Morphological heterogeneity of intratumoral macrophages in prostate tumors
Автор: Danilko K.V., Enikeeva K.I., Kabirov I.R., Maksimova S.Y., Vishnyakov D.S., Kzhyshkowska J.G., Pavlov V.N.
Журнал: Сибирский онкологический журнал @siboncoj
Рубрика: Лабораторные и экспериментальные исследования
Статья в выпуске: 6 т.21, 2022 года.
Бесплатный доступ
Background. Prostate cancer (PCa) is the most common human cancer worldwide. in the progression of prostate cancer, the total number of macrophages in the tumor tissue is associated with poor prognosis and increased risk of metastasis. However, the heterogeneity of intratumoral macrophages at various stages of PCa development, and the role of tumor-associated macrophages (TAMs) have been insufficiently investigated. The aim of the study was to analyze the morphological features, size and number of TAMs in PCa tissue samples, and to reveal their correlation with clinical data of patients. Material and Methods. immunohistochemical analysis of 36 paraffin blocks of patients with PCa (pT2a-3bN0-1M0) was performed using antibodies to the scavenger receptor CD68. Results. Foamy CD68+ macrophages were found in the tumor tissue. The indicator "number of macrophages per total number of fields of view with macrophages" was the lowest in patients with a Gleason score of 6 (5.8) (11.0 - in patients with a Gleason score > 8). Macrophages formed larger clusters in patients with severe PCa. Small but not large macrophages were significantly more common in patients with lymph node metastases (48 vs 24 in the N0 group; p=0.14). The number of small macrophages (smaller than 100 pm2) increased in a series of patients with Gleason scores of 6, 7 and > 8 (24, 47.5, 72, respectively, p=0.052). Conclusion. As the tumor process progressed and the risk of biochemical recurrence increased, there was a trend towards an increase in the total area of large, foamy TAMs, presumably rich in lipids, as well as wider distribution of small macrophages with a tendency to form clusters. We hypothesize that foamy macrophages are involved in the further recruitment of small TAMs, subsequently leading to metastasis and tumor progression.
Prostate cancer, tumor-associated macrophages, giant macrophages, tumor progression
Короткий адрес: https://sciup.org/140296697
IDR: 140296697 | УДК: 616.65-006:576.54 | DOI: 10.21294/1814-4861-2022-21-6-81-90
Текст научной статьи Morphological heterogeneity of intratumoral macrophages in prostate tumors
Prostate cancer (PCa) is the most common cancer among men worldwide, with approximately 1,600,000 cases and 366,000 deaths annually [1]. The incidence and mortality of PCa tends to increase due to an aging population and urbanization, thereby placing a significant social and financial burden on the global health care system [2]. Despite significant progress in therapy, prostate cancer remains a serious problem for men, mainly due to unwarranted treatment of initially benign disease and inadequate therapy for metastatic prostate cancer [3]. The lethality of progressive disease is due to the lack of therapeutic regimens capable of producing a stable response to therapy, which is due to the extreme heterogeneity of the tumor on the genetic and cellular-biological levels. Evidence suggests that the inflammatory microenvironment, especially tumor-associated macrophages (TAMs), are involved in prostate carcinogenesis and act as an important modulator of further malignant progression, metastasis and overall therapeutic response [4]. In turn, both the presence of the tumor itself and anticancer therapy can affect the programming of monocyte TAMs precursors [5, 6]. Studies on cohorts of patients of different ethnic groups indicate that the total number of macrophages in the tumor mass is associated with poor prognosis and increased risk of metastasis [7]. However, the heterogeneity of intratumoral macrophages in prostate cancer at different stages of disease development has not been sufficiently studied.
The aim of the investigation is to analyze the spectrum of macrophage characteristics (morphological features, size and amount) in prostate cancer tissue samples and to search their interrelation with clinical data of patients.
Material and Methods
A retrospective analysis of tumor tissues of patients with prostate cancer, who underwent surgical treatment in the Clinic of Bashkir State Medical University in the time period from 2014 to 2018, was performed. The study included 36 patients with pT2a–3bN0–1M0. Tissue samples in paraffin blocks were obtained from the Biobank of Bashkir State Medical University. The study was conducted in accordance with the requirements of the Declaration of Helsinki and was approved by the local ethical committee of the Federal State Medical University BSMU of the Ministry of Healthcare of Russia on 17.01.2017, protocol № 1. General characteristics of clinical data of patients are presented in Table 1.
One paraffin block containing at least 50 % of tumor tissue was pre-selected for each patient. The selection was performed by a pathomorphologist, for which hematoxylin and eosin stained sections from each block were prepared.
Subsequent immunohistochemical staining of sections of paraffin blocks was performed at the Institute of Transfusion Medicine and Immunology, Faculty of Medicine, Mannheim, Heidelberg University (Mannheim, Germany). Slices deparaffinized with xylene and ethanol and rehydrated were placed in Tris/EDTA buffer (pH 9.0) and heated in a water bath to 95°C for antigen recovery. After blocking endogenous peroxidase and nonspecific staining, samples were incubated for one hour with primary antibodies to CD68 (Zytomed systems # MSK005-55, dilution 1:100). The preparations were then incubated with secondary mouse HRP antibodies (Dako # K4001), AEC+substrate chromogen (Dako # K3461) and stained with hematoxylin solution (Hämalaun) and incubated under a coverslip in aqueous medium (Fara-mount Mounting Medium, Dako # S302580-2). Tumor tissue sections incubated with primary antibodies to IgG1 served as a control for specific staining.
Stained sections were visualized using a Leica DMRE light microscope, ×10 or ×40 objective and ×10 eyepiece. Staining of granular cytoplasmic structures or cell membranes of monocytic/macrophage origin in any intensity was considered positive for CD68.
Areas containing tumor tissue were examined on each preparation. From each patient, 10 random fields of view within the tumor tissue were analyzed at ×400 magnification. The following parameters were analyzed: presence of macrophages, stained with CD68 (yes/no); shape (rounded, irregularly shaped with outgrowths), size (large/small). The obtained images were analyzed using Image J program and Color Deconvolution plugin. The number of CD68positive cells, the area of each CD68+ cell, and the area of the image occupied by cells for each field of view were taken into account, since prostate tissue contains lumens. The following characteristics were then calculated in MS Excel for each patient:
-
1. Total number of CD68+ cells per 10 fields of view.
-
2. Average number of CD68+ cells per preparation.
-
3. Total area occupied by CD68+ cells per 10 fields of view.
-
4. Average area of CD68+ cells.
-
5. Proportion of the area covered by CD68+ cells from the area of all cells, %.
-
6. Number of visual fields with CD68+ cells.
-
7. Number of visual fields with CD68+ cells larger than 100 µm2.
-
8. Number of visual fields with CD68+ cells less than 100 µm2 in size.
-
9. Number of macrophages per total number of visual fields with macrophages.
-
10. Total number of macrophages greater than 100 μm2 in size.
-
11. Total number of macrophages less than 100 μm2 in size.
-
12. Area of macrophages larger than 100 μm2.
-
13. Area of macrophages less than 100 μm2 in size.
-
14. Total area of macrophages less than 100 µm2/ total area of macrophages greater than 100 µm2.
Comparisons for all indices were made for the following groups of patients: 1) without lymph node metastases at the time of surgery, N0, or with metastases, N1; 2) with a total Gleason score of 6 (3 + 3), 7 (3 + 4 or 4 + 3), 8–10 (4 + 4, 4 + 5, 5 + 4 or 5 + 5); 3) with a biochemical recurrence risk score of “low”, “intermediate”, “high”. Data distribution analysis using Kolmogorov-Smirnov test for all groups showed deviation from normal distribution in some groups. The median and interpercentile interval for each indicator were calculated using GraphPad Prism (V 6.01). To assess the statistical significance of the observed differences, we further used nonparametric Mann-Whitney tests to compare the 2 groups and Kruskal-Wallis tests for three groups.
Results
Our first step was to search for clusters of tumor-associated macrophages and characterized their morphology in the tumor tissue of patients with prostate cancer. The analyzed marker CD68 is highly specific for macrophages, belongs to scavenger receptor family, and is expressed on almost all TAMs [8]. The distri-
The morphology and pattern of macrophage distribution was heterogeneous in each patient: – there were both areas with large “foamy” round-shaped macrophages in ducts outside tumor tissue (Fig. 2A1), and massive clusters of irregularly shaped macrophages located in clusters inside tumor tissue (Figure 2A2). Figure 2B1 shows a rounded large “foamy” macrophage with a coarse-grained cytoplasm structure, a similar morphology found when the macrophage is localized inside the ductus. When examining a tissue sample of the same patient, there is a distribution of mediumsized macrophages in the parenchyma (Fig. 2B2). It is worth noting the presence of spindle-shaped
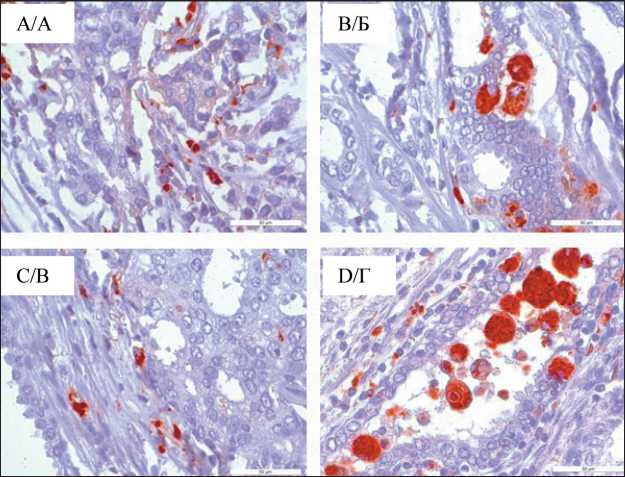
Fig. 1. Microphoto. IHC study, distribution of CD68+ macrophages in the tumor tissue, (A) intratumoral macrophages diffusely located in the tissue, (B) macrophages with predominant localization in the ducts of the tumor gland, (B) macrophages located in the stroma around the tumor parenchyma; (D) large, “foamy” macrophages in non-neoplastic lacunae; ×40
Рис. 1. Микрофото. ИГХ-исследование, распределение CD68+ макрофагов в опухолевой ткани, (А) внутриопухолевые макрофаги, диффузно расположенные в ткани, (Б) – макрофаги с преимущественной локализацией в протоках железы опухоли, (В) макрофаги, располагающиеся в строме вокруг паренхимы опухоли; (Г) крупные, «пенистые» макрофаги в неопухолевых лакунах; ×40
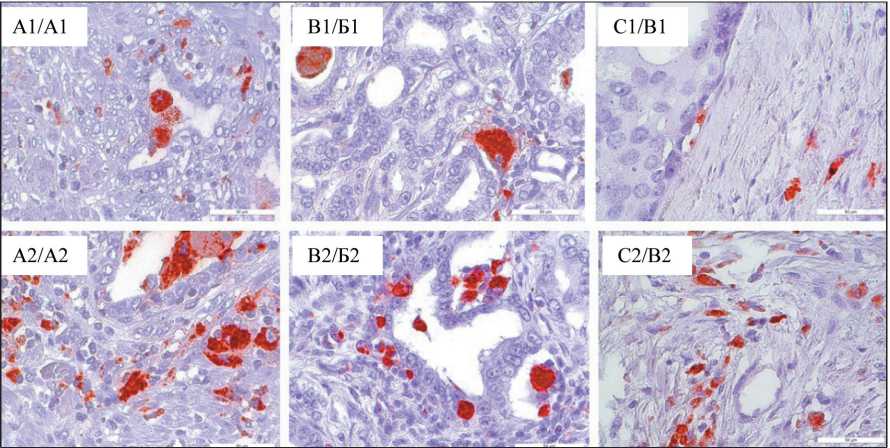
Fig. 2. Microphoto. Distribution of CD68+ macrophages in prostate adenocarcinoma tissue according to IHC staining, ×40 Рис. 2. Микрофото. Распределение CD68+ макрофагов в ткани аденокарциномы предстательной железы по данным ИГХ-окрашивания; ×40
macrophage bands in the examined samples, diffusely located in tumor tissue, locally abundantly infiltrating tumor area (Fig. 2B2).
The largest “foamy” macrophages were observed in tumor parenchyma of patient № 9 (Fig. 3A), here we detected macrophages measuring 600–710 µm2, having round shape with a pronounced granular structure, which formed clusters of more than 15 cells. Patient 17 also had macrophages larger than 600 μm2 in the tumor parenchyma (Fig. 3B), but not forming clusters.
In the next step we assessed the number and size of each CD68+ cell on 10 field-of-view images with tumor tissue from each patient, followed by calculation of the spectrum characteristics. The results are presented in Table 2.
Although there were no statistically significant differences between the clinical groups of patients, a number of trends are noteworthy. For example, the total number of CD68+ cells increase as the tumor process progresses. In the group with lymph node metastases the total number of macrophages was significantly higher, 68 against 42 in the group without lymph node metastases. The same index increases in a number of patients with a Gleason score of 6.7 and more than 8: 38, 64.5 and 74, respectively. The risk of biochemical recurrence correlates with the sum of Gleason scores and the number of macrophages increases in the tissues of patients at intermediate (64.5) and high risk (74) compared to the low risk group (38).
The total area occupied by CD68+ cells was also greater in N1 patients (6009 μm2) than in N0 patients (2698 μm2). In patients with low-differentiated prostate cancer the area (Gleason score ≥ 8) and high biochemical risk of recurrence was also the highest (5792 μm2 in both groups), while in the group with a Gleason score of 6 and low risk of recurrence it was 2219 μm2 in both groups.
Table 2/Таблица 2
ra 75
ra о Ъ о £ w Ф D) га
Q. О
О га Е о Е □ ф га 'К 2 Q. *О
to ф о га га
о га о
О) о о
Q. О Е *о с О га ф о О
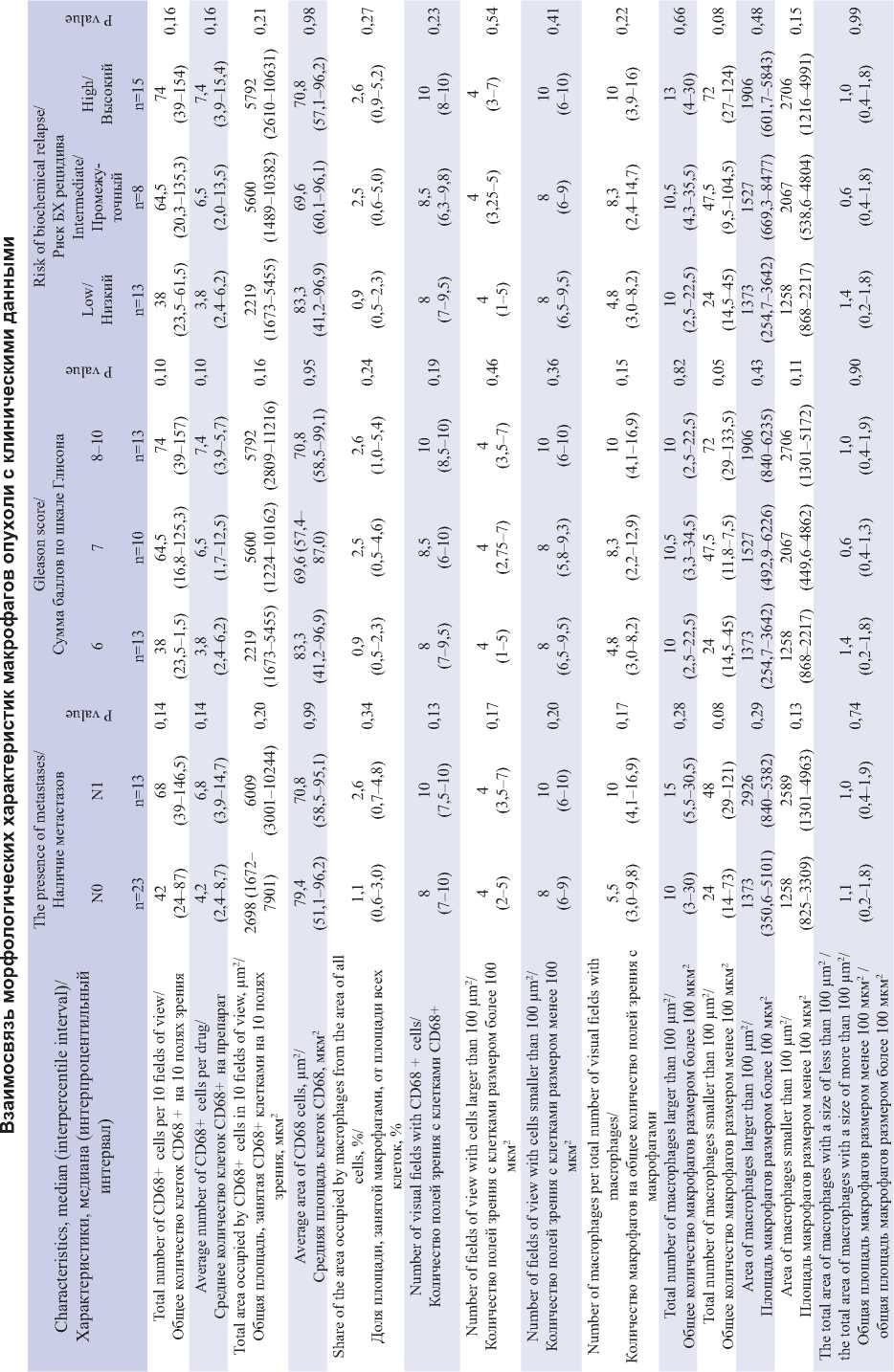
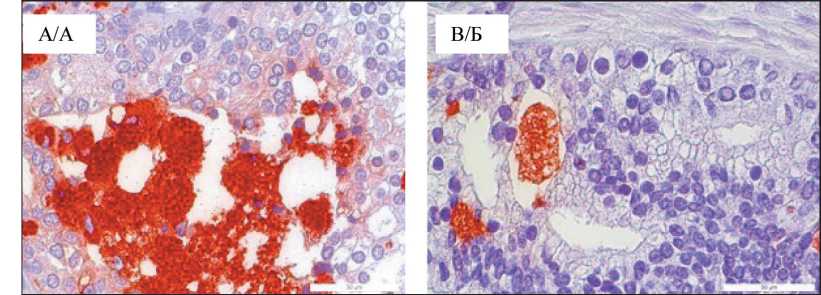
Fig. 3. Microphoto. “Foamy” CD68+macrophages of patients No. 9 (A), No. 17 (B), Gleason score 4 + 4, ×40
Рис. 3. Микрофото. «Пенистые» CD68+макрофаги пациентов № 9 (А), № 17 (Б), оценка по шкале Глисона 4 + 4; ×40
The average area of CD68+ cells, the proportion of the area of all cells occupied by CD68+ macrophages, the number of visual fields with CD68+ cells, the number of visual fields with CD68+ cells over 100 μm2, the number of visual fields with CD68+ cells under 100 μm2, and the ratio of the total area of less than 100 μm2 to the total area of macrophages over 100 μm2 showed no significant differences between the compared groups.
At the same time, the parameter “number of macrophages per total number of visual fields with macrophages” demonstrated an increase in values with the progression of the tumor process. It was lowest in patients with a Gleason score of 6 (4.8) and highest (10.0) in patients with a Gleason index ≥ 8. Thus, macrophages formed larger clusters in patients with advanced BPH.
It is important to note that the total number of macrophages larger than 100 μm2 did not practically differ between the groups. Whereas small macrophages were significantly more frequent in patients with lymph node metastases (48 vs 24 in N0 group; p=0.076). In addition, the number of small, apparently secreted macrophages increased in the series of patients with Gleason scores of 6, 7, and ≥ 8 (24, 47.5, 72, respectively, p=0.052), and as the risk of biochemical recurrence increased (24, 47.5, 72, respectively, p=0.079). Noteworthy is the increase in the number of macrophages, predominantly at the expense of small secreting cells less than 100 μm2 in size.
Both the areas of macrophages larger than 100 μm2 and the areas of macrophages smaller than 100 μm2 increased slightly as the prostate cancer progressed (Table 2). This observation requires further investigation.
Discussion
Our findings revealed characteristic single large “foamy” macrophages and clusters of similar macrophages in prostate cancer tissues, and correlations with clinical findings in patients were determined. The morphology of “frothy” macrophages is the most interesting, they are relatively large, their cytoplasm is loose and granular. Probably, such morphological features are a consequence of excessive internalization of lipids or lipoproteins. According to the published data, this is inherent to atherosclerotic macrophages and is also found in colorectal cancer [9–11]. Increased uptake and oxidation of fatty acids in tumor tissue may contribute to the secretion of proinflammatory cytokines from these cells, such as CXCL10, IL-1β and IL-10, which may subsequently increase the recruitment of effector T cells and natural killer (NK) cells or induce tumor cell migration [12–14].
Majority literature sources categorize the main polarization vectors of macrophages into two phenotypes: classically activated, proinflammatory macrophages M1 and alternatively activated, antiinflammatory, pro-tumor macrophages M2. However, it is worth noting that there are many intermediate variants [15], and the existing classifications are relevant in characterizing their role in cancer. Currently, biomarker systems are being developed to identify functional subpopulations of tumor-associated macrophages, which include both scavenger receptors and chitinase-like proteins [7, 16, 17]. Tumor-associated macrophages (TAMs) resembling M2 phenotype are involved in stimulation of angiogenesis, invasion and extravasation of tumor cells [18]. The metabolism of tumor-associated macrophages is a mixed form of M1 (glycolysis) and M2 (fatty acid oxidation) metabolism [19] It was shown that the number of M2-like TAMs positively correlates with increased tumor size, rapid proliferation and decreased overall survival in many cancer types, including prostate cancer [7]. Due to the fact that cancer cells have the ability to activate adipocytes, which leads to triglyceride lipolysis, and release free fatty acids in the intratumor environment [20], TAMs are induced to absorb fatty acids through phagocytosis, which in turn leads to lipid-laden TAMs [11]. Similar giant macrophages have been found, for example, in tumor tissue of ovarian cancer [21] Recent studies have begun to evaluate metabolically activated resident tissue macrophages, which cope with a high lipid load of dying hypertrophic adipocytes in adipose tissue in obese patients, which phenotypically differ from M1/M2 classifications [22]. It has been found that these cells are associated with the progression of triple-negative breast cancer [23]. Besides, recent studies describe macrophage subtypes in the prostate gland possessing a combination of M1 and M2 macrophage characteristics in various combinations [24, 25]. It is important to note that macrophage subtypes in tumor and non-tumor tissue did not differ, which reflects the possibility of tumor tissue factors influencing the distant non-tumor environment [25].
CD11b+CD68+ macrophages isolated from colorectal cancer tissue contained higher amount of lipids in comparison with macrophages in normal tissue [11] Lipid accumulation in tumor-associated macrophages of myeloma patients obtained from bone marrow is significantly associated with disease progression [24]. Our study has revealed that the size of large phagocytic cells continues to increase as the tumor progresses that may reflect the process of undigested lipid accumulation. It is known that during tumor progression macrophages can change their phenotype, accompanied by the recruitment of new monocytes from the bloodstream. Increased number of small macrophages relative to large, actively phagocytosing cells, combined with increased number of small macrophages may be one of the reasons of immune control failure and tumor progression.
Besides, according to literature data, in a number of studies it was shown the correlation of increased number of macrophages and their density in tumor tissue with more aggressive behavior of tumor and more unfavorable outcomes in prostate cancer [27–29]. Other data found an inverse correlation between the number of CD68+ OAM infiltrates in total tumor tissue and clinical TNM stage, while OAM density in the cancer
Список литературы Morphological heterogeneity of intratumoral macrophages in prostate tumors
- Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65(2): 87-108. https://doi.org/10.3322/caac.21262.
- Global Burden of Disease Cancer Collaboration, Fitzmaurice C., Abate D., Abbasi N., Abbastabar H., Abd-Allah F., Abdel-Rahman O., Abdelalim A., Abdoli A., Abdollahpour I., Abdulle A.S.M., Abebe N.D., Abraha H.N., Abu-Raddad L.J., at al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019; 5(12): 1749-68. https://doi.org/10.1001/jamaoncol.2019.2996. Erratum in: JAMA Oncol. 2020; 6(3): 444. Erratum in: JAMA Oncol. 2020; 6(5): 789. Erratum in: JAMA Oncol. 2021; 7(3): 466.
- Steele C.B., Li J., Huang B., Weir H.K. Prostate cancer survival in the United States by race and stage (2001-2009): Findings from the CONCORD-2 study. Cancer. 2017; 123 (Suppl 24): 5160-77. https://doi.org/10.1002/cncr.31026.
- Sfanos K.S., Yegnasubramanian S., Nelson W.G., De Marzo A.M. The inflammatory microenvironment and microbiome in prostate cancer development. Nat Rev Urol. 2018; 15(1): 11-24. https://doi.org/10.1038/nrurol.2017.167.
- Patysheva M., Larionova I., Stakheyeva M., Grigoryeva E., Iamshchikov P., Tarabanovskaya N., Weiss C., Kardashova J., Frolova A., Rakina M., Prostakishina E., Zhuikova L., Cherdyntseva N., Kzhyshkowska J. Efect of Early-Stage Human Breast Carcinoma on Monocyte Programming. Front Oncol. 2022; 11. https://doi.org/10.3389/fonc.2021.800235.
- Patysheva M., Frolova A., Larionova I., Afanas’ev S., Tarasova A., Cherdyntseva N., Kzhyshkowska J. Monocyte programming by cancer therapy. Front Immunol. 2022; 13. https://doi.org/10.3389/fmmu.2022.994319.
- Larionova I., Tuguzbaeva G., Ponomaryova A., Stakheyeva M., Cherdyntseva N., Pavlov V., Choinzonov E., Kzhyshkowska J. Tumor-Associated Macrophages in Human Breast, Colorectal, Lung, Ovarian and Prostate Cancers. Front Oncol. 2020; 10. https://doi.org/10.3389/fonc.2020.566511.
- Kzhyshkowska J., Neyen C., Gordon S. Role of macrophage scavenger receptors in atherosclerosis. Immunobiology. 2012; 217(5): 492-502. https://doi.org/10.1016/j.imbio.2012.02.015.
- Krawczyk K.M., Nilsson H., Allaoui R., Lindgren D., Arvidsson M., Leandersson K., Johansson M.E. Papillary renal cell carcinoma-derived chemerin, IL-8, and CXCL16 promote monocyte recruitment and diferentiation into foam-cell macrophages. Lab Invest. 2017; 97(11): 1296-305. https://doi.org/10.1038/labinvest.2017.78.
- Corn K.C., Windham M.A., Rafat M. Lipids in the tumor microenvironment: From cancer progression to treatment. Prog Lipid Res. 2020; 80. https://doi.org/10.1016/j.plipres.2020.101055.
- Wu H., Han Y., Rodriguez Sillke Y., Deng H., Siddiqui S., Treese C., Schmidt F., Friedrich M., Keye J., Wan J., Qin Y., Kühl A.A., Qin Z., Siegmund B., Glauben R. Lipid droplet-dependent fatty acid metabolism controls the immune suppressive phenotype of tumor-associated macrophages. EMBO Mol Med. 2019; 11(11). https://doi.org/10.15252/emmm.201910698.
- Zhang Y., Sun Y., Rao E., Yan F., Li Q., Zhang Y., Silverstein K.A., Liu S., Sauter E., Cleary M.P., Li B. Fatty acid-binding protein E-FABP restricts tumor growth by promoting IFN-β responses in tumor-associated macrophages. Cancer Res. 2014; 74(11): 2986-98. https://doi.org/10.1158/0008-5472.CAN-13-2689.
- Zhang Q., Wang H., Mao C., Sun M., Dominah G., Chen L., Zhuang Z. Fatty acid oxidation contributes to IL-1β secretion in M2 macrophages and promotes macrophage-mediated tumor cell migration. Mol Immunol. 2018; 94: 27-35. https://doi.org/10.1016/j.molimm.2017.12.011.
- Chiba S., Hisamatsu T., Suzuki H., Mori K., Kitazume M.T., Shimamura K., Mizuno S., Nakamoto N., Matsuoka K., Naganuma M., Kanai T. Glycolysis regulates LPS-induced cytokine production in M2 polarized human macrophages. Immunol Lett. 2017; 183: 17-23. https://doi.org/10.1016/j.imlet.2017.01.012.
- Martinez F.O., Sica A., Mantovani A., Locati M. Macrophage activation and polarization. Front Biosci. 2008; 13: 453-61. https://doi.org/10.2741/2692.
- Kzhyshkowska J., Yin S., Liu T., Riabov V., Mitrofanova I. Role of chitinase-like proteins in cancer. Biol Chem. 2016; 397(3): 231-47. https://doi.org/10.1515/hsz-2015-0269.
- Larionova I.V., Sevastyanova T.N., Rakina A.A., Cherdyntseva N.V., Kzhyshkowska J.G. Chitinase-like proteins as promising markers in cancer patients. Siberian Journal of Oncology. 2018; 17(4): 99-105. https://doi.org/10.21294/1814-4861-2018-17-4-99-105.
- Larionova I., Kazakova E., Gerashchenko T., Kzhyshkowska J. New Angiogenic Regulators Produced by TAMs: Perspective for Targeting Tumor Angiogenesis. Cancers (Basel). 2021; 13(13): 3253. https://doi.org/10.3390/cancers13133253.
- Larionova I., Kazakova E., Patysheva M., Kzhyshkowska J. Transcriptional, Epigenetic and Metabolic Programming of Tumor-Associated Macrophages. Cancers (Basel). 2020; 12(6): 1411. https://doi.org/10.3390/ cancers12061411.
- Dirat B., Bochet L., Dabek M., Daviaud D., Dauvillier S., Majed B., Wang Y.Y., Meulle A., Salles B., Le Gonidec S., Garrido I., Escourrou G., Valet P., Muller C. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011; 71(7): 2455-65. https://doi.org/10.1158/0008-5472.CAN-10-3323.
- Rakina M.A., Kazakova E.O., Sudaskikh T.S., Bezgodova N.V., Villert A.B., Kolomiets L.A., Larionova I.V. Giant foam-like macrophages in advanced ovarian cancer. Siberian Journal of Oncology. 2022; 21(2): 45-54. https://doi.org/10.21294/1814-4861-2022-21-2-45-54.
- Kratz M., Coats B.R., Hisert K.B., Hagman D., Mutskov V., Peris E., Schoenfelt K.Q., Kuzma J.N., Larson I., Billing P.S., Landerholm R.W., Crouthamel M., Gozal D., Hwang S., Singh P.K., Becker L. Metabolic dysfunction drives a mechanistically distinct proinfammatory phenotype in adipose tissue macrophages. Cell Metab. 2014; 20(4): 614-25. https://doi.org/10.1016/j.cmet.2014.08.010.
- Tiwari P., Blank A., Cui C., Schoenfelt K.Q., Zhou G., Xu Y., Khramtsova G., Olopade F., Shah A.M., Khan S.A., Rosner M.R., Becker L. Metabolically activated adipose tissue macrophages link obesity to triplenegative breast cancer. J Exp Med. 2019; 216(6): 1345-58. https://doi.org/10.1084/jem.20181616.
- Boibessot C., Molina O., Lachance G., Tav C., Champagne A., Neveu B., Pelletier J.F., Pouliot F., Fradet V., Bilodeau S., Fradet Y., Bergeron A., Toren P. Subversion of infltrating prostate macrophages to a mixed immunosuppressive tumor-associated macrophage phenotype. Clin Transl Med. 2022; 12(1). https://doi.org/10.1002/ctm2.581.
- Siefert J.C., Cioni B., Muraro M.J., Alshalalfa M., Vivié J., van der Poel H.G., Schoots I.G., Bekers E., Feng F.Y., Wessels L.F.A., Zwart W., Bergman A.M. The Prognostic Potential of Human Prostate Cancer-Associated Macrophage Subtypes as Revealed by Single-Cell Transcriptomics. Mol Cancer Res. 2021; 19(10): 1778-91. https://doi.org/10.1158/1541-7786.MCR-20-0740.
- Su P., Wang Q., Bi E., Ma X., Liu L., Yang M., Qian J., Yi Q. Enhanced Lipid Accumulation and Metabolism Are Required for the Diferentiation and Activation of Tumor-Associated Macrophages. Cancer Res. 2020; 80(7): 1438-50. https://doi.org/10.1158/0008-5472.CAN-19-2994. Erratum in: Cancer Res. 2022; 82(5): 945.
- Lissbrant I.F., Stattin P., Wikstrom P., Damber J.E., Egevad L., Bergh A. Tumor associated macrophages in human prostate cancer: relation to clinicopathological variables and survival. Int J Oncol. 2000; 17(3): 445-51. https://doi.org/10.3892/ijo.17.3.445.
- Erlandsson A., Carlsson J., Lundholm M., Fält A., Andersson S.O., Andrén O., Davidsson S. M2 macrophages and regulatory T cells in lethal prostate cancer. Prostate. 2019; 79(4): 363-9. https://doi.org/10.1002/pros.23742.
- Yuri P., Shigemura K., Kitagawa K., Hadibrata E., Risan M., Zulfqqar A., Soeroharjo I., Hendri A.Z., Danarto R., Ishii A., Yamasaki S., Yan Y., Heriyanto D.S., Fujisawa M. Increased tumor-associated macrophages in the prostate cancer microenvironment predicted patients’ survival and responses to androgen deprivation therapies in Indonesian patients cohort. Prostate Int. 2020; 8(2): 62-9. https://doi.org/10.1016/j.prnil.2019.12.001.
- Shimura S., Yang G., Ebara S., Wheeler T.M., Frolov A., Thompson T.C. Reduced infltration of tumor-associated macrophages in human prostate cancer: association with cancer progression. Cancer Res. 2000; 60(20): 5857-61.


