Myoinositol and its metabolites in abiotic stress tolerance in plants
Автор: Mukhia Raksha, Raj Chhetri Dhani
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 4 т.18, 2022 года.
Бесплатный доступ
Myo -inositol (MI) is a sugar-alcohol produced by most plants and animals. 1L-myo-Inositol- 1-phosphate synthase (MIPS) is the rate limiting enzyme that catalyzes the conversion of D-glucose 6- phosphate to 1L-myo-inositol-1-phosphate, the first step in the production of all inositol- containing compounds. The enzyme exists in a cytoplasmic form in a wide range of plants, animals, and fungi. In plants, a chloroplastic form of the enzyme is also widely known. The significance of MI and its direct and more downstream derivatives lies in their dual functions as signalling molecules as well as key metabolites under stress. The role of MI and its derivatives in aiding the plants to cope with various abiotic stress conditions through physiological and biochemical changes have been discussed in this paper.
Abiotic stress, myo-inositol, myo-inositol-1-phosphate synthase, osmolytes, stress tolerance
Короткий адрес: https://sciup.org/143179364
IDR: 143179364
Текст обзорной статьи Myoinositol and its metabolites in abiotic stress tolerance in plants
Plants tend to develop tolerance mechanism under adverse environmental conditions (Ashraf and Harris, 2004). Over-production of different kinds of soluble substances is one of the most common stress responses in plants (Serraj and Sinclair, 2002; Ashraf et al., 2012). Usually, plants tide over the stress condition through different mechanisms such as cellular osmotic regulation, detoxification of reactive oxygen species, protection of membrane integrity, and stabilization of proteins (Bohnert and Jensen, 1996; Mechri et al., 2015; Per et al., 2017).
Many studies demonstrate the importance of metabolite accumulation attributed to osmotic adjustment leading to water retention and protection of biochemical pathways. Such accumulations are called osmoprotectans which protect cellular components from osmotic damages. Thus, when single enzyme based characters were scrutinized in transgenic plants, the accumulation of mannitol, proline, fructans, trehalose, glycine betaine, or ononitol (Tarczynski et al., 1993; Kishor et al., 1995; Holmström et al., 1996; Hayashi et al., 1997; Sheveleva et al., 1997) were found to provide higher salinity and cold or drought tolerance. In plants, cold, drought, and salt stresses, all stimulate the accumulation of compatible osmolytes and antioxidants (Hasegawa et al., 2000). Cyclitols, such as quercitol, pinitol or quebrachitol, are well stable and common among the candidate classes of compatible solutes (Merchant et al., 2006).
Inositol is a cyclohexanehexol, a cyclic carbohydrate with six hydroxyl groups one on each of the ring carbons. Myo -inositol (MI) is the oldest known inositol and was first isolated from muscle extracts by Scherer in 1850 (Posternak, 1965). Among the nine possible geometrical isomers of inositol. The myo -isomer is the most abundant form in nature (Fig. 1). It occupies a unique place in inositol metabolism because this is the only isomer synthesized de novo from D-glucose-6-phosphate; all other isomers are derived from myoinositol (Loewus, 1990; Loewus and Murthy, 2000). MI can be divided into two mirror image halves (Fig. 2) – a perpendicular plane passes through C-2 and C-5
and splits the molecule into non-superimposable mirror images. Therefore, any modification that will disturb the symmetry of the molecule will render the molecule chiral, thus MI is prochiral.
MI and its stereo-forms, play a plethora of functional role in plants, in signal transduction, cell wall formation, regulation of tissue growth, osmotic adjustment, membrane transport (Stevenson-Paulik et al., 2005; Zhai et al., 2016) etc. The loss of viability called inositolless death was found in case of MI requiring mutants of Saccharomyces cerevisiae (Culbertson and Henry, 1975) and Neurospora crassa (Strauss, 1958). Increased activity of 1L-myo-Inositol-1-phosphate synthase (MIPS) and higher rate of inositol production in plants have been directed to stress resistance. They have been known to act as osmoprotectants. In this review we discuss the role of myo-inositol and its metabolites that plays critical role in abiotic stress tolerance in plants on having been produced as a response to such stressful conditions.
Inositols and ROS accumulation
One of the most commonly known effect of stress in plants is the increase of reactive oxygen species (ROS) level. ROS damage the photosynthetic pigments, cellular lipids, proteins and nucleic acids by oxidation and also regulates apoptosis (Gadjev et al. 2008). A systematic up-regulation of ROS-scavenging genes was observed in sweet potato plants overexpressing IbMIPS1- under salt and drought stresses (Zhai et al., 2016). Methylated derivatives of inositol have been found to be effective in checking ROS level and thereby protecting photosynthetic machinery (Patra et al., 2010). Various reports show that inositol caused decline in the negative effects of osmotic stress thereby increasing the tolerance to drought stress of plants (Cevik et al. 2014; Sambe et al., 2015). SOD (superoxide dismutase) is in the first line of defence against the toxic effects of elevated levels of ROS (Gill and Tuteja, 2010). Total SOD activities in pepper plants were affected by myoinositol treatment and drought stress. Cu,Zn-SOD II isoform is enhanced by myo-inositol treatment in droughted plants (Aytunç, et. al., 2018). Mechri et al. (2015) reported that sugar alcohols may function as scavengers of activated oxygen species, by preventing peroxidation of lipids and resulting cell damage. Perhaps, MI is effective as H2O2 scavenger in response to stress conditions. Accumulation of ROS also impacts the photosynthetic machinery and the total chlorophyll content of a plant (Sarkar et al., 2014). Chlorophyll reduction under abiotic stress denotes osmotic stress, which is an outcome of pigment photo-oxidation and chlorophyll degradation (Rai et al., 2013, Farooq et. al, 2009). Transgenic 7PcI O1-plants (Porteresia coarctata) expressing inositol, retained the chlorophyll content thus maintaining normal photosynthetic potential even under high salt concentration, while untransformed line showed maximum chlorophyll depletion (Mukherjee et al., 2019).
Malondialdehyde (MDA) is a widely used marker of lipid injury caused by environmental stress. MDA content was observed to be higher in drought stressed plants than well-watered plants. Fan et al. (2017) reported an increase in MDA content in cucumber during drought stress. Low MI treatment significantly increased the level of MDA in both droughted and well watered plants. In transgenic sweet potato plants, overexpression of IbMIPS1 significantly decreased their MDA content compared to watered plants, indicating a marked improvement of their salt tolerance (Wang et al., 2016) under stress, as has been postulated for other polyols (Loewus and Loewus, 1983; Bohnert et al., 1995). Inositols have also been reported to mimic the structure of water and maintain an artificial sphere of hydration around macromolecules (Mechri et al., 2015). MI is known to accumulate in response to stresses in many plants through an induced expression of MIPS ( elson et al., 1998, Majee et al. 2004, Tan et al., 2013). Therefore, an attempt to understand and determine the role and mechanisms of MI in providing abiotic stress tolerance in higher plants is significant.
Osmolyte accumulation as stress response
Several studies indicated that osmo-protective compounds play a role in mutants or transgenic plants with different capabilities, accumulating these metabolites for stress tolerance (Szabados et al., 2011). MI may function as a compatible solute for protection against abiotic stresses and can be converted to other compatible solutes as well (Taji et al., 2006). Omolytes help in osmotic adjustment of the cells and also protects the cells and macromolecules by maintaining membrane integrity, preventing protein degradation and protecting against oxidative damage by scavenging free radicals and lowering Tm value of nucleic acids (Crowe et al., 1987; omura et. al., 1995). Osmolytes not only help in osmotic adjustment in the cellular milieu but also act as scavengers of ROS. D-ononitol and myo-inositol are the potential protectants of enzymes and membranes from damage by ROS (Sheveleva et. al., 1997). Isomerization and methylation of MI leads to the formation of O-methyl inositol, which also directly participates in stress-related responses of plants (Loewus and Murthy, 2000). The plant species which are constantly exposed to saline conditions, accumulate cyclic sugar alcohols, pinitol and ononitol (Paul and Cockburn, 1989). Thus, Mesenbryanthemum crystallinum has been seen to accumulate these compounds when subjected to such stress (Bartels and elson, 1994). The upregulation of inositol biosynthesis by subjecting plants to salinity stress may be exploited for the enhanced production of myo-inositol from glucose-6phosphate (RayChaudhuri and Majumder, 1996).
Physiological, biochemical, metabolic and molecular changes occur in plants under various environmental stresses. Increased activity of the rate limiting enzyme, myo-Inositol synthase (MIPS) in addition to higher rate of inositol production is one such mechanism in plants to resist stress. The genes encoding MIPS have been isolated from several plant species, such as Arabidopsis (Johnson, 1994), rice (Yoshida et al., 1999), maize (Larson and Raboy, 1999), soya bean (Hegeman et al., 2001), smooth cordgrass (Joshi et al., 2013) and Medicago falcata (Tan et al., 2013). The upregulation of these genes confer stress tolerant character to plants. In higher plants, the inositol that accumulates in response to salt stress may function as an osmolyte, in addition to its critical roles concerning the cellular machinery (Bohnert et. al., 1995). The extent to which osmolyte levels rise is dependent on changes in the external osmotic potential (Hasegawa et at., 2000; Chen and Murata, 2002). Compounds such as gums, cell wall- located carbohydrates, glycoproteins and mucilages, which are involved in protective functions during stress, are also produced from inositol and inositol-1-phosphate (Shen et. al., 1997, Crowe et. al., 1992).
Myo-inositol in salinity stress tolerance
Polyols and sugar alcohols such as pinitol, mannitol, ononitol and inositol is known to play vital role as osmoprotectants against salt stress in various plants (Ghosh et. al., 2006). Tomato ( Lycopersicon esculentum Mill. cv. ew Yorker) plant subjected to aCl stress showed an increased MI content which remained elevated throughout (Sacher and Staples, 1985). Plants alternated daily between salt and control solutions accumulated less MI and exhibited less growth than the continuously salt stressed plants. The facultative halophyte, Mesenbryanthemum crystallinum has been seen to accumulate pinitol and ononitol when the plant is stressed (Bartels and elson, 1994). The content of free MI in Ulva lactuca was found to increase proportionately with the increase of surface salinity of the Chilika Lagoon, Odisha, India (Basu, 2019). It was proposed that myo -inositol could serve not only as a substitute for the production of compatible solutes, but also as a leaf-to-root signal that promotes sodium uptake ( elson et al ., 1999). When subjected to salinity stress by aCl treatment, the salt tolerant varieties of rice ( Oryza sativa ), exhibited enhanced activity in the chloroplast form of MIPS. The enhanced MIPS activity in the highly salt tolerant varieties of rice due to salinity stress was found to be comparable to that of Porteresia coarctata , a halophytic wild rice species. This ultimately suggests a role of inositol pathway in osmoregulation (RayChaudhury and Majumder, 1996).
MIPS gene isolated from the wild halophytic rice Porteresia coarctata, and introgressed into IR64 indica rice showed its role in conferring salt-tolerance. The PcI O1 transformed transgenic rice lines exhibited significantly higher salt tolerance with negligible compromises in other physiological parameters compared to the untransformed system grown without stress. Further introgression of PcI O1 in Brassica juncea also imparted a substantial tolerance to salt and oxidative stress and such plants could be a potential salt tolerant genetically modified crop.
Also, an attempt was made to generate salt-tolerant indica rice plants through transgenic functional cointrogression of both PcI O1 and PcIMT1 (Inositol-o-methyl transferase) which showed comparatively higher accumulation of inositol in stress conditions (Mukherjee et al., 2019). Overexpression of MIPS in B. juncea, Arabidopsis, tobacco and rice enhanced their tolerance to salt, dehydration and chilling respectively due to the increased production of inositol (Kaur et al., 2013; Majee et al., 2004; Tan et al., 2013).
MI has been implicated in salt tolerance of many plants as a facilitator of uptake and long-distance transport of sodium ( elson et al., 1999). Increased tolerance to salinity (150 mM aCl) were seen in transgenic Arabidopsis plants expressing SaI O1 ( Spartina alterniflora myo-inositol 1-phosphate synthase), at both germination level and during plant growth and development. ormal growth was observed after a week exposure to salt stress in transgenic plants whereas the wild type (WT) plants struggled to survive and eventually perished. Results indicated that under salinity stress, Arabidopsis transgenic plants were less sensitive to photoinhibition as compared to WT plants. Increased MI due to expression of SaI O1, served as the substrate for accumulating metabolic end products and that could facilitate sodium sequestration and protect photosynthesis ( elson et al., 1999, Bohnert et al., 1995). There was also indication of a substantial protection of photosystems especially PSII in SaI O1- Arabidopsis plants and the possible role of MI in protecting the chloroplast.
Does myo-inositol help in drought tolerance?
In plants, the limitations in available water imposes both hyperionic and hyperosmotic stress that can upset homeostasis both at cellular level and whole-plant level. Altered osmotic potential results in molecular damage that ultimately hinders growth and can even lead to death (Hasegawa et al., 2000). Water deficit or osmotic effects are probably the major physiological mechanisms for growth reduction as both stresses lower the soil water potential (Hu and Schmidhalter, 2005).
Plants respond to drought stress by synthesis of different metabolites including polyols in ripe olive fruit (Martinelli, 2013), grape berry (Conde et al. , 2014) etc. Six different polyols (mannitol, sorbitol, galactitol, myoinositol, glycerol and dulcitol) were significantly accumulated in the pulp of grape berries in response to water deficit. MI was the most abundant of the quantified polyols in mature leaves and tissues which helps the plant with water deficit, either directly as an osmolyte or indirectly as a precursor of galactinol and raffinose family oligosaccharides (Conde et al., 2014). MI is closely related to the accumulation of RFOs conferring further stress tolerance (Elsayed et al., 2014). There is a close relationship between the metabolism of MI and RFO and the yield performance of maize under drought stress. Galactinol synthase (Go1S EC 2.4.1.123) is the key enzyme that catalyzes the production of galactinol from MI which is the key regulatory factor in RFO biosynthesis (Taji et al., 2002; Kerner et al., 2004; Sengupta et al., 2015).
Expression of a stress responsive gene (SaI O1) for osmotic stress tolerance mentioned earlier was induced through accumulation of MI (Joshi et al., 2013). The findings of Wei et al. (2010) suggested that upregulation levels of the rcMIPS gene and their increased rcMIPS activities might be involved in tolerating drought stress in Ricinus communis. In the leaves, the activities increased by 88.5% under 30% PEG stress conditions for 72 h compared to the control (Wei et. al., 2010). There are seven Go1S-related genes in the Arabidopsis thaliana and named as AtGo1S 1, 2, 3, 4, 5 6 and 7 respectively. Among these, AtGo1S1 is the drought responsive gene, which mainly functions in drought stress tolerance (Taji et al., 2002). The model plant Ajuga reptans expresses two distinct Go1S, ArGo1S1 and ArGo1S2 which regulates raffinose family oligosaccharide (RFO) metabolism (Sprenger and Keller, 2000). RFOs have long been suggested to act as anti-stress agent in both generative and vegetative tissues (Taji et al., 2002). XvGo1S gene encoding galactinol synthase was also identified in the leaves of Xerophyta viscosa. This gene shows negative correlation between RFO accumulation and MI depletion which was reversed after rehydration. This suggests that myo- inositol is channelled into RFO synthesis during water deficit and channelled back to metabolic pathway during rehydration to repair desiccation-induced damages (Peter et al., 2007). In addition to Go1S, MIPS also control the levels of galactinol and raffinose because it controls the production of MI, the galactinol precursor. Therefore, both Go1S and MIPS are proved to play important roles in drought stress tolerance (Taji et al., 2002; Evers et al., 2010). Different genes associated with MIPS and stress tolerance in plants have been enumerated in table 1.
Myo-inositol in cold stress
Low and high temperature stress is very crucial for determining the health of plants. Plants exhibit a maximum rate of growth and development at an optimum temperature or over a diurnal range of temperatures (Fitter and Hay, 1981). Stress tolerance can be induced by exposure to reduced temperature and is known as chilling tolerance or cold acclimation. Chilling tolerance is the ability of a plant to tolerate low temperatures (0–15 ºC) without injury or damage (Somerville, 1995), while cold acclimation is an enhanced tolerance to the physical acclimation. Chilling tolerance involve an array of biochemical, molecular and metabolic processes (Thomashow, 1999; Larkindale et al., 2005; Kotak et al., 2007; Zhu et al., 2007).
Plants experience cold or chilling stress at temperatures from 0–15 °C. Under such conditions, plants try to uphold homeostasis to acquire freezing tolerance and this involves extensive reprograming of gene expression and metabolism (Thomashow, 1999; Cook et al., 2004). Accumulation of sugars and altered gene expression are two major processes that has been shown to confer freezing tolerance in plants. Among cold-responsive genes, cD A encoding MIPS had the highest abundance in the library, entailing that MIPS might play an important role in the cold tolerance of M. falcata (Tan et al., 2013). Expression analysis of MfMIPS1 ( Medicago falcata MIPS1) in tobacco exhibited an enhanced tolerance to chilling stress by increased levels of inositol, galactinol and raffinose sugars (Tan et al., 2013).
MI is known to induce galactinol synthase gene
(GolS) expression and participates in cold-induced MfGolS1 (galactinol synthase gene from Medicago falcata ) expression (Zhuo et al., 2013). A full length cD A encoding a MI transporter-like protein, MfI T-like, was cloned from Medicago sativa subsp. falcata a species with better cold tolerance than M. sativa subsp. sativa. Higher levels of MI was observed in leaves of transgenic tobacco plants overexpressing MfI T-like than the wild-type suggesting that transgenic plants had higher MI transport activity than the wild-type. Transgenic plants had a better endurance to freezing temperature, implied to have a higher maximal photochemical efficiency of photosystem II (Fv/Fm) after chilling treatment.
Under freezing conditions, the presence of cyclitol function as cryoprotective solutes. Accumulation of cryoprotectans prevents freeze induced shrinkage of by balancing the concentration of cryotoxic substance during ice formation. Cyclitols like pinitol, quebrachitol, quercitol, O-methyl-muco-inositol have all been found to be accumulated in low temperature (Diamantoglou, 1974; Ericsson, 1979; Popp et al., 1997). In mistletoe ( Viscum album ) more than 25% of its dry matter is occupied by the cyclitols during winter (Richter, 1989). Similarly enhanced storage of cyclitols in the living bark tissue and buds has been found in a number of tree species during the onset of cold season (Popp and Smirnoff, 1995; Popp et al., 1997). The transcription of the enzyme, myo -inositol-o-methyl-transferase, a key enzyme for ononitol and pinitol biosynthesis has been induced in the Mediterranean species M. crystallium when the plant was exposed to 4oC for 78hr. Similarly, accumulation of pinitol in chickpea in the thylakoid membrane functions as cryoprotective solutes (Orthen, 2000). Raffinose also plays a crucial role as compatible solutes in Arabidopsis in freezing tolerance (Hannah, et al., 2006).
Myo-inositol in high temperature stress
In plants, high temperature stress (HS) causes photosynthetic acclimation and alters physiological processes directly and changes the pattern ofdevelopment indirectly (Wahid et al., 2007).
It consequently influences the reproductive growth by increasing flower abortion and reducing seed size (Talwar et al., 1999). In response to HS, plants are endowed with different mechanisms and regulatory networks, viz. regulating vital genes, managing numerous physiological and biochemical adaptations and so forth. One such mechanism to counter the effect of HS is by increasing the level of inositol in plants.
Increasing the amount of cytoplasmic free Ca2+ under temperature stress could reduce the effect of stress and decrease the lipid peroxidation (Wu et al. 1997). Overexpression of Cicer arietinum MIPS (CaMIPS2) is known to enhance the tolerance of Saccharomyces pombe cells under salinity and high temperature (Kaur et al., 2008). Also Ca2+ levels were seen to contribute to the cell water use efficiency by exogenous MI treatments. TaMIPS2, identified from a heat subtractive cD A library from wheat for its tolerance to HS was found to be expressed during various developing seed stages upon infliction with HS. The transcript levels increased in unfertilized ovaries and significant amount of the same was found during the recovery period, indicative of the pivotal role of MIPS in heat stress recovery and flower development. Any abiotic stress condition, whether drought, cold, heat or salinity essentially deprives the plant from water. Therefore, osmotic stress is rather universal in all of these forms of stress. The plant cell utilizes different mechanisms to tide over the various stress conditions mentioned. They act by activating various genes, and synthesizing a plethora of chemicals. Some of these genes and biochemical molecules derived from MI is presented in fig. 3.
Khurana et al., 2017, studied the overexpression of TaMIPS2 in Arabidopsis under different abiotic stress conditions and revealed that TaMIPS2 transgenic plants have reduced sensitivity to HS, divulging a role of MI during this stress. Increased levels of inositol upon TaMIPS2 overexpression in Arabidopsis transgenics and increasd MI levels were correlated with enhanced tolerance towards HS and other abiotic stresses. TaMIPS2 transgenics when quantitatively analyzed showed better survival during heat as well as cold and salt stress. However, in salt stress, the length of transgenics were shorter compared to wild type plants
Increased membrane stability index, FV/FM ratio along with total chlorophyll content in the transgenics were found when compared to the wild type under stress conditions. On evaluation, TaMIPS2 overexpressing Arabidopsis transgenic during stress were found to have increased level of oligosaccharides. Significant increase in MI level was observed upon heat and ABA treatment that associated with decrease in stress sensitivity of transgenics. This might also be responsible for increased stachyose levels resulting in enhanced stress tolerance.
Table 1: Different genes coding for myo -inositol and its associated derivatives potentially responsible for abiotic stress tolerance in plants.
|
SL. NO. |
Type of Stress |
Chemical/Molecular Response |
Gene Responsible |
Reference |
|
1. |
Osmotic |
Myo-inositol o- methyl transferase |
IMT 1 |
Rammesmayer et al., 1995; Vernon & Bohnert, 1992 |
|
2. |
Salinity |
D-pinitol |
Imt 1, Inps 1 transcript |
Ishitani et al. 1996 |
|
3. |
Cold |
RFO |
AtGolS3 |
Taji et al., 2002 |
|
4. |
Drought & salinity |
RFO |
AtGolS1 andAtGolS2 |
Taji et al., 2002 |
|
5. |
Drought, salinity, cold |
Raffinose |
DREB1A/CBF3 and DREB1B/CBF1 |
Jaglo-Ottosen et al., 1998; Kasuga et al., 1999; Liu et al., 1998 |
|
6. |
High temperature |
Raffinose |
AtGolS1 |
Panikulangara et al., 2004) |
|
7. |
Salinity |
myo-inositol-O-methyl transferase |
Imt |
elson et al. 1998 |
|
8. |
Salinity, high temperature |
inositol |
MIP2 |
Kaur et al., 2008; Khurana et al. 2012 |
|
9. |
Drought |
up-regulation of many stress-responsive genes |
AKI 11 |
Umezawa et al., 2004 |
|
10. |
Drought and heat |
expression of downstream stressinducible genes |
DREB2A |
Mizoi et al., 2018 |
|
11 |
Heat Stress |
Heat shock factor (HSF)- |
Os02g0496100 |
Kikuchi et al., 2003 |
|
12 |
Cold stress |
Expression of the cold-Regulated genes |
WCOR410 |
Danyluk et al., 1994 |
|
13 |
Cold stress |
Expression of the cold-Regulated genes |
WCS120 |
Ganeshan et al., 2008 |
|
14 |
Salinity stress |
PM ADPH oxidase-dependent H(2)O(2) generation |
LOC543151 |
Yang et al., 2007 |
|
15 |
Salt stress |
Regulates the proline synthesis |
P5CS |
Karthikeyan et al., 2011 |
|
16 |
Salt stress |
Proline accumulation |
P5CSF129A |
Kumar et al., 2010 |
|
17 |
Salt stress |
Increases the level of spermidine and spermine |
SAMDC |
Roy and Wu, 2002 |
|
18 |
Salt stress |
Increased glycine betaine content |
betA |
He et al., 2010 |
|
19 |
Salt stress |
Biosynthesis of mannitol |
mtlD |
Abebe et al., 2003 |
|
20 |
Salt stress |
Increases the ion flux, germination rates, chlorophyll content and antioxidant enzyme activities |
SmCP |
Zheng et al., 2018 |
|
21 |
Salt stress |
Increases the level of proline biosynthesis enzyme (P5CS) |
DH -5 |
Saibi et al., 2015 |
|
22 |
Salt stress and oxidative stress |
Expression of stress-related genes were up-regulated, and antioxidant enzyme activity were increased |
PnF3H |
Li et al., 2017 |
|
23 |
Salt stress |
Increased proline synthesis |
ADC |
Espasandin et al., 2018 |
|
24 |
Salt stress |
Increased Glycine betaine content |
AhCMO |
Zhang et al., 2009 |
|
25 |
Salt stress and drought |
Increases proline and chlorophyll content |
BADH |
Rezaei et al., 2020 |
|
26 |
salt stress |
Phosphatidylinositol 5-phosphate |
PtdIns5P |
Pical et al., 1999 |
|
27 |
Salt stress |
Myo -inositol oxygenase |
OsMIOX |
Duan et al., 2012 |
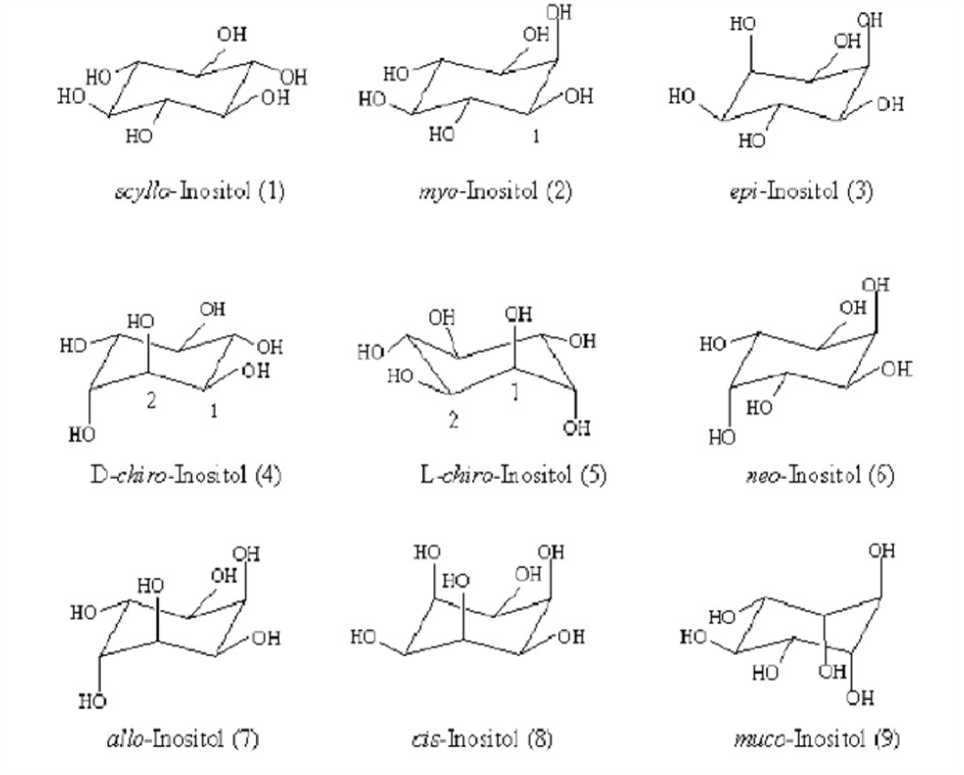
Figure 1: Structure of nine stereo isomers of inositol (*source– Murthy, 2006)
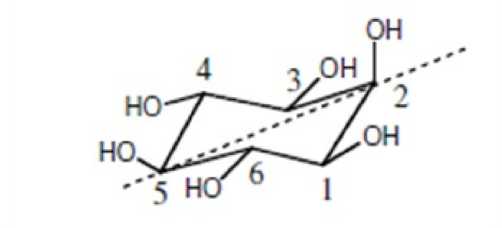
Figure 2: Mirror image of inositol (*source – Murthy, 2006)
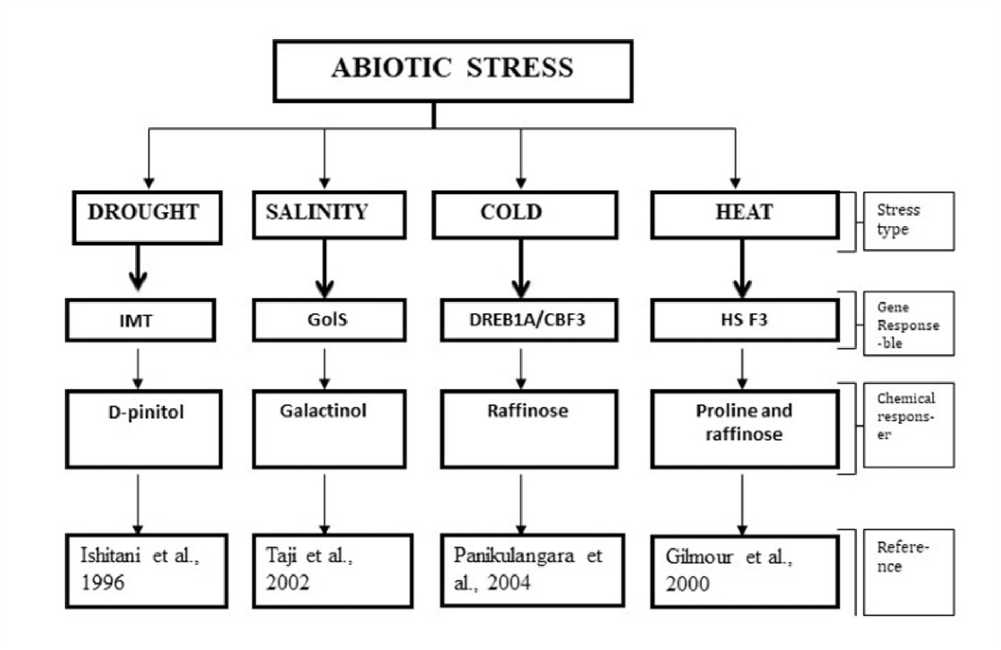
Figure 3: Some genes that regulates the expression of MI derivatives responsible for different types of abiotic stress tolerance in plants
ACKNOWLEDGMENTS
We acknowledge the Department of Botany, Sikkim University, Gangtok 737102, India for providing essential infrastructure and ambience for writing the paper.
CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest.
Список литературы Myoinositol and its metabolites in abiotic stress tolerance in plants
- Abebe T., Guenzi A.C., Martin B. and Cushman J.C. (2003) Tolerance of mannitol-accumulating transgenic wheat to water stress and salinity. Plant Physiol., 131, 1748-1755
- Ashraf M. and Harris P.J.C. (2004) Potential Biochemical Indicators of Salinity Tolerance in Plants. Plant Sci., 166, 3-16
- Ashraf M. Y., Awan A. R. and Mahmood K. (2012). Rehabilitation of saline ecosystems through cultivation of salt tolerant plants. Pak. J. Bot. 44, 69-75
- Aytung Y., Sertan C. and Serpil U. (2018) Effects of exogenous myo-inositol on leaf water status and oxidative stress of Capsicum annuum under drought stress. Acta Physiol. Plant., 40, 122
- Bartels D. and Nelson D. (1994) Approaches to improve stress tolerance using molecular genetics. Plant Cell Environ., 17, 659-667
- Basu S., Basak A, Mahanty S, Bhattacharjee S. and Adhikari J. (2019) Biosynthesis Of Myo-Inositol In Chloroplasts Of Salinity-Stressed Marine Macro Alga Ulva Lactuca. Botanica, 25(1), 32-40
- Bohnert H.J., Nelson D.E. and Jensen R.G. (1995) Adaptations to environmental stresses. Plant Cell., 7, 1099-1111
- Bohnert, H.J. and Jensen, R.G. (1996) Strategies for engineering water stress tolerance in plants. Trends Biotechnol., 14, 89-97
- Cevik S. and Yildizli A. (2014) Some synthetic cyclitol derivatives alleviate the effect of water deficit in cultivated and wild-type chickpea species. J Plant Physiol, 171, 807-816
- Chen T.H. and Murata N. (2002) Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr. Opin. Plant Biol., 5, 250-257
- Conde A., Regalado A., Rodrigues D., Costa J.M., Blumwald E., Chaves M.M. and Geros H. (2014) Polyols in grape berry: transport and metabolic adjustments as a physiological strategy for water-deficit stress tolerance in grapevine. J. Exp.Bot. 66, 1-18
- Cook D., Fowler S.F.O. and Thomashow M.F. (2004) A prominent role for the CBF cold response pathway in configuring the low-temperature metabolome of Arabidopsis. Proc Natl Acad Sci., USA.101, 15243-15248
- Crowe J.H., Crowe L.M., Carpenter J.F. and Wistrom A.C. (1987) Stabilization of dry phospholipid bilayers and proteins by sugars. Biochem J., 242, 1-10
- Crowe J.H., Hoekstra F.A. and Crowe L.M. (1992) Anhydrobiosis. Annu. Rev. Physiol., 54, 579599. doi: 10. 1146/annurev.ph.54.030192.003051 PMID: 1562184
- Culbertson M.R. and Henry S.A. (1975). Inositol-requiring mutants of Saccharomyces cerevisiae. Genetics., 80, 23-40.
- Danyluk J., Houde M., Rassart E., and Sarhan F. (1994) Differential expression of a gene encoding an acidic dehydrin in chilling sensitive and freezing tolerant gramineae species. FEBS Lett. 344, 20-24
- Diamantoglou S. (1974) Variation of contents of cyclitols in vegetative parts of higher plants. Biochem Physiol. Pflanz., 166: 511-523
- Duan J., Zhang M., Zhang H., Xiong H., Liu P., Ali, J., Li J. and Li Z. (2012) OsMlOX, a myo-inositol oxygenase gene, improves drought tolerance through scavenging of reactive oxygen species in rice (Oryza sativa L.). Plant Sci., 196, 143151
- Elsayed A.I., Rafudeen M.S. and Golldack D. (2014) Physiological aspects of raffinose family oligosaccharides in plants: protection against abiotic stress. Plant Biol. (Stuttg)., 16, 1-8
- Ericsson A. (1979) Effects of fertilization and irrigation on the seasonal changes of carbohydrate reserves in different age-classes of needle on 20 year-old scots pine trees (Pinus silvestris). Plant Physiol., 45, 270-280
- Espasandin F. D., Calzadilla P. I., Maiale S. J., Ruiz O.A. and Sansberro P.A. (2018) Overexpression of the arginine decarboxylase gene improves tolerance to salt stress in Lotus tenuis plants. J. Plant Growth Regul., 37, 156165.
- Evers D.L., Lefevre I., Legay S., Lamoureux D., Hausman J.F., Rosales R.O.G., Marca L.R.T., Hoffmann L., Bonierbale M. and Schafleitner R. (2010) Identification of droughtresponsive compounds in potato through a combined transcriptomic and targeted metabolite approach. J. Exp. Bot., 61, 2327-2343
- Fan H.F., Ding L., Xu Y.L. and Du C.X. (2017) Antioxidant system and photosynthetic characteristics responses to short-term PEG-induced drought stress. Russ. J. Plant Physiol., 64(2), 162-173
- Farooq M., Wahid A., Kobayashi N., Fujita D. and Basra S.M.A. (2009) Plant drought stress: effects, mechanisms and management. Agron. Sustain. Dev., 29, 185-212
- Fitter A.H. & Hay R.K.M. (1981) Environmental Physiology of Plants, Academic Press, London.
- Gadjev I., Ston J.M. and Gechev T.S. (2008) Programmed cell death in plants: new insights into redox regulation and the role of hydrogen peroxide. Int. Rev. Cell. Mol. Biol. 270, 87-144
- Ganeshan S., Vitamvas P., Fowler D. B. and Chibbar R. N. (2008) Quantitative expression analysis of selected COR genes reveals their differential expression in leaf and crown tissues of wheat (Triticum aestivum L.) during an extended low temperature acclimation regimen. J. Exp. Bot., 59(9), 2393-2402
- Ghosh D.K., Maitra S., Goswami L., Roy D., Das K.P. and Majumder A.L. (2006) An insight into the molecular basis of salt tolerance of L-myo-inositol 1-P synthase (PcINO1) from Porteresia coarctata (Roxb.) Tateoka, a halophytic wild rice. Plant Physiol., 140, 12791296
- Gill S.S. and Tuteja N. (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem., 48, 909-930
- Gilmour S.J., Sebolt A.M., Salazar M.P., Everard, J.D. and Thomashow M.F. (2000) Overexpression of the Arabidopsis CBF3 transcriptional activator mimics multiple biochemical changes associated with cold acclimation. Plant Physiol., 124 18541865
- Hannah M.A., Zuther E., Buchel K. and Heyer A.G. (2006) Transport and metabolism of raffinose family oligosaccharide in transgenic potato. J. Exp. Bot. 57, 3801-3811
- Hasegawa P.M., Bressan R.A., Zhu J.K., and Bohnert H.J. (2000) Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Mol. Plant Physiol., 51, 463-499
- Hayashi H., Alia, Mustardy L., Deshnium P., Ida M. and Murata N. (1997) Transformation of Arabidopsis thaliana with the codA gene for choline oxidase: Accumulation of glycinebetaine and enhanced tolerance to salt and cold stress. Plant J., 12, 133-142
- He C., Yang A., Zhang, W.; Gao, Q.; Zhang, J. (2010) Improved salt tolerance of transgenic wheat by introducing betA gene for glycine betaine synthesis. Plant Cell Tiss. Org. Cult., 101, 65-78
- Hegeman C.E., Good L.L. and Grabau E.A. (2001) Expression of D-myoinositol-3-phosphate synthase in soybean. Implications for phytic acid biosynthesis. Plant Physiol., 125, 1941-1948
- Holmstrom K.O., Mântylâ E., Welin B., Mandal A., Palva E.T., Tunnela O.E. and Londesborough J. (1996) Drought tolerance in tobacco. Nature., 379, 683-684
- Hu Y. and Schmidhalter U. (2005) Drought and salinity: A comparison of their effects on mineral nutrition of plants. J. Plant Nutr. Soil Sci., 168, 541-549
- Ishitani M., Majumder A.L., Bornhouser A., Michalowski C.B., Jensen R.G., and Bohnert H.J. (1996) Coordinate transcription induction of myo-inositol metabolism during environmental stress. Plant J., 9, 537-548
- Jaglo-Ottosen K.R., Gilmour S.J., Zarka D.G, Schabenberger O. and Thomashow M.F. (1998) Arabidopsis CBF1 Overexpression Induces COR Genes and Enhances Freezing Tolerance. Science, 280(5360), 104-106
- Jang I.C., Oh S.J., Seo J.S., Choi W.B., Song S.I, Kim C.H, Kim Y.S., Seo H.S., Choi Y.D., Nahm B.H. and Kim J.J. (2003) Expression of a Bifunctional Fusion of the Escherichia coli Genes for Trehalose-6-Phosphate Synthase and Trehalose-6-Phosphate Phosphatase in Transgenic Rice Plants Increases Trehalose Accumulation and Abiotic Stress Tolerance without Stunting Growth. Plant Physiol., 131(2), 516-24
- Johnson M.D. (1994) The Arabidopsis thaliana myo-inositol 1-phosphate synthase (EC 5.5.1.4). Plant Physiol., 105, 1023-1024
- Joshi R., Ramanarao M.V. and Baisakh N. (2013) Arabidopsis plants constitutively overexpressing a myoinositol 1-phosphate synthase gene (SaINO1) from the halophyte smooth cordgrass exhibits enhanced level of tolerance to salt stress. Plant Physiol. Biochem., 65, 6166
- Karthikeyan A., Pandian S.K. and Ramesh M. (2011) Transgenic indica rice cv. ADT43 expressing a 1-pyrroline-5-carboxylate synthetase (P5CS) gene from Vigna aconitifolia demonstrates salt tolerance. PCTOC, 107(3): 383-395
- Kasuga M., Liu Q., Miura S., Yamaguchi-Shinozaki K. and Shinozaki K. (1999) Improving plant drought, salt and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nat. Biotechnol., 17, 287291
- Kaur H., Shukla R.K., Yadav G., Chattopadhya D. and Majee M. (2008) Two divergent genes encoding L-myo-inositol-1-phosphate synthase 1 (CaMIPS1) and 2 (CaMIPS2) are differentially expressed in chickpea. Plant Cell Env., 3, 1701-1716
- Kaur H., Verma P., Petla B.P., Rao V., Saxena S.C. and Majee M. (2013) Ectopic expression of the ABA-inducible dehydration-responsive chickpea L-myo-inositol 1-phosphate synthase 2 (CaMIPS2) in Arabidopsis enhances tolerance to salinity and dehydration stress. Planta., 237, 321-335
- Kerner U., Peterbauer T., Raboy V., Jones D.A., Hedley C.L. and Richter A. (2004) Myo-inositol and sucrose concentrations affect the accumulation of raffinose family oligosaccharides in seeds. J. Exp. Bot., 55, 1981-1987
- Khurana N, Sharma N and Khurana P (2017) Overexpression of a heat stress inducible wheat myo-inositol-1-phosphate synthase 2 (TaMIPS2) confers tolerance to various abiotic stresses in Arabidopsis thaliana. Agri. Gene, 6, 24-30
- Kikuchi K., Terauchi K., Wada M. and Hirano H. (2003) The plant MITE mPing is mobilized in anther culture. Nature., 421, 167-170
- Kishor P.B.K., Hong Z.L., Miao G.H., Hu C.A.A. and Verma D.P.S. (1995) Overexpression of D-pyrroline-5-carboxylate synthetase increases proline production and confers osmotolerance in transgenic plants. Plant Physiol., 108, 13871394
- Kotak S, Vierling E, Bäumlein H., and Koskull-Döring P. (2007) A novel transcriptional cascade regulating expression of heat stress proteins during seed development of Arabidopsis. Plant Cell, 19, 182-195
- Kumar V., Shriram V., Kishor P.K., Jawali N. and Shitole M.G. (2010) Enhanced proline accumulation and salt stress tolerance of transgenic indica rice by over-expressing P5CSF129A gene. Plant Biotechnol. Rep., 4, 37-48
- Larkindale J., Hall J.D., Knight M.R. and Vierling E. (2005) Heat Stress Phenotypes of Arabidopsis Mutants Implicate Multiple Signaling Pathways in the Acquisition of Thermotolerance. Plant Physiol., 138(2):882-97
- Larson S.R. and Raboy V. (1999) Linkage mapping of maize and barley myoinositol 1-phosphate synthase DNA sequences: correspondence with a low phytic acid mutation. Theor. Appl. Genet., 99, 27 -36
- Li H., Chang J., Chen H., Wang Z., Gu X., Wei C., Zhang Y., Ma J., Yang J. and Zhang X. (2017) Exogenous melatonin confers salt stress tolerance to watermelon by improving photosynthesis and redox homeostasis. Front. Plant Sci., 8, 295
- Liu Q., Kasuga M., Sakuma Y., Abe H., Miura S., Yamaguchi-Shinozaki, K. and Shinozaki, K. (1998) Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain, separate two cellular signal transduction pathways in drought- and low temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell., 10, 1391-1406
- Loewus F.A. (1990) Structure and occurrence of inositols in plants. In Morre D.J., Boss W.F. and Loewus F.A. (ed.), In Inositol Metabolism in Plants. Wiley-Liss, New York pp. 1-11.
- Loewus F.A. and Loewus M.W. (1983) Myo-Inositol: Its biosynthesis and metabolism. Annu. Rev. Plant Physiol., 34, 137-161
- Loewus F.A. and Murthy P.P.N. (2000) Myo-Inositol metabolism in plants. Plant Sci., 150, 1-19
- Majee M., Maitra S., Dastidar K.G., Pattnaik, S., Chatterjee A., Hait N.C., Das K.P. and Majumder A.L. (2004) A novel salt-tolerant L-myo-inositol-1phosphate synthase from Porteresia coarctata (Roxb.) Tateoka, a halophytic wild rice: molecular cloning, bacterial overexpression, characterization, and functional introgression into tobacco-conferring salt tolerance phenotype. J. Biol. Chem., 279, 28539-28552.
- Martinelli F., Remorinic D., Saiab S., Massaic R. and Tonuttiaa P. (2013) Metabolic profiling of ripe olive fruit in response to moderate water stress. Scientia. Horti., 159, 52-58.
- Mechri B., Tekaya M., Cheheb H. and Hammami M. (2015) Determination of mannitol sorbitol and myo-inositol in olive tree roots and rhizospheric soil by gas chromatography and effect of severe drought conditions on their profiles. J. Chromatogr. Sci., 53(10), 1631-1638
- Merchant A., Tausz M., Arndt S. and Adams M. (2006) Cyclitols and carbohydrates in leaves and roots of 13 Eucalyptus species suggest contrasting physiological responses to water deficit. Plant, Cell & Environ., 29, 2017- 2029
- Mizoi, J., Kanazawa, N., Kidokoro, S., Takahashi, F., Qin, F., Morimoto, K., et al. (2018). Heat-induced inhibition of phosphorylation of the stress-protective transcription factor DREB2A promotes thermotolerance of Arabidopsis thaliana. J. Biol. Chem. 294, 902-917. doi:
- 10.1074/jbc.RA118.002662 Mukherjee R., Mukherjee A., Bandyopadhyay S., Mukherjee S., Sengupta S., Ray S. and Majumder A.L. (2019) Selective manipulation of the inositol metabolic pathway for induction of salt-tolerance in indica rice variety. Sci. Rep., 9, 5358
- Nelson D.E., Koukoumanos M. and Bohnert H.J. (1999) Myo-inositol-dependent sodium uptake in ice plant. Plant Physiol., 119,165-172
- Nelson D.E., Rammesmayer G. and Bohnert H.J. (1998) Regulation of cell specific inositol metabolism and transport in plant salinity tolerance. Plant Cell, 10, 753-764
- Nomura M., Ishitani M., Takabe T., Rai A.K. and Takabe T. (1995) Synechococcus sp. PCC7942 transformed with Escherichia coli bet genes produces glycine betaine from choline and acquires resistance to salt stress. Plant Physiol,. 107, 703-708
- Orthen B. and Popp M. (2000) Cyclitols as cryoprotectants for spinach and chickpea thylakoids. Environ. Exp. Bot., 44, 125-132
- Panikulangara T.J., Eggers-Schumacher G., Wunderlich M., Stransky H. and Schoffl F. (2004) Galactinol synthase1: a novel heat shock factor target gene responsible for heat-induced synthesis of raffinose family oligosaccharides in Arabidopsis. Plant Physio.,l 136, 3148-3158
- Patra B., Ray S., Richter A. and Majumder A. L. (2010) Enhanced salt tolerance of transgenic tobacco plants by co-expression of PcINO1 and McIMT1 is accompanied by increased level of myo-inositol and methylated inositol. Protoplasma., 245, 143-52
- Paul M.J. and Cockburn W. (1989) Pinitol, a Compatible Solute in Mesembryanthemum crystallinum. J. Exp. Bot., 219, 1093-1098
- Per T.S., Khan N.A., Reddy P.S., Masood A., Hasanuzzaman M., Khan M.I.R. and Anjum N.A. (2017) Approaches in modulating proline metabolism in plants for salt and drought stress tolerance: Phytohormones, mineral nutrients and transgenics. Plant Physiol Biochem., 115, 126-140
- Peter S., Mundree S.G., Thomson J.A., Farrant J.M. and Keller F. (2007) Protection mechanisms in the resurrection plant Xerophyta viscosa (Baker): both sucrose and raffinose family oligosaccharides (RFOs) accumulate in leaves in response to water deficit. J. Exp. Bot., 58, 1947-1956
- Pical C., Westergren T., Dove S.K., Larsson C. and Sommarin M. (1999) Salinity and hyperosmotic stress induce rapid increases in phosphatidylinositol 4,5-bisphosphate, diacylglycerol pyrophosphate, and phosphatidylcholine in Arabidopsis thaliana cells. J. Biol. Chem. 274, 38232-38240
- Popp M. and Smirnoff N. (1995) Polyol accumulation and metabolism during water deficit. In Smirnoff, N. (ed.), Environment and plant metabolism: Flexibility and acclimation environment plant biology series, BIOS, Oxford, pp. 199-215
- Popp M., Lied W., Bierbaum U., Gross M., Grobe-schulte T., Hams S., Oldenettel J., Schuler S. and Wiese J. (1997) Cyclitol-stable osmotic in trees. In Rennenberg, H., Escherich, W. and Ziegler H. (ed.), Trees-Contribution to modern tree physiology, Backhuys, Leiden, pp. 257270.
- Posternak T. (1965) The cyclitols. Holden Day, San Francisco, Hermann, Paris Rai A. C., Singh M. and Shah K. (2013) Engineering drought tolerant tomato plants over-expressing BcZAT12 gene encoding a C2H2 zinc finger transcription factor. Phytochem., 85, 44-50
- Rammesmayer G., Pichorner H., Adams P., Jensen R.G., and Bohnert H.J. (1995) Characterization of IMT1, myoinositol O-methyltransferase, from Mesembryanthemum crystallinum. Arch. Biochem. Biophys., 322, 183-188
- RayChaudhuri A. and Majumder A.L. (1996) Salinity induced enhancement of L-myo-inositol-1-phosphate synthase in rice (Oryza sativa L.). Plant Cell Environ., 19, 1437-1442
- Rezaei QBF., Solouki A., Tohidfar M., Mehrjerdi M.Z., Izadi-Darbandi A. and Vahdati K. (2020) Agrobacterium-mediated transformation of Persian walnut using BADH gene for salt and drought tolerance. J. Hortic. Sci. Biotechnol., 96, 162-171
- Richter A. (1989) Osmotisch wirksame Inhaltsstoffe in einheimischen Mistelarten and ihren Wirten. University of Vienna Ph.D. Thesis
- Roy M. and Wu R. (2002) Overexpression of S-adenosylmethionine decarboxylase gene in rice increases polyamine level and enhances sodium chloride-stress tolerance. Plant Sci., 163, 987992
- Roy M. and Wu R. (2002) Overexpression of S-adenosylmethionine decarboxylase gene in rice increases polyamine level and enhances sodium chloride-stress tolerance. Plant Sci. J., 163(5), 987-992
- Sacher R.F. and Staples R.C. (1985) Inositol and sugars in adaptation of tomato to salt. Plant Physiol., 77(1) 206-210
- Saibi W., Feki K., Mahmoud R.B. and Brini F. (2015) Durum wheat dehydrin (DHN-5) confers salinity tolerance to transgenic Arabidopsis plants through the regulation of proline metabolism and ROS scavenging system. Planta., 242(5), 118794
- Sambe M.A.H., He X., Tu Q. and Guo Z. (2015) A cold-induced myo-inositol transporter-like gene confers tolerance to multiple abiotic stresses in transgenic tobacco plants. Physiol Plant., 153, 355-364
- Sarkar T., Tankappan R., Kumar A., Mishra G. P. and Dobaria J.R. (2014) Heterologous Expression of the AtDREB1A Gene in Transgenic Peanut-Conferred Tolerance to Drought and Salinity Stresses. PLoS One., 9, 110-507
- Sengupta S., Mukherjee S., Basak P. and Majumder A.L. (2015) Significance of galactinol and raffinose family oligosaccharide synthesis in plants. Front Plant Sci., 6, 1-11
- Serraj R. and Sinclair T.R. (2002) Osmolyte accumulation: Can it really help increase crop yield under drought conditions?. Plant Cell Environ., 25(2), 333-341
- Shen B., Jensen R.G. and Bohnert H.J. (1997) Increased resistance to oxidative stress in transgenic plants by targeting mannitol biosynthesis to chloroplasts. Plant Physiol., 113, 1177-1183.
- Sheveleva E., Chmara W., Bohnert H.J. and Jensen R.G. (1997) Increased salt and drought tolerance by D-ononitol production in transgenic Nicotiana tabacum L. Plant Physiol., 115, 1211-1219.
- Somerville C. (1995) Direct tests of the role of membrane lipid composition in low-temperature-induced photoinhibition and chilling sensitivity in plants and cyanobacteria. Proc. Natl. Acad. Sci. 92, 6215-6218
- Sprenger N. and Keller F. (2000) Allocation of raffinose family oligosaccharides to transport and storage pools in Ajuga reptans: the roles of two distinct galactinol synthase. Plant J., 21, 249-258
- Stevenson-Paulik J., Bastidas R.J., Chiou S.T., Frye R.A. and York JD (2005) Generation of phytate-free seeds in Arabidopsis through disruption of inositol polyphosphate kinases. PNAS102: 12612-12617
- Strauss B.S. (1958) Cell death and unbalanced growth in Neurospora. J. Gen. Microbiol., 18, 658-669
- Szabados L., Kovacs H., Zilberstein A. and Bouchereau A. (2011) Plants in extreme environments. Adv Bot Res. 57, 105-150 https://doi.org/10.1016/ B978-0-12-387692-8.00004-7
- Taji T., Ohsumi C., luchi S., Seki M., Kasuga M., Kobayashi M., Yamaguchi-Shinozaki K. and Shinozaki K. (2002) Important roles of drought- and cold-inducible genes for galactinol synthase in stress tolerance in Arabidopsis thaliana. Plant J., 29, 417-426
- Taji T., Ohsumi C., luchi S., Seki M., Kasuga M., Kobayashi M., Yamaguchi-Shinozaki K. and Shinozaki K. (2002) Important roles of drought- and cold-inducible genes for galactinol synthase in stress tolerance in Arabidopsis thaliana. Plant J., 29, 417-426
- Taji T., Ohsumi C., Luchi C. and Seki M. (2002) Important roles of drought- and cold-inducible genes for galactinol synthase in stress tolerance in Arabidopsis thaliana. Plant J., 29(4), 417-26
- Taji T., Takahashi S. and Shinozaki K. (2006) Inositol ad their metabolism in abiotic and biotic stress responses. Subcell. Biochem., 39, 239-264
- Talwar H.S., Takeda H., Yashima S. and Senboku T. (1999) Growth and photosynthetic responses of groundnut genotypes to high temperature. Crop Sci., 39, 460-466
- Tan J.L., Wang C.Y., Xiang B., Han R.H. and Guo Z.F. (2013) Hydrogen peroxide and nitric oxide mediated cold- and dehydration-induced myo-inositol phosphate synthase that confers multiple resistances to abiotic stresses. Plant, Cell Environ. 36, 288-299
- Tarczynski M., Jensen R. and Bohnert H. (1993) Stress protection of transgenic tobacco by production of the osmolyte mannitol. Science, 259, 508-510
- Thomashow M.F. (1999) Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Physiol., 50, 571599
- Umezawa T., Yoshida R., Maruyama K., Yamaguchi-Shinozaki K. and Shinozaki K. (2004) SRK2C, a SNF1-related protein kinase 2, improves drought tolerance by controlling stress-responsive gene expression in Arabidopsis thaliana. Proceedings of the National Academy of Sciences of the United States of America. 101, 17306- 17311
- Vernon D., and Bohnert H.J. (1992) A novel methyl transferase induced by osmotic stress in the facultative halophyte Mesembryanthemum crystallinum. EMBO J., 11, 2077-2085
- Wahid A., Gelani S, Ashraf M. and Foolad M.R., (2007) Heat tolerance in plants: An overview. Environ. Exp. Bot., 61, 199-223
- Wang F.B., Zhai H., An Y.Y., Si Z.Z., He S.Z. and Liu Q.C. (2016) Overexpression of IbMIPS1 gene enhances salt tolerance in transgenic sweetpotato. J Integr Agric., 15, 271-281
- Wei W., Dai X., Wang Y., Chuan Y., Gou C.B. and Chen F. (2010) Cloning and expression analysis of 1 L-myo-Inositol-1-phosphate synthase gene from Ricinus communis L. Z. Naturforsch. C., 65, 501-507
- Wu Y., Kuzma J., Marechal E., Graeff R., Lee H.C., Foster R. and Chua N.H. (1997) Abscisic acid signaling through cyclic ADP-ribose in plants. Science., 278, 2126-2130
- Yang Y., Xu S., An L. and Chen N. (2007) NADPH oxidase-dependent hydrogen peroxide production, induced by salinity stress, may be involved in the regulation of total calcium in roots of wheat. J. Plant Physiol., 164(11), 1429-1435
- Yoshida K.T., Wada T., Koyama H., Mizobuchi-Fukuoka R. and Naito S. (1999) Temporal and spatial patterns of accumulation of the transcript of myo-inositol-1-phosphate synthase and phytin-containing particles during seed development in rice. Plant Physiol., 119, 65 -72.
- Zhai S, Jinxi H, Lei X, Yanyan A, Shaozhen H, Quingchang L (2016) A myo-inositol-1-phosphate synthase gene, IbMIPS1, enhances salt and drought toleranceand stem nematode resistance in transgenic sweet potato. Plant Biotechnol. J. 14, 592-602
- Zhai S, Jinxi H, Lei X, Yanyan A, Shaozhen H, Quingchang L (2016) A myo-inositol-1-phosphate synthase gene, IbMIPS1, enhances salt and drought toleranceand stem nematode resistance in transgenic sweet potato. Plant Biotechnol. J. 14, 592-602
- Zhang H., Dong H., Li W., Sun Y., Chen S. and Kong X. (2009) Increased glycine betaine synthesis and salinity tolerance in AhCMO transgenic cotton lines. Mol. Breed., 23, 289-298
- Zheng L., Chen S., Xie L., Lu Z., Liu M. and Han X., Qiao G., Jiang J., Zhuo R., Qiu W. and He, Z. (2018) Overexpression of cysteine protease gene from Salix matsudana enhances salt tolerance in transgenic Arabidopsis. Environ. Exp. Bot., 147, 53-62.
- Zhu Y., Zhang L., Fan J. and Han S. (2007) Neural basis of cultural influence on self-representation. NeuroImage, 34, 1310-16
- Zhuo C., Wang T., Lu S., Zhao Y, Li X. and Guo Z. (2013) A cold responsive galactinol synthase gene from Medicago falcata (MfGolS1) is induced by myo-inositol and confers multiple tolerances to abiotic stresses. Physiol. Plant., 149, 67-78


