Molecular docking of fisetin as a multi-target drug in the treatment of multiple sclerosis
Автор: Malathi R., Vailina Dsouza, Puja, Rithika R., Sneha P.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 4 т.18, 2022 года.
Бесплатный доступ
Multiple Sclerosis (MS), is an autoimmune disorder of the CNS a long-lasting disorder that can attack the brain, spinal cord, and eyes. The severity of this disorder varies from person to person. Demyelination and lesion formation is the major pathological changes of MS, due to which there is no significant generation of the action potential along the axon of the nerves. In turn, leads to delayed propagation and perception of the chemical signals required for varieties of function in the body. Several theories have been emerging to apprehend the origin of MS such as genes, smoking, viral infection caused by Epstein-Barr virus, or the human herpes virus may trigger the disorder or cause relapses, Vitamin D deficiency etc. Statistical reports show Canada is the country having the highest rate with 1 in 400 people suffering from MS. In the present study, the drug targets of MS were analyzed by understanding its interaction with a plant flavonoid Fisetin having neuroprotective properties. Molecular docking of plant flavonoid fisetin with the enzyme targets of MS was performed using auto-dock 4.2. The minimum binding energy obtained from docking explains the efficiency of the ligand binding with the therapeutic target proteins. Three proteins were selected based on their action and function they play in the progression of Multiple sclerosis namely Caspase 1 (PDB Id: 1lBC), Calpain-1(PDB Id: 2ARY), and Cathepsin B (PDB Id: 1GMY). The docking of fisetin with Caspase 1 (PDB Id: 1lBC), Calpain-1(PDB Id: 2ARY), and Cathepsin B (PDB Id: 1GMY) displayed the minimum binding energy score as, Cathepsin B = -10.01 kcal/mol, Calpain-1= -9.95 kcal/mol, Caspase 1= -8.18 kcal/mol respectively and also the number of hydrogen bonds: 20, 18, 23 respectively. The target proteins Cathepsin B and Calpian 1, showed the strongest interaction with Fisetin with the least minimum binding energy. Molecular properties & drug-likeness, biological activity, and toxicity of Fistein were analyzed using the Way2drug bio tool. Lipinski's benchmark rule of five (RO5)5 defines desirable drug candidate physicochemical property which was successfully shown by the ligand fistein : log P:1.35, HBD: 4, HBA:6 The following are the obtained results showing biological activity and toxicity of ligand fistein: • Biological Activity: 0.966- Membrane intergrity; 0.959- Aryl-alcohol dehydrogenase (NADP+) inhibitor; 0.950- kinase inhibitor • Toxicity: Vascular toxic (0.755 pa), ulcer, aphthous (0.713 pa). where pa [pharmacologically active] is greater than 0.7.
Autodock, calpian 1, caspase-1, cathepsin b, fisetin, therapeutic target proteins
Короткий адрес: https://sciup.org/143179237
IDR: 143179237
Текст научной статьи Molecular docking of fisetin as a multi-target drug in the treatment of multiple sclerosis
Encephalomyelitis disseminate also known as Multiple Sclerosis is an autoimmune disorder caused due to demyelination, inflammation, and axonal loss in the central nervous system(Leray et al. , 2016). The French neurologist Jean-Martin Charcot was the first scientist to describe MS by the appearance of developed glial scars or lesions on the white matter of the brain and spinal cord. As the disease occurs from the age of 20 to 60 years the symptoms differ from one and another, but at the peak, it results in physical and mental instability, blurred or double vision, loss of coordination, muscle weakness, etc. A close up to one million estimated by The National Sclerosis Society is being diagnosed with MS although the women with MS outnumber men. The diagnosis is been done by tools like MRI imaging, evoked potential test, and spine fluid analysis. Contrasting to the present study, the National Multiple Sclerosis Society and Multiple Sclerosis International Federation have introduced 4 types of MS such as CIS (Clinically isolated syndrome), RRMS (Relapsing-remitting MS), PPMS (Primary Progressive MS), SPMS (Secondary progressive MS). Though there is a lack of evidence to cure Multiple sclerosis, technology, and Research scientists still pertain us with hope to find alternative treatments like rehabilitation and physical therapy which intend to slow the progression of Multiple sclerosis.
One of the ways to understand and overcome MS is by inhibiting the enzyme that is potentially causing damage, especially to the myelin sheath, limb function.
Many studies have shown the enzyme belonging to the Protease family play a critical role in the development of MS pathologies(Scarisbrick, 2008).
In the current study, we have focused on three proteins that have been shown to have an elevated level that causes various effects ultimately that leads to the development or progression of MS.
Caspase-1 a protease enzyme that is responsible for the inflammatory response of IL-1 Beta, many studies show blocking caspase-1 potentially decreases the injury in various tested models(McKenzie et al. , 2018; Ming et al. , 2002).
Oligodendrocytes help in the production of myelin sheath in the CNS. Oligodendrocytes cell death caused by cytokines results in MS has been observed through in-vitro studies.
From these cytokines, tumor necrosis factor (TNF) mediates the damage of Oligodendrocytes in MS which is mediated by the Caspase-1 pathway (Hisahara et al. , 2000).
Calpain-1 is another major enzyme found to be responsible for the breakdown of all main myelin proteins, the elevated activity & expression of this proteinase may play a significant role in myelinolysis (Shields et al. , 1999).
Relating to modern studies, Fisetin a natural flavonoid having anti-inflammatory, anti-oxidative and neuroprotective properties could play a huge role in treating neurodegenerative diseases like MS (Ahmad et al. , 2019).
The objective of the present study is to focus on docking the therapeutic targets proteins of Multiple Sclerosis with the plant flavone fisetin which further computationally analyzed.
MATERIALS AND METHODS
Ligand Generation:
Ligand fisetin having Molecular formula C15H10O6 and a molecular weight of 286.2. The log P value for fisetin is 2.282. The ligand structure was extracted from 1/) and downloaded in SDF format which needs to be converted into PDB format for docking; this was done using Open Babel Software. SDF file was converted into PDB format.
Preparation of therapeutic target proteins:
The selected docking macromolecules (target enzymes) for Multiple sclerosis are Caspase 1, Calpain-1, and Cathepsin B. Protein Data Bank is a global repository for 3D structural data of proteins from which, structures of all our three target proteins were extracted.
The 3D crystal structures were downloaded from (ww.rscb.org) in a Protein Data bank format (PDB) having the following ids Caspase 1 (PDB Id: 1lBC), Calpain-1(PDB Id: 2ARY), and Cathepsin B (PDB Id: 1GMY) respectively. The extracted protein structures had ligand interaction which all were extracted out and a clean structure of the protein was put through energy minimization using a Swiss PDB viewer.
RESULTS AND DISCUSSION
Continuous and rapid advances of computational techniques in science have led to various discoveries. It is entirely based on the prediction of binding affinities of the target molecule with crystallographic X-ray structures as well as potential drugs used for docking analysis. The surface grid-based docking method was used to analyze the different linkages in the active site of the therapeutic target proteins Caspase 1(PDB Id: 1lBC), Calpain-1(PDB Id: 2ARY), and Cathepsin B (PDB Id: 1GMY) respectively that were subjugated by the fisetin.
The following results have been discussed in Tables 1 and 2.
The five best minimum binding energy of the ligand (fisetin) with the 3 therapeutic target proteins are mentioned in the following table.
The lowest binding energy conformation of the ligand Fisetin with the three therapeutic target proteins
Calpain-1 (PDB Id: 2ARY), Caspase 1 (PDB Id: 1lBC), and Cathepsin B (PDB Id: 1GMY) were shown in the following figures 1-3. Visualization of these structures was done using the chimera 11- program that is used for analyzing 3D protein structures.
Cathepsin B and Calpain-1, showed the best interaction with Fisetin having the lowest binding energy with No. of hydrogen bonds: 20 and 18 respectively.
Molecular Properties of fisetin was analyzed using molsoft
Lipinski's benchmark rule of five (RO5)5 defines desirable drug candidate physicochemical property space as MW < 500 Da, log P < 5, HBD < 5, and HBA < 10.
The Biological Activity and Toxicity of the ligand fisetin were analyzed by Way2Drug Bio tool.
The biological activity and toxicity of Fisetin were analyzed using the way2drug bio tool, where pa [pharmacologically active] relates to the probability of the activity (pa >0.7).
Table 1: Minimum Binding Energy of fisetin with the 3 drug targets of the top hits
|
1GMY - Cathepsin B |
||
|
S.no |
Run |
Minimum binding energy (kcal/mol) |
|
1 |
4 |
-10.01 |
|
2 |
9 |
-9.35 |
|
3 |
8 |
-9.3 |
|
4 |
3 |
-9.32 |
|
5 |
10 |
-9.29 |
|
2ARY : Calpain-1 |
||
|
S.NO |
Run |
Minimum binding energy (kcal/mol) |
|
1 |
5 |
-9.95 |
|
2 |
2 |
-8.92 |
|
3 |
1 |
-8.91 |
|
4 |
4 |
-8.91 |
|
5 |
3 |
-8.89 |
|
lBC - Caspase 1 |
||
|
S. No |
Run |
Minimum binding energy (kcal/mol) |
|
1 |
10 |
-8.18 |
|
2 |
2 |
-8.11 |
|
3 |
4 |
-7.99 |
|
4 |
1 |
-7.99 |
|
5 |
8 |
-7.96 |
Table 2: Lowest binding energy of fisetin with the 3 drug targets of the top hits
|
S.No. |
Protein |
Final Intermolecular Energy (vdW + Hbond + desolv Energy+Electrostatic Energy) (kcal/mol) |
Final Total Internal Energy (kcal/mol) |
Torsional Free Energy (kcal/mol) |
Unbound System's Energy (kcal/mol) |
|
1 |
Calpain-1 |
-10.23 |
-0.52 |
0.3 |
-0.5 |
|
2 |
Cathepsin B |
-10.01 |
-0.56 |
0.3 |
-0.31 |
|
3 |
Caspase 1 |
-8.54 |
-0.46 |
0.3 |
-0.52 |
Table 3: Analysis score
Analysis of the score for Fistein
Log P: 1.35
HBD: 4
HBA: 6
Table 4: Biological activity
|
Sr.NO |
Pa |
Biological activity |
|
1 |
0.966 |
Membrane integrity agonist |
|
2 |
0.959 |
Aryl-alcohol dehydrogenase (NADP+) inhibitor |
|
3 |
0.950 |
Kinase inhibitor |
|
4 |
0.865 |
2 Enoate reductase inhibitor |
|
5 |
0.844 |
UGT 1A substrate |
|
6 |
0.808 |
CYP2B5 |
|
7 |
0.725 |
vasoprotector |
Table 5: Toxicity
|
Sr.no |
Pa |
Toxicity |
|
1 |
0.755 |
Vascular toxic |
|
2 |
0.713 |
Aphthous ulceration |
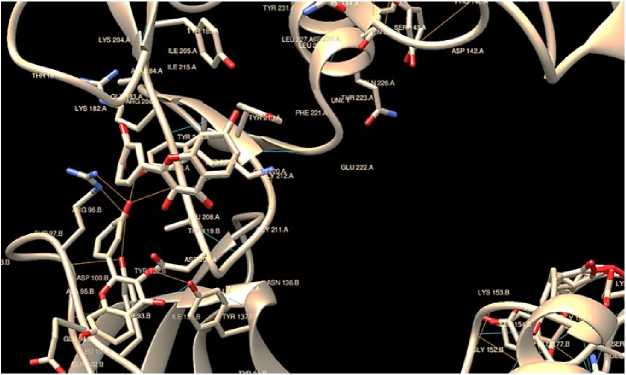
Figure 1: Calpain1. The oxygen atom in the image is signified as red in color in the ligand structure fisetin. The blue lines signify the hydrogen bonds between the ligand fisetin and the therapeutic target protein (calpain1). No. of hydrogen bond = 18
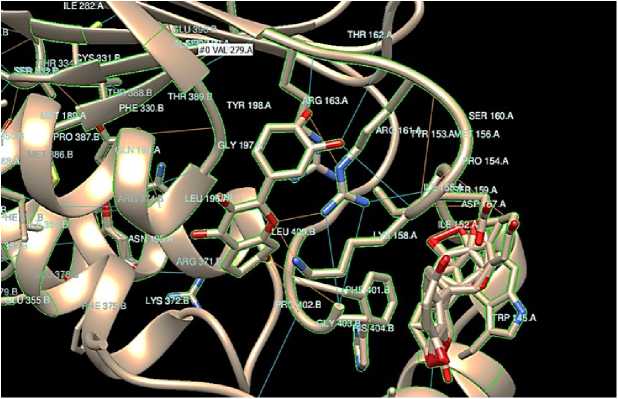
Figure 2: Capase 1. The oxygen atom in the image is signified as red in color in the ligand structure fisetin. The blue lines signify the hydrogen bonds between the ligand fisetin and the therapeutic target protein (capase1). No. of hydrogen bond = 23.
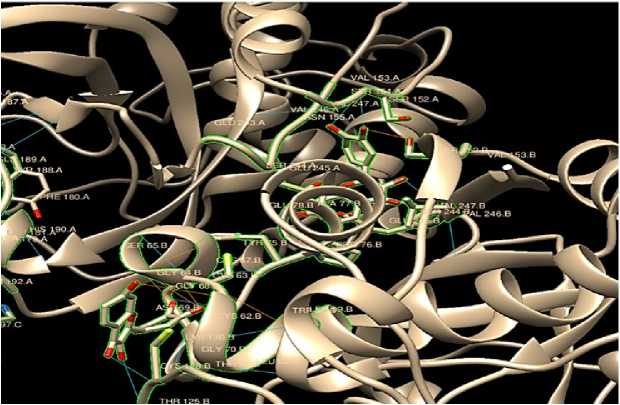
Figure 3: Cathepsin B. The oxygen atom in the image is signified as red in color in the ligand structure fisetin. The blue lines signify the hydrogen bonds between the ligand fisetin and the therapeutic target protein (cathepsin B). No. of hydrogen bond = 20.
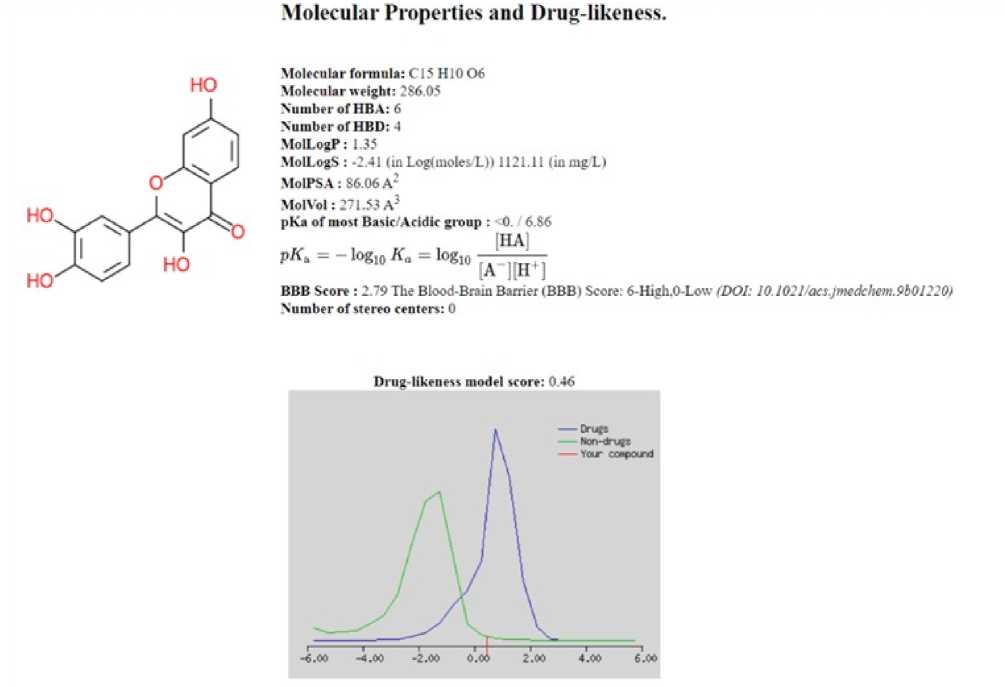
Molecular Properties and Drug-likeness.
Molecular formula: CIS H10 06
Molecular weight: 286.05
Number of HE A: 6
Number of HBD: 4
MolLogP: 1.35
MolLogS : -2.41 (in Loglinolesll) 1121.11 (in mg 11
MolPSA: 86.06A*
MolVol: 271.53 A3
pKe of most Basic/Acidir group : 0. 6 86
[HA]
pKM = - log,0 Ka = logla
[A ]|H + ]
Number of stereo centers: 0
Drug-likeness model score: 0 46
4.00
6.00
____J2 -2.00 0.W
Figure 4: Molecular properties & drug-likeness.
CONCLUSION
In the present study, the effectiveness of fisetin showing the characteristic binding ability to the therapeutic target proteins and their effective progressiveness against the disease multiple sclerosis was assessed.
To the three therapeutic target proteins caspase 1 (PDBId:1IBC), Calpain-1 (PDB id:2ARY), and cathepsin B(PDB Id: 1GMY) fisetin, exhibited satisfactory docking output to all of them.
The primary cause for the disease MS as known is due to the formation of lesions in CNS and destruction in the myelin sheath that leads to multiple pathological defects, from tremors to complete loss of coordination. As fisetin can interact with the target proteins of Multiple sclerosis, this can be a potent candidate for treating and reducing the symptoms of Multiple Sclerosis.
ACKNOWLEDGMENTS
The authors would like to thank the support rendered by Kristu Jayanti College, Autonomous.
CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest.
Список литературы Molecular docking of fisetin as a multi-target drug in the treatment of multiple sclerosis
- Ahmad, A., Ali, T., Rehman, S. U., & Kim, M. O. (2019). Phytomedicine-Based Potent Antioxidant, Fisetin Protects CNS-Insult LPS-Induced Oxidative Stress-Mediated Neurodegeneration and Memory Impairment. Journal of Clinical Medicine, 8(6).
- Bever, C. T., & Garver, D. W. (1995). Increased cathepsin B activity in multiple sclerosis brain. Journal of the Neurological Sciences, 131(1), 71-73.
- Hisahara, S., Takano, R., Shoji, S., Okano, H., & Miura, M. (2000). Role of caspase-1 subfamily in cytotoxic cytokine-induced oligodendrocyte cell death. Journal of Neural Transmission. Supplementum, 58(58), 135-142.
- Leray, E., Moreau, T., Fromont, A., & Edan, G. (2016). Epidemiology of multiple sclerosis. Revue Neurologique, 172(1), 3-13.
- McKenzie, B. A., Mamik, M. K., Saito, L. B., Boghozian, R., Monaco, M. C., Major, E. O., … Power, C. (2018). Caspase-1 inhibition prevents glial inflammasome activation and pyroptosis in models of multiple sclerosis. Proceedings of the National Academy of Sciences of the United States of America, 115(26), E6065-E6074.
- Ming, X., Li, W., Maeda, Y., Blumberg, B., Raval, S., Cook, S. D., & Dowling, P. C. (2002). Caspase-1 expression in multiple sclerosis plaques and cultured glial cells. Journal of the Neurological Sciences, 197(1-2), 9-18.
- Scarisbrick, I. A. (2008). The Multiple Sclerosis Degradome: Enzymatic Cascades in Development and Progression of Central Nervous System Inflammatory Disease. Current Topics in Microbiology and Immunology, 318, 133.
- Shields, D. C., Schaecher, K. E., Saido, T. C., & Banik, N. L. (1999). A putative mechanism of demyelination in multiple sclerosis by a proteolytic enzyme, calpain. Proceedings of the National Academy of Sciences of the United States of America, 96(20), 11486-11491.


