Молекулярные основы современных подходов к анализу продуктивной функции тимуса
Автор: Сайдакова Е.В.
Журнал: Вестник Пермского университета. Серия: Биология @vestnik-psu-bio
Рубрика: Медико-биологические науки
Статья в выпуске: 2, 2015 года.
Бесплатный доступ
Рассмотрена информация о тимусе - центральном органе иммунной системы, обеспечивающем микроокружение, необходимое для созревания и селекции CD4+ и CD8+ Т-лимфоцитов. Дана справка об основных событиях в истории изучения вилочковой железы. Продемонстрирована высокая значимость органа в восстановлении иммунной системы при опустошении пула Т-лимфоцитов. Представлены данные о молекулярных механизмах формирования разнообразия антигенраспознающих рецепторов Т-клеток, которые легли в основу современных подходов к анализу продуктивной функции вилочковой железы. Приведены примеры использования методов определения функциональной активности тимуса в науке и клинической практике.
Тимус, т-клеточный рецептор, реаранжировка сегментов генов, пцр
Короткий адрес: https://sciup.org/147204723
IDR: 147204723 | УДК: 571.27
Текст научной статьи Молекулярные основы современных подходов к анализу продуктивной функции тимуса
Тимус — центральный орган иммунной системы — является основным местом созревания и селекции Т-лимфоцитов. Его функция состоит в создании и поддержании большого репертуара периферических CD4^ и CD8+ Т-клеток различной специфичности. Нарушение функции вилочковой железы приводит к развитию оппортунистических инфекций, иммунодефицитных состояний, аутоиммунных заболеваний, и значительно осложняет восстановление репертуара иммунных клеток в условиях их дефицита.
Историческая справка
Вилочковая железа, или тимус. впервые был описан в работах древнегреческих авторов. Ему7
придавали особое значение, считая вместилищем д)гши и эмоциональным центром человека, Во 11 в. н.з. Гален описал морфологию органа. Однако затем. на многие века, ученые потеряли интерес к вилочковой железе. считая ее простым наростом, не имеющим важных функций в организме. Позже - в эпоху Ренессанса - вновь были совершены попытки установить роль тимуса. Его описывали как подушку’ для предотвращения повреждений сердца, как разрастание легких у новорожденных, как орган кроветворения, как центр контроля метаболизма и тд В тот исторический период железу начали изображать в анатомических атласах. Впервые связь междуг опухолью вилочковой железы и заболеванием человека обнаружил Карл Вайгерт в 1901 г. [Weigert. 1901]. Он установил связь нарушений работы тимуса с развитием миастении. На сегодняшний день аномалии органа связывают уже с не-
(С Сайдакова Е. В . 2015
сколькими заболеваниями (гипертиреоз, лимфома, гипоплазия костного мозга, неспецифический язвенный колит, гиперпаратиреоз, синдром Кушинга и т.Д.).
Б 50-х гг XX в. тимус был признан местом образования Т-лимфоцитов - важнейших клеток адаптивной иммунной системы [Billingham, Brent, Medawar, 1956; Gowans, Gesner, McGregor, 1961]. Неоценимый вклад в понимание функции вилочковой железы внес Френсис Миллер, Проводя серии экспериментов с неонатально тимэкгомированными мышами, он показал, что вилочковая железа в большей степени необходима в ранний период жизни. так как ее удаление у взрослых животных не приводило к серьезным последствиям. [Miller, 1960]. В его экспериментах мыши, подвергнувшиеся тимэктомии сразу после рождения, отличались от контрольных особей существенно меньшим количеством Т-клсток, как в тканях, так и в циркулирующей крови. У них наблюдались аномалии при формировании иммунной системы. Структура лимфоидных органов тим эктомирова иных мышей была значительно менее развита, чем у их интактных сородичей. [Millen 1962]. Дальнейшие эксперименты показали. что особи, лишенные тимуса в разное время после рождения, отличаются по способности отторгать аллогенные кожные трансплантаты. [Miller, 1962]. Эти и некоторые другие работы позволили установить ключевую роль тимуса в развитии Т-лимфоцитов. В дальнейшем было показано. что именно тимус предоставляет клеткам особое микроокружение для реаранжировки генов Т-клеточных рецепторов (ТкР), а также созревания и селекции тимоцитов, формирующих пул периферических клеток иммунной системы [Cooper et at. 1991: Kisielow. von
Продукция Т-лимфоцитов для нужд периферии (продуктивная функция) — не единственная, из описанных функций вилочковой железы. Как известно, тимус также продуцирует несколько полипептидов, оказывающих влияние на дифференцировку лимфоцитов in vitro и in vivo (эндокринная функция). [Bach, 1979]. Некоторые из этих пептидов (ct 1-тимозин, тимопоэтин, сывороточный фактор тимуса) были секвернированы, искусственно синтезированы и тщательно изучены. В работах 70-х-80-х гг. показано, что ЭТИ пептиды — гормоны тимуса - оказывают различное влияние на субпопуляции Т-лимфоцитов [Wara el al., 1979, Tan, Shore. 1984]. связываясь co специфичными клеточными рецепторами и реагируя с аденилатцик-лазой [Bach, 1979]. Исследование роли гормонов тимуса продолжается и сегодня, хотя эта тема характеризуется сравнительно небольшим числом публикаций.
Таким образом, современное понимание функции тимуса складывается из двух компонентов: продукции наивных Т-лимфоцитов и секреции гормонов. Мы рассмотрим продуктивный компонент функциональной активности вилочковой же лезы. его роль в организме, методы анализа и применение полученных данных в науке и медицине.
Роль тимуса в реставрации иммунной системы
Вилочковая железа является основным поставщиком наивных Т-лимфоцитов. Важность этого органа наиболее высока в первые шесть месяцев после рождения человека, когда формируется основная часть репертуара периферического пула Т-клеток [Haynes et al-. 2000], функциональная активность тимуса непрерывно растет вплоть до пятнадцатого месяца жизни, когда она достигает своего максимума, после чего начинает постепенно снижаться [Taub, Longo, 2005]. За 80 лет количество наивных Т-лимфоцитов, продуцируемых тимусом. падает примерно в 100 раз [Steinmann, Klaus. Muller-Hermelink. 1985]. Таким образом, в течение жизни вилочковая железа претерпевает значительную инволюцию [Aspinall, Andrew, 2000]. при этом существенная часть ее периваскулярного пространства замещается жировой тканью [Flores et al. 1999], Уменьшается размер корковой зоны органа. Теряется четкая граница между’ корой и мозговым веществом [Taub, Longo. 2005]. и хотя видимый размер тимуса меняется не сильно, эпителиальное пространство органа, ответственное за генерацию новых Т-лимфоцитов, значительно сокращается. Обследование людей старшего возраста показало, что у них присутствуют лишь небольшие островки активного эпителиального пространства. поддерживающего функциональную активность тимуса. Наблюдая эти морфологические изменения, многие исследователи в течение долгого времени придерживались мнения о малом вкладе тимуса в реставрацию пула Т-клеток в постпубертатный период. Такое положение вещей не является удивительным. На периферии существуют еще два механизма тимуснезависимого поддержания численности лимфоцитов: гомеостатическая пролиферация Т-клеток и их антигензависимое деление [Mackall et al.. 1996; Min, Paul, 2005] Гомеостатическая пролиферация определяется цитокинами и сигналами, поступающими в Т-клетку через ТкР [Surh, spent. 2008] Антигензависимое деление осуществляется через взаимодействие рецепторов Т-лимфоцитов с экзогенными и эндогенными пептидами, презентированными в составе гл а вного ком плекса гистосовместимости [М i п, Paul. 2005; Ernst et al.. 1999]. Таким образом, не только тимус, но и другие лимфоидные органы обеспечивают постоянство численности периферического пула Т-клеток. Вклад каждого определяется возрастом организма, присутствующими на периферии субпопуляциями Т-клеток. концентрацией гомеостатических цитокинов и эндогенной стиму- ляцией клеток антигенами [Mackall et al., 1997; Mackall, Gress, 1997]. Однако следует отметить, что разнообразие репертуара Т-лимфоцитов создается исключительно при участии вилочковой железы, что говорит о важности данного органа в восстановлении периферического пула Т-клеток в любом возрасте [Hakim et al., 2005].
Несмотря на инволюцию* тимус сохраняет свою функциональную активность на протяжении всей жизни организма [Poulin et at, 1999]* Экспериментальные данные свидетельствуют о том* что, хотя у взрослых животных периферическая экспансия клеток имеет наибольшее значение для поддержания численности Т-лимфоцитов* их тимусы по-прежнему активно продуцируют наивные клетки (эффективность работы железы взрослой мыши может достигать половины эффективности работы тимуса детеныша) [Mackall et at* 1998]. Клинические наблюдения подтверждают значительный вклад тимуса в восстановление уровня периферических Т-клеток у людей старше 30 лет. Показано* что у взрослых первые три месяца после химиотерапии .характеризуются преобладанием Т-лимфоцитов с фенотипом эффекторных клеток памяти (CD62L"CCR7”CD45RA”) Однако эта периферическая экспансия лишь временно увеличивает популяцию периферических Т-клеток. Продукция наивных Т-лимфоцитов восстанавливает нормальные количества CD4 Т-клеток к концу второго года реабилитации* Без активации тимопоэза уровень CD4 Т-лимфоцитов может оставаться ниже нормы даже спустя 4-5 лет после трансплантации [Hakim el at* 2005]. Данные* полученные при исследовании разнообразия ТкР клеток периферической крови* количества тимических мигрантов* двуцепочечных разрывов ТкР|3 генов в клетках тимуса и т.д. также убедительно говорят о непре-кращающемся поступлении новых наивных Т-клеток из тимуса взрослых и даже пожилых людей [Douek et al** 1998]*
Следует отметить* что в механизмах восстановления CD4 и CD 8 Т-клеток после деплеции существуют значительные отличия [Mackall el al* 1997]* У людей отмечена отрицательная корреляционная зависимость между возрастом и способностью иммунной системы восстанавливать уровень CD4 Т-лимфоцитов после интенсивной химиотерапии* трансплантации клеток костного мозга или введения моноклональных антител при лечении ревматоидного артрита [Moreland et al* 1994; Storek et al * 1995]. В то же время* корреляционной зависимости междуг темпами репопуляции периферического отдела цитотоксическими Т-клетками и возрастом не обнаружено. Известно* что численность CD8 Т-клеток может вернуться к контрольным значениям всего через три месяца после деплеции* в то время как CD4 Т- лимфоциты к этому времени восстановят лишь около 35% изначального количества. Столь эффективное увеличение числа цитотоксических Т-клеток опосредуется делением периферических лимфоцитов с фенотипом клеток памяти. При этом субпопуляция наивных CD8+ Т-лимфоцитов восстанавливается с интенсивностью, характерной для CD4+ Т-клеток [Mackall et al., 1997]. Эти данные свидетельствуют о том, что восстановление уровня CD4+ Т-лимфоцитов может проходить только по тимусзависимому пути, эффективность которого падает параллельно с инволюцией вилочковой железы* Но для восстановления количества CD8 Т-клеток после деплеции более значимы ти-муснезависимые механизмы. Следует отметить* что только тимусзависимый путь репопуляции периферического пула может обеспечить широкое разнообразие ТкР* Тимусиезависимая пролиферация отдельных субпопуляций CD8 Т-клеток приводит к формированию пула Т-лимфоцитов с низкой степенью разнообразия рецепторов* что ограничивает эффективность его работы [Mackall el al** 1997]. Опосредованное возрастом уменьшение числа вновь формирующихся наивных CD4” и CD8 Т-клеток является основным лимитирующим фактором тимусзависимой регенерации Т-клеток после деплеции [Donek et al** 1998; Mackall el al** 1995; Weinberg et al * 1995].
Таким образом* продуктивная функция вилочковой железы обеспечивает формирование пула наивных Т-лимфоцитов в раннем возрасте* а также восстановление CD4” и отдельных субпопуляций CD8 Т-лимфоцитов при деплеции в течение всей ЖИЗНИ*
Современные подходы к изучению продуктивной функции тимуса
На сегодняшний день существуют несколько подходов к прижизненной оценке продуктивной функции вилочковой железы. Данные об объеме тимуса* получаемые при томографии грудной клетки и данные о структуре ткани* доступные при ультразвуковом исследовании* дают представление о работе органа на основе косвенных признаков [Bogot, Quint, 2005; Lee et al., 2009]. Объективным показателем эффективности работы железы можно считать количество тимических мигрантов (RTE -recent thymic emigrants) в периферической крови [Douek et al., 2001; Pennar et al., 2003]* Эти клетки прошли все этапы созревания и селекции в тимусе* покинули железу, но еще не являются зрелыми наивными Т-лимфоцитами. В моделях на животных определение числа RTE реализуется прямым введением флуоресцентных красок (FITC) в тимус [Staton et al * 2004] или добавлением бромдезоксиуридина в питьевую воду [Tough, Sprent, 1994] с последующим обнаружением меченых Т-клеток на периферии* Однако данные методы являются не- точными и не могут быть использованы на ЛЮДЯХ. Для получения сведений о субпопуляциях Т-лимфоцитов человека используют цитометрические методы исследований с определением поверхностных маркеров, характерных для фенотипически наивных Т-лимфоцитов. Однако на сегодняшний день вопрос о составе набора маркеров, полностью описывающих наивную популяцию Т-клеток, остается открытым, поэтому их сочетание варьирует от эксперимента к эксперименту [Kim-mig S., 2002; Haines C.J., 2009; Opiela S.J., 2009; Prelog M.. 2009]. Такое разнообразие говорит о сложности определения пула именно наивных Т-клеток только по фенотипическим признакам. Таким образом, ни один из представленных методов не дает возможности напрямую выявить и проанализировать изменения продуктивной функции вилочковой железы.
Разработка метода, не полагающегося на косвенные показатели продуктивной функции тимуса, долгое время не была возможна ввиду отсутствия молекулярных маркеров, достоверно отличающих RTE от долгоживущих наивных Т-клеток [Steffens et al., 2000; Ye, Kirschner, 2002]. Предложенный в 1998 г. Д. Дуеком и коллегами [Douek et ak 1998] оригинальный метод определения одного из побочных продуктов реаранжировки генов цепей ТкР - aTREC (Т cell receptor excision circles - эписо-мальные кольца ТкР) - в качестве молекулярного маркера RTE открыл новые возможности в изучении динамики продуктивной функции вилочковой железы.
Молекулярные основы метода определения продуктивной функции тимуса
Для „лучшего понимания метода анализа продуктивной функции тимуса по количеству aTREC, следует внимательнее рассмотреть механизмы, обеспечивающие продуктивную функцию вилочковой железы: процессы реаранжировки сегментов генов ТкР и селекции Т-лимфоцитов.
Созревание и селекция Т-клеток
Предшественники Т-клеток мигрируют в вилочковую железу из костного мозга [Donskoy, Goldschneider, 1992]. На начальном этапе они обозначаются как дубль-негативные (DN - double-negative cells) клетки, не экспрессирующие такие характерные для Т-лимфоцитов поверхностные маркеры, как ТкР, CD 3-комплекс, молекулы CD4 и CD8 [Germain, 2002]. В этот период тимоциты активно пролиферируют. Последующая активация белков RAG1 и RAG2 (recombinase-activating gene) приводит к запуску реаранжировки сегментов генов ТкР [Spits. 2002].
Небольшая часть DN-тимоцитов (<5%) успешно реаранжирует у- и 6-цепи рецептора, в то время как основная масса клеток формирует офТкР. Первым ключевым этапом в созревании ар-тимоцитов является успешная перестройка сегментов генов р-цепи. Клетки, экспрессирующие на своей поверхности белковую P-цепь, подвергаются р-селекции [Petrie et al.. 2000]. Они формируют пре-Т-клеточный рецепторный комплекс, соединяясь с гликопротеином (33kDa), известным как пре-Та-цепь. и группой молекул СОЗ [von Boehiner, 2005]. Предположительно такой пре-ТкР распознает некий внутритимический лиганд, что приводит к получению клеткой сигнала на выживание и дальнейшее развитие (в т ч. подавляет сигнал на перестройку второй аллели p-цепи. индуцирует экспрессию молекул CD4 и CDS. обусловливает пролиферацию). В случае. если реаранжировка Р-цепи была произведена некорректно клетка устраняется путем апоптоза [Alvarez-Vallina el al.. 1993].
Последующая успешная реаранжировка гена а-цепи рецептора обусловливает экспрессию арТкР [Hazenberg. Verschuren. 2001]. Такие клетки несут на своей поверхности CD4 и CD 8 корецепторы. и называются дубль-позитивными (DP - double-positive cells) [Gascoigne. Palmer. 2011]. Они вступают в процесс положительной селекции, взаимодействуя с комплексом пептид-МНС на эпителиальных клетках тимуса. Тимоциты, несущие рецепторы. способные к такому взаимодействию, проходят позитивную селекцию, так как они ДОЛЖНЫ быть способны проводить сигналы, опосредованные связыванием с антигенпрезентирующими клетками [Takahama et al.. 2010]. Клетки, не получившие сигнал на выживание и дальнейшее развитие, умирают в течение 3-4 дней. В процессе позитивной селекции тимоциты становятся прекомми-тированными развиваться в CD4+, либо CD8+ субпопуляции.
Тимоциты, экспрессирующие на своей поверхности только один из корецепторов. называются монопозитивными (SP - single positive). Благодаря наличию хемокинового рецептора CCR7 они мигрируют в мозговое вещество вилочковой железы, где подвергаются процессу негативной селекции, в котором из общей популяции устраняются клетки, несущие высокоаффинные рецепторы к аутоантигенам- что предотвращает развитие аутоиммунных процессов [Cheng et al.. 2007].
CD4+ и CD8+ зрелые Т-клетки. несущие рецепторы с низкой аффинностью к аутоантигенам, покидают тимус и ПОПОЛНЯЮТ периферический пул лимфоцитов крови (рис. 1).
Реаранжпровка сегментов генов ТкР и формирование TREC
Все разнообразие генов, кодирующих белковые цепи сф- и уб-ТкР, формируется при реаранжировке четырех локусов: D (3-цепь). G (у-цепь). В ф-
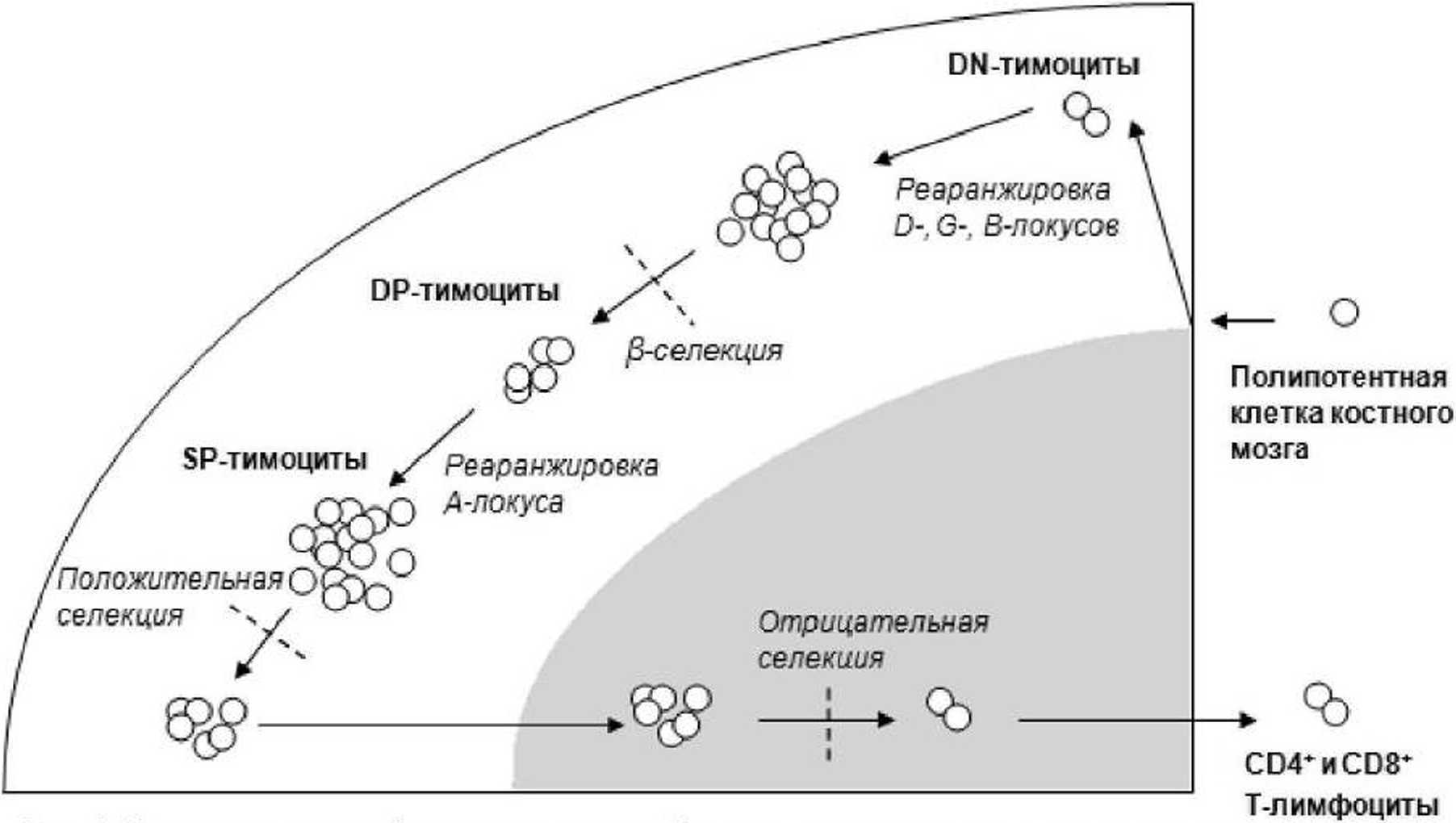
Рис. 1. Схематическое изображение тимуса с обозначенными этапами реаранжировки и селекции тимоцитов.
Белая область - корковый слой тимуса; серая область - мозговое вещество
цепь) и А (a-цепь). Они перестраиваются последовательно. подчиняясь строгому порядку; Первыми в процесс реаранжировки вступают сегменты генов ТкР-D локуса. Следом начинается рсаранжи-ровка ТкР-G. Эти два этапа рассматриваются как ранние, так как происходят до начала экспансии тимоцитов [Ramio et al.. 1996]. Следующим шагом в формировании генов ТкР является реаранжировка сегментов ТкР-B. Этот этап созревания является ключевым, гак как на нем клетки подвергаются р-селекции [Trigueros el al, 1998]. В последнюю очередь происходит рсаранжировка ТкР-А. На 14qll хромосоме сегменты ТкР-D расположены внутри ТкР-А (ТкР-AD локус). По этой причине последовательная реаранжировка А-локуса приводит к удалению генов 8-цепи. Таким образом, одна из аллелей всех арТ-лимфоцитов не несет в своем составе генов D-локуса, а 80% клеток удаляют эти гены и на второй аллели [Van Dongen. Wolvers-Tettero. 1991]. После вырезания 8-участка происходит продуктивная перестройка сегментов генов А-локуса, за которой следует экспрессия арТкР на поверхности тимоцитов [Verschuren et al., 1997].
Аффинность ТкР определяется результатом реаранжировки сегментов генов в каждом из локусов. Несмотря на стру кту рную схожесть этих генетических элементов, их реорганизация позволяет создать все разнообразие репертуара ТкР. Это является возможным благодаря тому; что рекомбинация происходит между крупными участками ДНК. состоящими из V (Variable), D (Diversity) и J (Joining) генетических сегментов [Oltz, 2001]. Ко личество V, D и J сегментов в каждом локусе, а также возможность ну клеотидных вставок и деле-ций в местах их сочленений, определяют потенциальное комбинативнос разнообразие.
Порядок реаранжировки сегментов генов ТкР контролируется на различных уровнях организации. Для того чтобы генетические сегменты могли включиться в процесс перестройки, они должны стать доступными для действия V(D)J-рекомбиназы [Bassing et al.. 2002]. Генетическая регуляция этого процесса связана с наличием особых рекомбинационных сигнальных последовательностей (RSS - recombination signal sequences). Они состоят из консервативных участков в шесть пар нуклеотидов (гептамер) и девять пар нуклеотидов (нонамер). разделенных относительно неконсерватив-ными 12 или 23 парами нуклеотидов. Эти рекомбинационные сигнальные последовательности фланкируют генетические сегменты антигенрас-познающего рецептора и обозначаются как 12RSS и 23RSS соответственно [Tonegawa, 1983]. Рекомбинация инициируется связыванием RSS со специфичными белками: RAG-1 и RAG-2 [Fugmann et al, 2000]. За этим шагом следует формирование комплекса между двумя RSS. прежде находившимися на удалении друг от друга (расстояние до 10б пар нуклеотидов) [Fugmann et al.. 2000]. Внутри комплекса RAG-1 и 2 создают двуцепочечные разрывы ДНК на обеих границах RSS. тем самым освобождая два «сигнальных» и два «кодирующих» конца нуклеиновой кислоты. Эти концы процессируются и соединяются белками репарации ДНК.
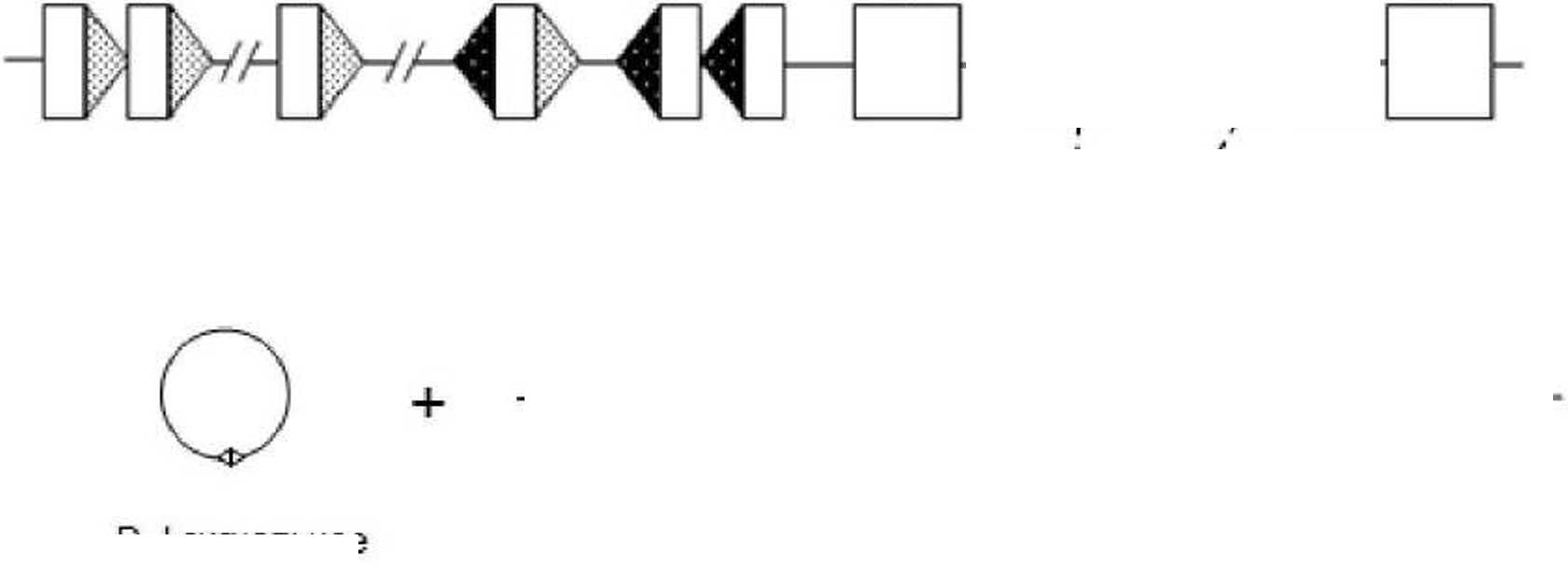
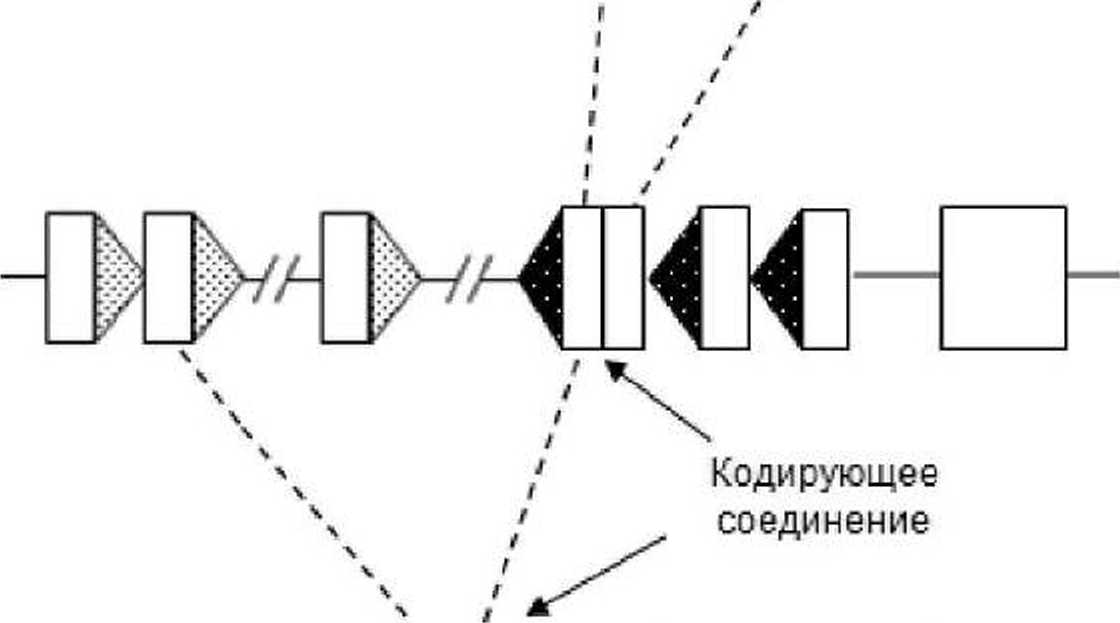
формируя последовательность, кодирующую цепь рецептора, и эписомальный кольцевой участок ДНК - TREC [Bassing ct al., 2002]. Эффективное формирование комплексов происходит только ме-жду 12RSS и 23RSS [Fugmann et al., 2000]. Это ограничение. названное правилом 12/23. приводит к тому, что рекомбинация происходит исключительно между генетическими сегментами, фланкированными RSS со спейсерами различной длины, а следовательно, находящимися на большом удалении друг от друга [Tonegawa, 1983] (рис. 2).
Как уже было указано выше, суть рекомбинации сводится к «вырезанию» участка ДНК между удаленными сегментами, что приводит к их сближению и формированию гена, кодирующего белковую цепь рецептора. Вырезанные фрагменты замыкаются в кольца и остаются в клетке в виде внехромосомных образований, получивших название TREC. Это стабильные эписомальные молекулы [Guazzi V.. 2002]. Так как. в отличие от остальной ДНК. они не ду плицируются во время клеточного деления, только одна дочерняя клетка наследует TREC [Lewin S.R.. 2002]. Соответственно, количество маркерных молекул в популяции Т-клеток периферической крови обратно пропорцио нально пролиферативной истории, следующей за генерацией кольцевой молекулы. В случае, когда TREC образуется на поздних этапах тимопоэза, большинство делений происходит на периферии и соответствует гомеостатической пролиферации зрелых наивных Т-клеток.
Из четырех генов рецепторных цепей ТкР последним формируется ген a-цепи [Spits Н., 1998]. Образующийся при этом aTREC является результатом удаления 5-гена из ТкР-AD локуса. Так как вырезание сегментов происходит у всех клеток в одинаковой области, кольцевые молекулы aTREC характеризуются единообразием [De Villartay, et at. 1988]. В связи с этим и тем, что разбавление aTREC при делении тимоцитов оказывается минимальным. определяя количество TREC a-цепи в периферических Т-лимфоцитах, мы можем примерно оценить выход тимических мигрантов, а следовательно, провести оценку* продуктивной функции тимуса [Chavan et al., 2001].
Для количественного анализа aTREC в клетках периферической крови используется метод полимеразной цепной реакции в реальном времени (ПЦР-РВ). Так как определяемая область представляет собой участок ДНК, общий для всех кле- vpi vp:
vpn
Dpi
jpi ... Jp6
cpi
op:
jp?.._ jpi2 cp:
HH№
D-J сигнальное соединение
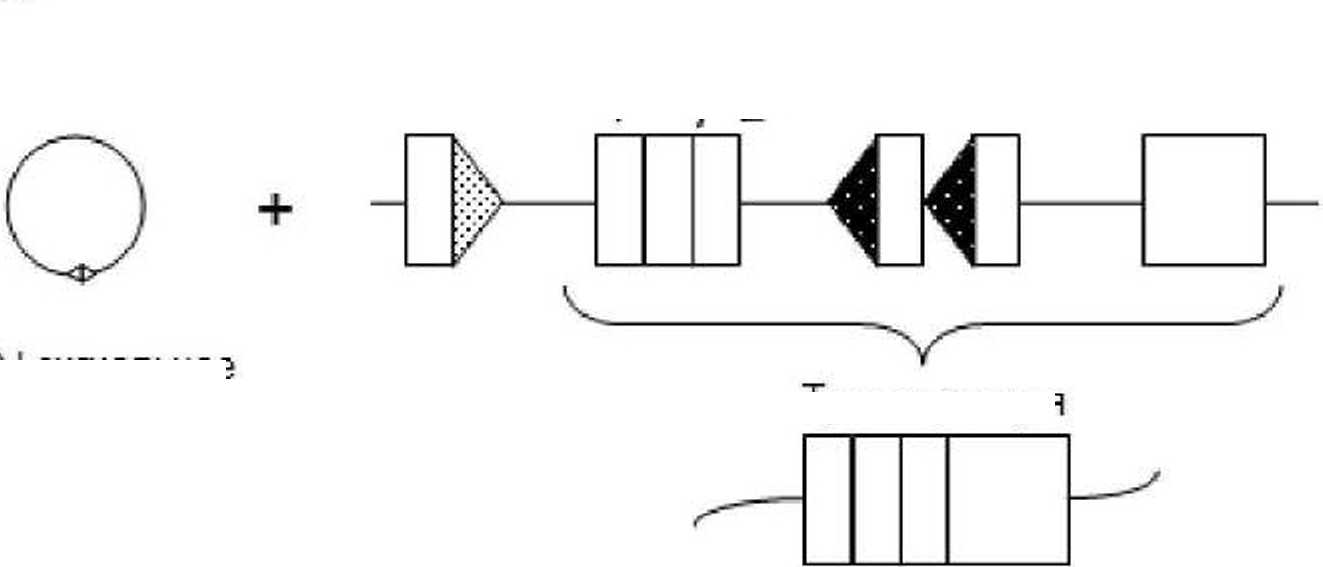
V-DJ сигнальное соединение
Рис. 2. Схематическая диаграмма последовательной реаранжировки p-цепи ТкР.
Прямоугольниками обозначены V-, D-, J-сегменты гена и константные домены. Треугольниками обозначены рекомбинационные сигнальные последовательности (RSS): серая штриховка - 23RSS; черная штриховка - I2RSS. Кольцевые молекулы - TREC
Транскрипция
ток, в ее нуклеотидном составе отсутствуют уникальные последовательности. Для того чтобы специфично определить интересующий участок, праймеры для реакции амплификации подбираются таким образом, чтобы места их «посадки» располагались по разные стороны «сигнального» соединения кольца aTREC Отжиг таких праймеров на линейную последовательность ДНК, где места их гибридизации разнесены на 89000 п*н. [*nih*gov/; NT 026437.12], не способен дать продукт амплификации* Следовательно, наличие в системе ДНК клеток, в которых не была проведена рекомбинация генетических сегментов a-цепи* не влияет на качество прохождения реакции.
Детекция результатов амплификации может осуществляться с использованием любых зондов* комплементарных определяемому фрагменту (гибридизационные, гидролизные* и т.п ). В конце каждого круга ПЦР интенсивность генерируемого флуоресцентного сигнала пропорциональна количеству зондов, связавшихся с мишенью, поэтому увеличение детектируемого сигнала пропорционально увеличению количества продукта реакции. Детекция интенсивности флуоресцентного сигнала позволяет с высокой точностью установить момент начала экспоненциальной фазы реакции* Номер порогового цикла, во время которого значение флуоресценции пересекает пороговый уровень* зависит от количества целевой последовательности в исследуемом образце* Проще говоря, чем больше искомой последовательности (aTREC) содержится в пробе, тем раньше наступит пороговый цикл амплификации [Dion el al** 2007]*
Для анализа данных количественных исследований молекул aTREC в периферической крови разработан ряд математических моделей [Ye* Kirschner* 2002]* Они учитывают факторы, оказывающие влияние на концентрацию TREC. а именно: общее число Т-лимфоцитов и общее количество кольцевых молекул ДНК* Общее число Т-лимфоцитов зависит от продуктивной функции ВИ ЛОЧКОВОЙ железы (количества RTE)* пролиферации клеток вне тимуса и клеточной смерти* Молекулы aTREC формируются в тимоцитах и экспортируются на периферию в составе RTE. следовательно* количество маркерных молекул определяется про-ду ктиБной функцией железы* смертью Т-клеток и внутриклеточной деградацией кольцевой молекулы. Таким образом, математические модели учитывают все четыре фактора: продуктивную функцию тимуса* пролиферацию клеток* смерть Т-лимфоцитов и деградацию кольцевой молекулы ДНК.
Влияние каждого из описанных факторов на концентрацию aTREC оказалось неодинаковым* В своей работе Е и Киршнер [Ye* Kirschner* 2002]
показали, что эмиграция RTE, скорость клеточного деления, смерть клеток и деградация маркерной кольцевой молекулы имеют достоверную корреляцию с концентрацией aTREC на протяжении всей жизни (р<0_05). Вклад продуктивной функции ти-муса, деления и смерти клеток значительно увеличивается с возрастом, в то время как значимость деградации кольцевой молекулы остается постоянной* Выход тимуса, деление Т-клеток и деградация кольца - более критичные факторы в регуляции концентрации aTREC, нежели клеточная смерть (р<0.005)* Статистически значимых отличий между эффектом продуктивной функции тимуса и деления Т-клеток на концентрацию aTREC не обнаружено (р>0.05)* Если предположить* что период полураспада маркерной молекулы константен, то выход тимуса* и клеточное деление оказывают равнозначное влияние на концентрацию aTREC в течение жизни. Эти данные правомерны как для CD4 * так и для CD 8 Т-лимфоцитов.
Применение в медицинской и исследовательской практике
В настоящее время перечень клинических исследований, в рамках которых проводится определение количества aTREC, ограничен. Одна из сфер применения - установление природы и тяжести иммунодефицитов человека [Seraпа F., 2011]. Метод является полезным, так как зачастую данные о количестве Т-клеток в периферическом кровотоке недостаточны для диагностирования заболевания. Некоторые медицинские центры предлагают учитывать показатель количества aTREC при проведении высокоактивной антиретровирусной терапии для оценки эффективности лечения ВИЧ-инфекции [Weinberg А., 2008; Anselmi А.: 2007]. Также функциональное состояние тимуса оценивают перед проведением трансплантации гемопоэтических стволовых клеток. Учет этого показателя позволяет избежать развития иммунодефицита у пациентов [Reiff А., 2009; Junge S.,2007].
В науке оценка продуктивной функции вилочковой железы нашла более широкое применение. Об этом свидетельствует все взрастающее число публикаций. Показатель количества aTREC используется в различных исследованиях, затрагивающих вопросы нарушения работы иммунной системы [Joshi et al., 2011; Horvath et al., 2010]. Количество aTREC является прогностическим при анализе развития заболеваний, в том числе раковых [Ducloux et al., 2011], оно используется для оценки восстановления ткани тимуса [van Gent et al, 2011]. Некоторые авторы также предлагают включить этот показатель в обязательный скрининг новорожденных на наличие дефектов иммунной системы [Comeau А.М., 2010].
Заключение
Вилочковая железа является источником наивных иммунокомпетентных клеток, несущих на своей поверхности рецепторы различной специфичности. Несмотря на постепенную возрастную инволюцию приводящую к значительным перестройкам функционально активных тканей органа и их замещение жировой тканью* функция тимуса не теряет своей значимости даже в зрелом возрасте. Хотя количество периферических Т-клеток в основном поддерживается за счет механизмов гомеостатической пролиферации, функция тимуса необходима для восстановления полноценного репертуара Т-лимфоцитов при деплеции.
Так как многие факторы способны оказывать влияние на продуктивную функцию ВИЛОЧКОВОЙ железы, адекватная оценка этого показателя может Стать эффективным инструментом иммунологических исследований. Определение количества RTE с использованием молекулярного маркера aTREC открыло новые возможности для науки и лабораторной клинической практики.
Список литературы Молекулярные основы современных подходов к анализу продуктивной функции тимуса
- Alvarez-Vallina L., Gonzalez A., Kreisler M., Diaz-Espada F. Delimitation of the proliferative stages in the human thymus indicates that cell expansion occurs before the expression of CD3 (T cell receptor)//J. Immunol. 1993. Vol. 150. P. 8-16
- Aspinall R., Andrew D. Thymic involution in aging//J. Clin. Immunol. 2000. Vol. 20. P. 250-256
- Bach J.F. Thymic hormones//J. Immunopharmacol. 1979. Vol. 1(3). P. 277-310
- Bassing C.H., Swat W., Alt F. W. The mechanism and regulation of chromosomal V(D)J recombination//Cell. 2002. Vol. 109. P. S45-S55
- Billingham R.E., Brent L., Medawar P.B. Quantitative studies on tissue transplantation immunity. III. Actively acquired tolerance//Philos. Trans. R. Soc. Lond. 1956. Vol. 239B. P. 357-412
- Bogot N.R., Quint L.E. Imaging of thymic disorders//Cancer Imaging. 2005. Vol. 5. P. 139-149
- Chavan S., Bennuri B., Kharbanda M., et.al. Evaluation of T cell receptor gene rearrangement excision circles after antiretroviral therapy in children infected with human immunodeficiency virus//J. Infect. Dis. 2001. Vol. 183. P. 1445-1454
- Cheng M.H., Shum A.K., Anderson M.S. What's new in the Aire?//Trends in Immunology. 2007. Vol. 28, Iss. 7. P. 321-327
- Cooper M.D., Chen C.H., Bucy R.P., Thompson C.B. Avian T cell ontogeny//Adv. Immunol. 1991. Vol. 50. P. 87-117
- De Villartay J.P., Hockett R.D., Coran D., Kors-meyer S.J., Cohen D.I. Deletion of the human T cell receptor 5-gene by a site specific recombination//Nature. 1988. Vol. 335. P. 170-174
- Dion M.L., Sekaly R.P., Cheynier R. Estimating thymic function through quantification of T-cell receptor excision circles//Methods Mol. Biol. 2007. Vol. 380. P. 197-213
- Donskoy E., Goldschneider I. Thymocytopoiesis is maintained by blood-borne precursors throughout postnatal life. A study in parabiotic mice//J. Immunol. 1992. Vol. 148. P. 1604-1612
- Douek D.C., Betts M.R., Hill B.J. et al. Evidence for increased T cell turnover and decreased thymic output in HIV infection//J. Immunol. 2001. Vol. 167. P. 6663-6668
- Douek D.C., McFarland R.D., Keiser P.H. et al Changes in thymic function with age and during the treatment of HIV infection//Nature. 1998. Vol. 396. P. 690-695
- Ducloux D., Bamoulid J., Courivaud C., Gaugler B., Rebibou J.M., Ferrand C. et al. Thymic function, anti-thymocytes globulins, and cancer after renal transplantation//Transpl. Immunol. 2011. Vol. 25(1). P. 56-60
- Ernst B, Lee D.S., Chang J.M., Sprent J., Surh C.D. The peptide ligands mediating positive selection in the thymus control T cell survival and homeo-static proliferation in the periphery//Immunity. 1999. Vol. 11. P. 173-181
- Flores K.G., Li J., Sempowski G.D., Haynes B.F., Hale L.P. Analysis of the human thymic perivas-cular space during aging//J. Clin. Invest. 1999. Vol. 104(8). P. 1031-1039
- Fugmann S.D., Lee A.I., Shockett P.E., Villey I.J., Schatz D.G. The RAG proteins nd V(D)J recombination: complexes, ends, and transposition//Annu. Rev. Immunol. 2000. Vol. 18. P. 495-527
- Gascoigne N.R.J., Palmer E. Signaling in thymic selection//Current Opinion in Immunology. 2011. Vol. 23, Iss. 2. P. 207-212
- Germain R.N. T-cell development and the CD4-CD8 lineage decision//Nat. Rev. Immunol. 2002. Vol. 2(5). P. 309-322
- Gowans I.L., Gesner B.M., McGregor D.D. The im-munological activities of lymphocytes//Wolsten-holmeGEW, O'ConnorM, eds. Biological activity of leucocyte. Ciba Foundation Study Group. London: Churchill, 1961. P. 32-34
- Hakim F.T., Memon S.A., Cepeda R., Jones E.C., Chow C.K., Kasten-Sportes C. et al. Age-dependent incidence, time course, and consequences of thymic renewal in adults//J. Clin. Invest. 2005. Vol. 115(4). P. 930-939
- Haynes B.F., Markert M.L., Sempowski G.D. et al. The role of the thymus in immune reconstitution in aging, bone-marrow transplantation and HIV-1 infection//Annu. Rev. Immunol. 2000. Vol. 18. P. 529-560
- Hazenberg M.D., Verschuren M.C.M. T cell receptor excision circles as markers for recent thymic emigrants: basic aspects, technical approach, and guidelines for interpretation//J. Mol. Med. 2001. Vol. 79. P. 631-640
- Horvath D., Kayser C., Silva C.A., Terreri M.T., Hilario M.O., Andrade L.E. Decreased recent thymus emigrant number in rheumatoid factor-negative polyarticular juvenile idiopathic arthritis//Clin. Exp. Rheumatol. 2010. Vol. 28(3). P. 348-353
- Joshi A.Y., Abraham R.S., Snyder M.R., Boyce T.G. Immune evaluation and Vaccine responses in Down syndrome: Evidence of immunodeficiency?//Vaccine. 2011. Vol. 29(31). P. 5040-5046
- Kisielow P., von Boehmer H. Development and selection of T cells: facts and puzzles//Adv. Immunol. 1995. Vol. 58. P. 87-209
- Lee W.K., Duddalwar V.A., Rouse H.C. et al. Extran-odal lymphoma in the thorax: cross-sectional imaging findings//Clin. Radiol. 2009. Vol. 64, № 5. P. 542-549
- Mackall C.L., Bare C.V., Granger L.A., Sharrow S.O., Titus J.A., Gress R.E. Thymic-independent T cell regeneration occurs via antigen-driven expansion of peripheral T cells resulting in a repertoire that is limited in diversity and prone to skewing//J. Immunol. 1996. Vol. 156(12). P. 46094616
- Mackall C.L., Fleisher T.A., Brown M.R., Andrich M.P., Chen C.C., Feuerstein I.M. et al. Distinctions between CD8+ and CD4+ T-cell regenerative pathways result in prolonged T-cell subset imbalance after intensive chemotherapy//Blood. 1997. Vol. 89(10). P. 3700-3707
- Mackall C.L., Fleisher T.A., Brown M.R. et al Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy//N. Engl. J. Med. 1995. Vol. 332. P. 143-149
- Mackall C.L., Gress R.E. Pathways of T-cell regeneration in mice and humans: implications for bone marrow transplantation and immunotherapy//Immunol. Rev. 1997. Vol. 157. P. 61-72
- Mackall C.L., Punt J.A., Morgan P., Farr A.G., Gress R.E. Thymic function in young/old chimeras: substantial thymic T cell regenerative capacity despite irreversible age-associated thymic involution//Eur. J. Immunol. 1998. Vol. 28(6). P. 1886-1893
- Miller J.F.A.P. Analysis of the thymus influence in leukaemogenesis//Nature. 1960. Vol. 191. P. 248-249
- Miller J.F.A.P. Immunological significance of the thymus of the adult mouse//Nature. 1962. Vol. 195. P. 1318-1319
- Min B., Paul W.E. Endogenous proliferation: burstlike CD4 T cell proliferation in lymphopenic set tings//Semin Immunol. 2005. Vol. 17(3). P. 201-207
- Moreland L. W., Pratt P. W., Bucy R.P., Jackson B.S., Feldman J.W., Koopman W.J. Treatment of refractory rheumatoid arthritis with a chimeric anti-CD4 monoclonal antibody. Long-term followup of CD4+ T cell counts//Arthritis Rheum. 1994. Vol. 37(6). P. 834-838
- Oltz E.M. Regulation of antigen receptor gene assembly in lymphocytes//Immunol. Res. 2001. Vol. 23. P. 121-133
- Permar S.R., Moss W.J., Ryon J.J. et al. Increased thymic output during acute measles virus infection//Journal of Virology. 2003. Vol. 77, № 14. P. 7872-7879
- Petrie H.T., Tourigny M., Burtrum D.B. et al. Precursor thymocyte proliferation and differentiation are controlled by signals unrelated to the pre-TCR//J. Immunol. 2000. Vol. 165. P. 3094-3098
- Poulin J.F., Viswanathan M.N., Harris J.M., Koman-duri K.V., Wieder E., Ringuette N. et al. Direct evidence for thymic function in adult humans//J. Exp. Med. 1999. Vol. 190(4). P. 479-486
- Ramio A.R., Trigueros C., Toribio M.L., San Milan J.L. Regulation of pre-T cell (pTa-TCRP) receptor gene expression during human thymic development//J. Exp. Med. 1996. C. 184. P. 519-530
- Shortman K., Wu L. Early T lymphocyte progenitors//Annu. Rev. Immunol. 1996. Vol. 14. P. 29-47
- Spits H. Development of ap T cells in the human thy-mus//Nature reviews: immunology. 2002. Vol. 2. P. 760-772
- Staton T.L., Johnston B., Butcher E.C., Campbell D.J. Murine CD8+ recent thymic emigrants are alphaE integrin-positive and CC chemokine ligand 25 responsive//J. Immunol. 2004. Vol. 172. P. 7282-7288
- Steffens C.M., Al-Harthi L., Shott S. et al. Evaluation of thymopoesis using T cell receptor excision circles (TRECs): differential correlation between adult and pediatric TRECs and naive phenotypes//Clinical Immunology. 2000. Vol. 97, № 2. P. 95-101
- Steinmann G.G., Klaus B., Muller-Hermelink H.K. The involution of the ageing human thymic epithelium is independent of puberty. A morphomet-ric study//Scand. J. Immunol. 1985. Vol. 22. P. 563-575
- Storek J., Witherspoon R.P., Storb R. T cell reconsti-tution after Bone Marrow Transplantation into adult patients does not resemble T cell development in early life//Bone Marrow Transplant. 1995. Vol. 16(3). P. 413-425
- Surh C.D., Spent J. Homeostasis of Naive and memory T cells//Immunity. 2008. Vol. 29. P. 848862
- Takahama Y., Nitta T., Mat R.A, Nitta S., Murata S., Tanaka K. Role of thymic cortex-specific self-peptides in positive selection of T cells//Semin. Immunol. 2010. Vol. 22(5). P. 287-293
- Tan P.L., Shore A. Thymosin induces helper function in OKT3-positive, E-rosette-negative human cord blood T cells//Scand. J. Immunol. 1984. Vol. 20(1). P. 27-34
- Taub D.D., Longo D.L. Insights into thymic aging and regeneration//Immunol. Rev. 2005. Vol. 205. P. 7293
- Tonegawa S. Somatic generation of antibody diversity//Nature. 1983. Vol. 302. P. 575-581
- Tough D.F., Sprent J. Turnover of naive-and mem-ory-phenotype T cells//J. Exp. Med. 1994. Vol. 179. P. 1127-1135
- Trigueros C., Ramio A.R., Carrasco Y.R., De Yebenes V.G., Albar J.P., Toribio M.L. Identification of a late stage of small noncycling pTa-pre-T cells as immediate precursors of T cell receptor ap+ thymocytes//J. Exp. Med. 1998. Vol. 188. P. 1401-1412
- Van Dongen J.J.M., Wolvers-Tettero I.L.M. Analysis of immunoglobulin and T cell receptor genes. Part II: Possibilities and limitations in the diagnosis and management of lymphoproliferative diseases and related disorders//Clin. Chim. Acta. 1991. Vol. 198. P. 93-174
- van Gent R., Schadenberg A. W., Otto S.A., Nievelstein R.A., Sieswerda G.T., HaasF. et al. Long-term restoration of the human T-cell compartment after thy-mectomy during infancy: a role for thymic regeneration?//Blood. 2011. Vol. 118(3). P. 627-634
- Verschuren M.C.M., Wolvers-Tettero I.L.M., Breit T.M., Noordzij J., Van Wering E.R., Van Dongen J.J.M. Preferential rearrangements of the T cell receptor-5-deleting elements in human T cells//J. Immunol. 1997. Vol. 158. P. 1208-1216
- von Boehmer H. Unique features of the pre-T-cell receptor a-chain: not just a surrogate//Nat. Rev. Immunol. 2005. Vol. 5. P. 571-577
- Wara D.W., Barrett D.J., Ammann A.J., Cowan M.J. In vitro and in vivo enhancement of mixed lymphocyte culture reactivity by thymosin in patients with primary immunodeficiency disease//Ann. N. Y. Acad. Sci. 1979. Vol. 32. P. 128-134
- Weigert C. Pathologisch-anatomischer beitrag zur erb'schen krankheit (Myasteia gravis)//Neurol. Centralbl. 1901. Vol. 20. P. 597-601
- Weinberg K., Annett G., Hashyap A. et al. The effect of thymic function on immunocompetence following bone marrow transplantation//Biol. Blood Marrow Transplant. 1995. Vol. 1. P. 18-23
- Ye P., Kirschner D.E. Reevaluation of T cell receptor excision circles as a measure of human recent thymic emigrants//The Journal of Immunology. 2002. Vol. 169. P. 4968-4979


