind of increase in MDA suggesting the existence of active repair mechanisms during the stress.
Environmental stresses triggers the accumulation of ROS in plant cells (Mahajan and Tuteja 2005; El-Shabrawi et al., 2010; Karuppanapandian et al., 2011). Enzymatic scavenging of ROS could be efficiently achieved through the complex and elaborated coordination among the anti-oxidative enzymes. Enzymatic defenses include catalase and peroxidase enzymes that remove H2O2 and superoxide dismutase that catalyze the disproportionation of superoxide radicals to hydrogen peroxide and dioxygen (Mittler 2002; Apel and Hirt 2004). SOD is reported to play an important role in cellular defense against oxidative stress since its activity directly modulates the amount of O˙2¯ and H2O2, the two Haber-Weiss reaction substrates (Bower et al., 1992). Activities of the antioxidant enzymes SOD, CAT and POD, were increased in safflower leaves and the increase was more significant and consistent at severe stress indicating a positive correlation with the drought tolerance in safflower. Among the twelve SOD isoforms detected in stressed samples, six (SOD 3, 4, 6, 7, 8 and 9) are commonly expressed at all stress levels but SOD 1 and SOD 2 were expressed at mild stress. Isoform SOD 5, 10, 11 and 12 were expressed during moderate and severe stress. Re-hydration brought about a transient decrease in the activities of SOD isoforms. This increase in the enzyme activity and the expression as various isoforms might be enhancing the rate of free radical scavenging and thereby preventing membrane damage. The increased SOD activity under drought stress was reported in common bean (Turkan et al., 2005) and cowpea (Manivannan et al., 2007). Similarly Mn SOD type isoforms with with increased activity were identified from leaves of desiccated Ramonda serbica with a remarkable decrease on re-hydration and also Fe SOD isoform which is reported to be as an ancient form of SOD localized in the chloroplasts of several plants found to induce more intense bands of Mn SOD in senescized leaves, proposed to be the part of protective mechanism under stress conditions (Alscher et al., 2002; Parida et al., 2004; Veljovic-Jovanovic et al., 2006; Vijayalakshmi et al., 2016; Biradar et al., 2019). Over expression of SOD in transgenic plants also reported to be enhancing the protection against enhanced oxidative stress (Samis et al., 2002).
The ubiquitously present catalase is an oxidoreductase that decomposes H 2 O 2 to water and molecular oxygen and one of the key enzymes involved in removal of toxic peroxides formed during photorespiration or during β-oxidation of fatty acids (Apel and Hirt 2004; Negre-Salvayre et al., 2008). The same kind of an activity elevation is reported during water stress as part of the tolerance mechanisms (Mahajan and Tuteja 2005) in glyoxisomes (Dat et al., 2000). Increase in catalase activity is supposed to be an adaptive trait possibly helps to overcome the damage on tissue metabolism by reducing toxic levels of H 2 O 2 produced during cell metabolism (Rasheed and Mukerji 1991; Eraslan et al., 2008). In the present study catalase activity increased gradually and decreased upon relief from stress. But 48 h of recovery showed 20% increase in CAT activity than the control value suggesting a possible role in membrane repair/synthesis during the recovery as reported earlier (Dat et al., 2000). Among the three isoforms detected on gel, CAT 1 was significantly induced at all levels of stress and its intensity decreased significantly on re-hydration. However CAT 3 was induced during moderate and severe stress and CAT 2 was specifically expressed upon severe treatments. In stress-recovered plants, the absence of CAT 2 and 3 activities suggest their specificity to the stress intensity.
Peroxidases are widely accepted as ‘stress enzymes’ (Gaspar et al., 1991) and the induction of peroxidase activity has been documented under a variety of stressful conditions such as water stress (Turkan et al., 2005), chilling (Prasad et al., ., 1995), salinity (Sreenivasulu et al., 1999), high temperature
(Biradar et al., 2019) and under toxic levels of Al, Cu, Cd, Zn (Tamas et al., 2008; Eraslan et al., 2008). Increased peroxidase activity caused by water stress (Turkan et al., 2005; Manivannan et al., 2007), salt stress (Sudhakar et al., 2001; Sekmen et al., 2007) is also demonstrated. In tolerant plant species, peroxidase activity was found to be higher enabling them to protect themselves against oxidative stress (Scalet et al., 1995; Eraslan et al., 2008). An increase in total peroxidase activity and its isoform patterns were observed in a salt tolerant cultivar of foxtail millet due to NaCl stress and by jasmonic acid treatments in peanut seedlings (Sreenivasulu et al., 1999; Kumari et al., 2006). In concurrence to the above observations, peroxidase activity was found increased and seven isoforms of the enzyme were detected in safflower. POD 1 didn’t show any change under all stress levels, whereas POD 2, 4, 6 and 7 were showed differential response. POD 3 and 5 were expressed during moderate and severe stress and activity was found to be decreasing according to the severity of stress and totally disappeared on re-hydration after 48h, indicating that these isoforms could be specific to stress and involved in scavenging free radicals generated during oxidative stress.
The cell components of the relative water content and cell membrane stability under the different drought stress levels alone are convincing to show the drought tolerant capacity of safflower. The observations on lipid peroxidation and antioxidant enzymes strongly imply a possibility that the enzyme system is utilized in safflower to defend the oxidative stress caused by drought and thereby protecting the cells from oxidative damage. Even though the observed parameters are reliable to reveal the mechanisms of drought tolerance in safflower, more specific gene expression level studies and gene transfer studies of osmo-regulatory pathway can improve our understanding regarding the adaptation.
ACKNOWLEDGEMENTS
The authors Dr. M. Thippeswamy and Dr. V. Rajasreelatha acknowledge the Dr. D. S. Kothari postdoctoral fellowship from the University Grants Commission (UGC), New Delhi, India.
Список литературы Modulation of Cell Components and Specific Isoforms of Antioxidant Enzymes in Safflower Under Water Stress and Recovery
- Aghaei K., Ehsanpour A. A. and Komatsu S. (2009) Potato responds to salt stress by increased activity of antioxidant enzymes. J. Integr. Plant. Biol., 51, 1095–1103.
- Alscher R. G., Erturk N. and Heath L. S. (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot., 53, 1331–1341.
- Amirkhiz K. F., Dehaghi M. A. and Heshmati S. (2015) Antioxidant enzymes and physiological responses of safflower (Carthamus tinctorius L.) to iron application, under water deficit condition. Not. Sci. Biol., 7(2), 203-209.
- Apel K. and Hirt H. (2004) Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant. Biol., 55, 373-399.
- Azevedo-Neto A. D., Prisco J. T., Eneas Filho J., Aberu C. E. B. and Gomes Filho E. (2006) Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ. And Exp. Bot., 56, 87-94.
- Baisak R., Rana D., Acharya P. B. B. and Kar M. (1994) Alterations in the activities of active oxygen scavenging enzymes of wheat leaves subjected to water stress. Plant. Cell. Physiol., 35, 489-495.
- Bartels D. and Sunkar R. (2005) Drought and salt tolerance in plants. Crit. Rev. Plant. Sci., 24, 23-58.
- Beauchamp C. and Fridovich I. (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem., 44, 276-287.
- Beers R. F. and Sizer I. W. (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem., 195, 133-140.
- Biradar G., Laxman R. H., Namratha M. R., Thippeswamy M., Shivashankara K. S., Roy T. K., Sadashiva A. T. (2019) Induction temperature enhances antioxidant enzyme activity and osmoprotectants in Tomato. Int. J. Curr. Microbiol. App. Sci., 8(3), 1284-1293.
- Bower C., Van Montagu M. and Inze D. (1992) Superoxide dismutase and stress tolerance. Annu. Rev. Plant. Physiol. Plant. Mol. Biol., 43, 81-116.
- Cheong Y. H., Kim K. N., Pandey G. K., Gupta R., Grant J. J. and Luan S. (2003) CLB1, a calcium sensor that differentially regulates salt, drought, and cold responses in arabidopsis. Plant Cell., 15, 1833–1845.
- Dada O. A., Jude A. O. and Adeniyi O. T. (2020) Water stress at anthesis and storage temperature affected growth and germinability of rice (Oryza spp.). J. Stress Physiol. Biochem., 16, 5-20.
- Dat J., Vandenabeele S., Vranova E., Van Montagu M., Inze D. and Van Breusegem D. F. (2000) Dual action of the active oxygen species during plant stress responses. Cell. Mol. Life. Sci., 57, 779–795.
- El-Shabrawi H., Kumar B., Kaul T., Reddy M. K., Singla-Pareek S. L. and Sopory S. K. (2010) Redox homeostasis, antioxidant defense, and methylglyoxal detoxification as markers for salt tolerance in Pokkali rice. Protoplasma., 245, 85-96.
- Eraslan F., Inal A., Pilbeam D. J. and Gunes A. (2008) Interactive effects of salicylic acid and silicon on oxidative damage and antioxidant activity in spinach (Spinacia oleracea L. cv. Matador) grown under boron toxicity and salinity. Plant. Growth. Regul., 55, 207-219.
- Farooq S. and Azam F. (2006) The use of cell membrane stability (CMS) technique to screen for salt tolerant wheat varieties. J. Plant. Physiol., 163, 629-637.
- Foyer C. H. and Noctor G. (2003) Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol. Plant., 119, 355-364.
- Galle A. and Feller U. (2007) Changes of photosynthetic traits in beech saplings (Fagus sylvatica) under severe drought stress and during recovery. Physiol. Plant., 131, 412-421.
- Gaspar Th., Penel C., Hagege D. and Greppin H. (1991) Peroxidases in Plant Growth, Differentiation and Development Processes, Biochemical, Molecular, and Physiological Aspects of Plant Peroxidases, Lobazzewski, G. et al., Eds., Lublin: Univ. M. Curie-Sklodowska., 249-280.
- Giannopolitis C. N. and Ries S. K. (1977) Superoxide dismutases. I. Occurrence in higher plants. Plant. Physiol. 59, 309-314.
- Gilca M., Stoian I., Atanasiu V. and Virgolici B. (2007) The oxidative hypothesis of senescence. J. Postgrad. Med. 53(3), 207-213.
- Gill S. S. and Tuteja N. (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant. Physiol. Biochem., 48, 909-930.
- Heath R. L. and Packer L. (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys., 125, 189-198.
- Hossain M. A., Hossain M. Z. and Fujita M. (2009) Stress-induced changes of methylglyoxal level and glyoxalase I activity in pumpkin seedlings and cDNA cloning of glyoxalase I gene. Aust. J. Crop. Sci., 3, 53-64.
- Karuppanapandian T., Moon J. C., Kim C., Manoharan K. and Kim W. (2011) Reactive Oxygen Species in Plants: Their Generation, Signal Transduction, and Scavenging Mechanisms. Aust. J. Crop Sci., 5, 709-725.
- Kumari G. K., Reddy A. M., Naik S. T., Kumar S. G., Prasanthi J., Sriranganayakulu G., Reddy P. C. and Sudhakar C. (2006) Jasmonic acid induced changes in protein pattern, antioxidative enzyme activities and peroxidase isozymes in peanut seedlings. Biol. Plant., 50, 219-226.
- Laemmli U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage 4. Nature., 227, 680-685.
- Leopold A. C., Musgrave M. E. and Williams K. M. (1981) Solute leakage resulting from leaf desiccation. Plant. Physiol., 68, 1222-1225.
- Lowry O. H., Rosebrough N. J., Farr A. L. and Randall R. S. (1951) Protein measurements with the Folin-Phenol reagent. Biol. Chem., 193, 265-275.
- Mahajan S. and Tuteja N. (2005) Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys., 444, 139-158.
- Manivannan P., Abdul Jaleel C., Sankar B., Kishorekumar A., Somasundaram R., Sreedharan R. and Panneerselvam R. (2007) Changes in antioxidant metabolism of Vigna unguiculata (L) Walp. by propiconazole under water deficit stress. Colloids. Surf. B., 57, 69-74.
- Matsuo U., Yoko T., Chihaya N., Satomi S., Anzu M. and Yukio K. (2006) Responses of the plasma membrane to low temperatures. Physiol. Plant., 126, 81-88.
- Meloni D. A., Oliva M. A., Martinez C. A. and Cambraia J. (2003) Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ. Exp. Bot., 49, 69-76.
- Mittler R. (2002) Oxidative stress, antioxidants and stress tolerance. Trends. Plant. Sci., 7, 405-410.
- Munne-Bosch S. and Penuelas J. (2003) Photo and antioxidative protection, and a role for salicylic acid during drought and recovery in field-grown Phillyrea angustifolia plants. Planta, 217, 758-766.
- Negre-Salvayre A., Coatrieux C., Ingueneau C. and Salvayre R. (2008) Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. Br. J. Pharmacol., 153, 6-20.
- Oh M. M., Trick H. N. and Rajashekar C. B. (2008) Secondary metabolism and antioxidants are involved in environmental adaptation and stress tolerance in lettuce. J. Plant. Physiol., 166, 180-191.
- Parida A. K., Das A. B. and Mohanty P. (2004) Defense potentials to NaCl in a mangrove, Bruguiera parviflora: Differential changes of isoforms of some antioxidative enzymes. J. Plant. Physiol. 161, 531-542.


