Morphological and molecular variation of five rice varieties to ultra violet-B radiation stress
Автор: John De Britto A, Mary Sujin R, Roshan Sebastian Steena
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 3 т.7, 2011 года.
Бесплатный доступ
India is one of the major rice producing countries. Rice cultivation is affected by several factors such as climate, soil pollution, UV radiation etc. The present study is an attempt made to understand the morphological variation and molecular variation through SDS-PAGE in five different rice varieties induced with UV-B stress. Five varieties of rice were irradiated with UV-B for 10, 20 and 30 min and a set was maintained as control for every variety. Morphological variations were estimated using morphometric analysis that showed significant variation in stressed and controlled sets. The leaf protein was separated through SDS-PAGE gel electrophoresis and molecular weight markers were used to calculate the molecular weight of the proteins. UV treated varieties had the lowest record based on the banding pattern than the control. These results show that the UV radiation could induce both phenotypic and genotypic changes in rice.
Morphological parameters, molecular variation, oryza sativa l., sds-page, uv stress
Короткий адрес: https://sciup.org/14323540
IDR: 14323540
Текст научной статьи Morphological and molecular variation of five rice varieties to ultra violet-B radiation stress
The solar energy from sun is essential to support the life on our planet. However, the sunlight also contains a small amount of short wavelength ultraviolet (UV) light irradiation, which is harmful to the life on the planet. Fortunately most of this harmful UV irradiation is filtered out by the stratospheric ozone layer, which strongly absorbs UV light, but this protective shield is being continually damaged by human activity (Longstreth et al., 1995). UV-B exposure to living organism has several harmful effect, among which its capacity to induce the genetic mutations is the foremost (Bais et al., 1996). In brief the increased irradiation of UV-B would endanger all forms of life and have devastating effect by reducing the yield of crops (Kerr, 1988). UV-B can potentially interfere with growth, development, photosynthesis, flowering, pollination, and changes the ability of crop plants (Jansen et al., 2001; Kliebenstein et al., 2002).
Rice is one of the world's most important food crop and grown mostly in tropical and subtropical countries. The demand for rice is increasing tremendously as the population in rice consuming areas is increasing at an alarming rate. However, increasing rice production is becoming more difficult because of biotic and abiotic stresses (Hert, 1991).
In the present study, analysis of morphology and protein variability of the five rice varieties induced with UV-B (280–320 nm) stress through morphometric analysis and SDS-PAGE has been carried out.
MATERIALS AND METHODS
Materials
The seeds of rice varieties were procured from seed testing centre, Palayamkottai. Five popular, medium duration varieties namely IR 32, IR 36, IR 45, IR 46 and IR 50 were selected for the present study.
Methodology
A pot culture experiment was conducted in Plant Molecular Biology Research Unit, Tirunelveli during 2010. The seeds were surface sterilized and soaked overnight in double distilled water (ddw) and then kept in dark for 24 hr for germination. After soaking, the seeds were sown in pots (paper cup), which were watered adequately. After 15 days the samples were exposed to UV-B for 10, 20, 30 min and a control was maintained for all the varieties.
Morphometric analysis
After 30th day, the shoot length, root length and leaf area was measured using a metric scale. Its fresh and dry weights were also measured.
Protein profiling
The separation of leaf protein was carried out at -50°C at 100V thereafter for 3-5 hours. SDS-PAGE electrophoresis preparation was followed by (Laemmli, 1970).
Data analysis:
The obtained morphometric data was tabulated and correlated using SPSS software and one way ANOVA was done using Origin Pro 7.5 software. The molecular weight of the unknown proteins and their Rf values were calculated using TL100 software.
RESULTS
The effect of UV-B on morphological and molecular parameters was studied in five rice varieties.
Morphometric analysis
The pearson’s correlation coefficient data of stressed and control set revealed that shoot length of IR 32 and IR 45 at 20 min was highly significant, The root length of IR 50 at 20, 30 min was highly significant, whereas IR 45 and IR 50 at 10 min was less significant, Leaf area of IR 32 at 10, 20 and 30 min; IR 45 at 10 and 20 min were highly significant, whereas IR 36 and IR 46 at 10 min were less significant, fresh weight of IR 32 at 10, 20, 30 min; IR 46 and IR 50 at 30 min were highly significant, Dry weight of IR 32 at 10 and 30 min; IR 45 and IR 46 at 10 min were less significant (Table 2).
Protein profiling
The UV- B exposed seedlings of rice exhibited substantial changes in protein levels. The protein pattern was studied in 30 days old UV-B stressed rice seedlings at different time intervals of 10, 20 and 30 min. Protein pattern differed under both control and stressed condition. SDS denatured protein gels could resolve a total of 42 bands in 10 min, 39 bands in 20 min and 37 bands in 30 min of UV-B irradiated seedlings. More number of protein bands was observed in seedlings treated with UV-B for 10 minutes. The total number of bands was more in control than the seedlings treated with UV-B These SDS protein bands belong to different molecular weights ranging from 3.62 KDa to 153.5 KDa. The molecular weights ranged from 76.98 to 3.62 KDa, 153.5 to 3.74 KDa and 77.75 to 3.78 KDa for seedlings treated with 10, 20 and 30 min of UV-B stress respectively.
Using Total Lab 100 protein analyzer software the molecular weights and the Rf values of each band was calculated. The least molecular weight protein with 3.62 KDa was present in IR 45 stressed plant (10 min UV treatment) with highest Rf value (0.91) and highest molecular weight protein with 153.5 KDa was found in IR 32 (20 min UV treatment) with lowest Rf value (0.19).
Table 1. Effect of UV on shoot length, Leaf length, Leaf width, Root length, Fresh and Dry weight of the 30 days old ten rice varieties
|
Sample |
Treatment of UV (mts) |
Shoot length (cm) |
Root length (cm) |
Leaf area (cm2) |
Fresh weight (g) |
Dry Weight (g) |
|
IR 32 |
Control |
14±0.38 |
5.5±0.41 |
2.4±2.44 |
1.410±0.007 |
0.635±0.01 |
|
10 |
11±0.38 |
5.0±0.79 |
0.82±2.22 |
1.512±0.007 |
0.672±0.008 |
|
|
20 |
10.5±0.41 |
4.8±0.32 |
0.43±2.04 |
1.420±0.007 |
0.432±0.010 |
|
|
30 |
9±0.30 |
5.0±0.66 |
0.94±2.35 |
1.528±0.007 |
0.410±0.410 |
|
|
IR 36 |
Control |
12±3.4 |
5.3±0.38 |
2.45±0.07 |
1.431±0.013 |
0.640±0.015 |
|
10 |
9±1.5 |
4.9±0.88 |
1.2±0.31 |
1.448±0.006 |
0.528±0.006 |
|
|
20 |
8±1.5 |
4.7±0.41 |
0.39±0.07 |
1.397±0.005 |
0.443±0.004 |
|
|
30 |
7±1.5 |
4.9±0.69 |
0.36±0.04 |
1.448±0.007 |
0.378±0.006 |
|
|
IR 45 |
Control |
15±2.54 |
5.1±0.57 |
1±0.38 |
1.528±0.006 |
0.618±0.004 |
|
10 |
9±1.58 |
5.1±0.47 |
0.86±0.03 |
1.510±0.007 |
0.580±0.007 |
|
|
20 |
10±2.54 |
4.9±0.31 |
0.45±0.04 |
1.423±0.006 |
0.473±0.002 |
|
|
30 |
8±2.54 |
4.7±0.58 |
0.82±0.05 |
1.391±0.004 |
0.419±0.084 |
|
|
IR 46 |
Control |
17±2.9 |
4.9±0.57 |
0.96±0.35 |
1.632±0.004 |
0.712±0.005 |
|
10 |
9±1.5 |
5.0±0.79 |
1.2±0.51 |
1.574±0.003 |
0.489±0.006 |
|
|
20 |
8.5±0.7 |
4.9±0.47 |
0.42±0.05 |
1.549±0.007 |
0.458±0.006 |
|
|
30 |
10±2.5 |
4.8±0.66 |
0.41±0.065 |
1.517±0.005 |
0.315±0.007 |
|
|
IR 50 |
Control |
14±2.54 |
5.3±0.25 |
0.9±0.31 |
1.502±0.001 |
0.643±0.003 |
|
10 |
10.5±0.79 |
4.9±0.69 |
0.8±0.25 |
1.4309±0.006 |
0.328±0.006 |
|
|
20 |
10±2.54 |
4.8±0.54 |
0.41±0.07 |
1.397±0.003 |
0.499±0.003 |
|
|
30 |
9±1.58 |
4.7±0.53 |
0.4±0.15 |
1.358±0.006 |
0.473±0.006 |
Table 2. Pearson Correlation coefficient between different treatment of rice varieties compared with control
|
Sample |
Treatment of UV (mts) |
Shoot length (cm) |
Root length (cm) |
Leaf area (cm2) |
Fresh weight (g) |
Dry Weight (g) |
|
IR 32 |
10 |
1.000** |
0.614 |
1.000** |
1.000** |
-910* |
|
20 |
0.589 |
0.500 |
1.000** |
1.000** |
.658 |
|
|
30 |
-0.850 |
0.591 |
1.000** |
1.000** |
.919* |
|
|
IR 36 |
10 |
0.996** |
-0.469 |
0.900* |
0.021 |
0.105 |
|
20 |
0.543 |
-0.159 |
0.178 |
0.502 |
-0.380 |
|
|
30 |
-272 |
-0.773 |
0.200 |
0.721 |
-0.105 |
|
|
IR 45 |
10 |
0.868* |
-0.823* |
0.966** |
-0.262 |
0.0926* |
|
20 |
1.000** |
0.274 |
0.701 |
0.164 |
-0.453 |
|
|
30 |
0.385 |
-0.171 |
0.995** |
-0.619 |
-0.497 |
|
|
IR 46 |
10 |
0.533 |
0.666 |
0.852* |
0.281 |
0.839* |
|
20 |
-0.533 |
0.166 |
-0.069 |
0.276 |
0.209 |
|
|
30 |
0.463 |
0.250 |
-0.803 |
0.974** |
-0.799 |
|
|
IR 50 |
10 |
-0.620 |
0.845* |
0.124 |
0.500 |
0.073 |
|
20 |
0.538 |
0.978** |
0.200 |
0.0997** |
-0.025 |
|
|
30 |
0.124 |
0.983** |
-0.300 |
0.733 |
-0.690 |
P<0.05**- highly significant, P<0.01*- less significant
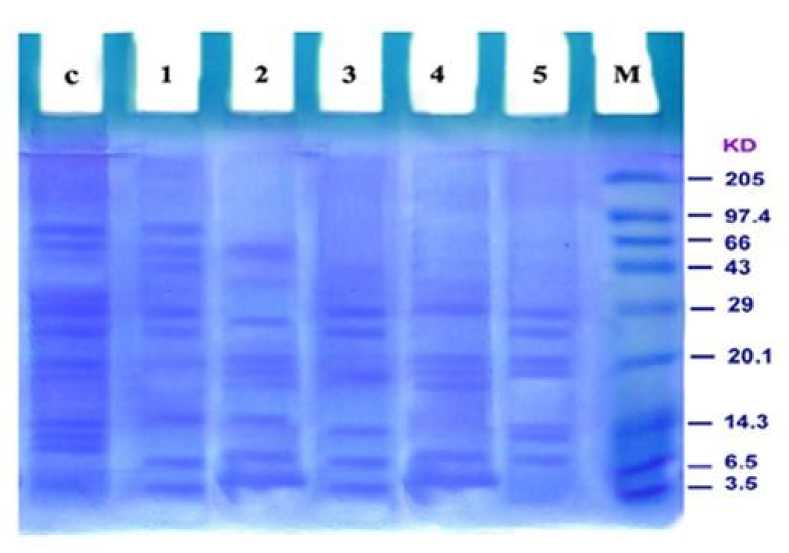
Figure 1. SDS-PAGE gel revealing stress proteins (30 mts UV-treatment) on 30 days old rice varieties. 1- IR 32; 2- IR 36; 3-IR 45; 4- IR 46; 5- IR 50; M- marker.
DISCUSSION
Morphometric analysis
Prasad (1995) reasoned that the decreased growth may be due to the reduced mitotic division of the meristematic zone and also due to the reduction in both new cell formation and cell elongation in the extension region of the root. UV-B radiation causes a variety of morphological responses in higher plants, such as reduction in plant height, fresh mass, shortening of internodes, secretion of wax and leaf thickening. Besides this, UV-B radiation affects many physiological and biochemical parameters like CO2 uptake, RUBPC activity, photosynthetic electron transport chain, dark respiration, stomatal behavior, pigment content and endogenous level of phytohormones (Nedunchezhian and Kulandaivelu, 1991).
Similar work was done by Olsson et al., 1999 in Brassica napus L. to investigate the effect of UV-B radiation on photosynthesis in atrazine tolerant and sensititve cultivars. Predicted increase in UV-B radiation may have adverse impacts on growth and yield of rice ( Oryza sativa L.), as has been found in a study done by Kim et al. (2006). Extensive studies of the physiological, biochemical and morphological effects of UV-B in rice plants, as well as the mechanisms of UV-B resistance have been carried out by Hidema and Kumagai, 2006. Finckh et al., 1999 studied the effect of enhanced UV-B radiation on the growth of the rice and observed the reduction of leaf area and dry weight.
Protein profiling
Kosakivska et al. (2008) observed distinctions in protein synthesis patterns which suggest that stress response proteins could be used as biomarkers. Casati et al. (2005) explained the synthesis of additional protein in response to UV stress. As explained by Yost and Lindquist (1988) the commonality of the proteins repressed or induced in response to UV-B stress indicates that the proteins have an important role in the maintenance of vital cellular function, they could be abnormal proteins synthesized in response to stress. UV damaged specific binding proteins are considered to play an important role in early responses of cell irradiated with UV. UV-B as was found to cause a decline in total RNA, enzyme activity and proteins levels of several key photosynthesis proteins including RUBISCO (Jordan et al., 1992).
Similar work was done in UV-B exposed seedlings of Oryza sativa L. var. ADT(R) – 45, that exhibited substantial changes in protein levels, which was manifested as tolerant, sensitive and induced proteins (Dooslin et al., 2010). Nedunchezhian et al. (2008) reported at the effect of UV-B enhanced protein synthesis in Vigna Sinensis L., which could have possible protective role against UV radiation.
CONCLUSION
From the present study it may be concluded that rice seedlings treated with UV-B radiation at different duration reduced the growth and may lead to the reduced productivity. Moreover there were changes in the occurrence of protein bands indicating changes at genotypic level. Some new protein bands appeared in the stressed plants when compared to the control, these proteins may be used as molecular markers synthesized in response to UV-B stress and this may also have a protective role against the stress.
Список литературы Morphological and molecular variation of five rice varieties to ultra violet-B radiation stress
- Bais A.F., Zerefos C.S. and McElroy C.T. (1996). Solar UVB Measurements with the Double-and Single-monochromator Brewer Ozone Spectrophotometers. Geophy. Res. Lett., 23(8), 833-836
- Casati P., Zhang X., Burlingame A.L. and Walbot V. (2005). Analysis of leaf proteome after UV-B irradiation in maize lines differing in sensitivity. Mol. &Cellular Proteo., 4, 1673-1685.
- Dooslin M.D., Johnson M. and Gerardin J. (2010). UV-B Response of Oryza sativa L. var. ADT(R) 45 Seedlings. Int. J. Biotech. Biochem., 6(3), 411-418.
- Finckh M.R., Chavez A.Q., Dai Q., Teng P.S. (1999). Effects of enhanced UV-B radiation on the growth of rice and its susceptibility to rice blast under glasshouse conditions. Agriculture Ecosystems & Environment., 52(2-3), 223-233.
- Hert R.W. (1991). Research priorities for rice biotechnology. In: G.S. Khush and G.H. Toenniessen (ed.), Rice Biotechnology, CAB Intemational, UK, pp.19-54.
- Hidema J. and Kumagai T. (2006). Sensitivity of Rice to Ultraviolet-B Radiation. Ann. Bot., 97, 933-942.
- Jansen M.A.K., Van Den Noort R.E., Tan M.Y.A., Prinsen E., Lagrimini L.M. and Thorneley R.N.F. (2001). Phenol-oxidizing peroxidases contribute to the protection of plants from ultraviolet radiation stress. Plant Physiol., 126, 1012-1023.
- Jordan B.R., He J., Chow W.S. and Anderson J.M. (1992). Changes in mRNA levels and polypeptide subunits of ribulose 1, 5-bisphophate carboxylase in response to supplementary ultraviolet-B radiation. Plant Cell Environ., 15, 91-98.
- Kerr R.A. (1988). Stratospheric ozone is decreasing. Science., 239, 1489-1491.
- Kim H.Y., Kobayashi K., Nouchi I. and Yoneyama T. (2006). Enhanced UV-B radiation has little effect on growth, δ13C values and pigments of pot-grown rice (Oryza sativa) in the field. Physiologia Plantarum., 96(1), 1-5.
- Kliebenstein D.J., Lim J.E., Landry L.G. and Last R.L. (2002). Arabidopsis UVR8 regulates Ultraviolet-B signal transduction and tolerance and contains sequence similarity to Human Regulator of Chromatin Condensation. Plant Physiol., 130(1), 234-243
- Kosakivska I., Klymchuk D., Negretzky V., Bluma D. and Ustinova A. (2008). Stress proteins and ultrastructural characteristics of leaf cells of plants with different types of ecological strategies. Gen. Appl. Plant Physiol., 34(3-4), 405-418.
- Laemmli U.K. (1970). Cleavage of structural proteins during the assembly of head of bacteriophage T4. Nature., 227, 680-85.
- Longstreth J.D., De Gruijl F.R., Kripke M.L., Takizawa Y. and Van der Leun J.C. (1995). Effects of increased solar ultraviolet radiation on human health. Ambio., 24, 153-165.
- Nedunchezhian N., Amamalainathan K., Kulandaivelu G. (2008). Induction of heat shock-like proteins in Vigna sinensis seedlings growing ultraviolet-B (280-320 nm) enhanced radiation. Physiol. Plant., 85(3), 503-506.
- Olsson L.C., Fraysse L. and Bornman J.F. (1999). Influence of high light and UV‐B radiation on photosynthesis and D1 turnover in atrazine‐tolerant and sensitive cultivars of Brassica napus. J. Experi. Bot., 51(343), 265-274.
- Prasad M.N.V. (1995). Cadmium toxicity and tolerance in vascular plants. Environ and Exp Bot., 35, 525-45.
- Yost H.J. and Lindquist S.L. (1988). Translation of unspliced transcripts after heat shock. Science., 242, 1544-1548.


