NADP-malate dehydrogenase isoforms of wheat leaves under drought: their localization, and some physicochemical and kinetic properties
Автор: Babayev H.G., Mehvaliyeva U.A., Aliyeva M.N., Guliyev N.M., Feyziyev Y.M.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 3 т.11, 2015 года.
Бесплатный доступ
Changes in sub-cellular localization, isoenzyme spectrum and kinetic characteristics of NADP-malate dehydrogenase (NADP-MDH, EC 1.1.1.82) in Triticum durum Desf. genotypes with contrasting drought tolerance have been studied. In chloroplast and cytosol fractions of mesophyll cells of wheat flag leaves 70-75% and 25-30% of the total NADP-MDH activity were found to be localized, respectively. Three isoforms of NADP-MDH with molecular weights of 66, 74 and 86 kDa were revealed in the chloroplast fraction, whereas in the cytosolic fraction molecular weights of the isoenzymes were found to be 42, 66 and 74 kDa. Drought caused the formation of a new 90 kDa isoform of the enzyme in the chloroplast fraction in anthesis phase of ontogenesis. In the drought-tolerant genotype the appearance of the new isoform in the chloroplast fraction was accompanied by a more rapid increase in K m and V max contrary to the chloroplast fraction of the drought-sensitive genotype manifesting a slight decrease in these parameters, suggesting one of the adaptive traits in forming drought tolerance in C 3 plants.
Drought, enzyme isoforms, nadp-malate dehydrogenase, wheat
Короткий адрес: https://sciup.org/14323923
IDR: 14323923
Текст научной статьи NADP-malate dehydrogenase isoforms of wheat leaves under drought: their localization, and some physicochemical and kinetic properties
Water deficit is known to be one of the essential factors limiting plant productivity and the yield loss caused by water stress exceeds the total loss caused by other extreme factors (Passioura, 2007; Rampino et al ., 2006). The degree of plant tolerance to water stress is varied among different species (Quartacci et al ., 1995; Lizana et al ., 2006). To prevent water loss during drought, plants are forced to close their stomata, causing in turn CO 2 uptake decline and violation of Calvin cycle reactions leading to the decrease in photosynthesis rates (Chaves et al ., 2002; Chaves and Oliveira, 2004; Cornic and Massacci, 1996). C 4 -acids, such as malate, aspartate, oxaloacetate, etc. and enzymes of the PEP-system controlling their metabolism perform important functions in creation of the defence mechanisms in response to extreme conditions. Thus, enzymes involved in C 4 -acid metabolism in C 3 and C 4 plants have been extensively investigated recently (Häusler et al ., 2002).
Isoforms of malate dehydrogenase (MDH) enzymes involved in metabolic processes in higher plants differ in their subcellular localization and cofactor specificity (Gietl, 1992; Goward and Nicholls, 1994). Complex molecular forms of MDH are in a certain dynamic equilibrium in cells. To meet the requirement of plant cell in energy and carbon skeleton dexarboxylating and oxidizing MDHs reduce the dependence from glycolysis. At the same time by the mobilization of organic acids they respond to environmental changes (Scheibe, 1990).
NADP-MDH catalyses reversible conversion of oxaloacetate to malate using NADPH as cofactor (Edwards and Andreo, 1992; Johansson et al., 1999). NADP-MDH has been isolated and characterized from leaves of C4 plants ‒ maize (Jacquot et al., 1981; Kagawa and Bruno, 1988; Agostino et al., 1992), spinach (Ferte et al., 1986), pea (Scheibe and Fickenscher, 1985; Fiskenscher and Scheibe, 1988) and sorghum (Johansson et al., 1999). Malate formed during NADP-MDH reaction in mesophyll cells of C4 plants is transported to chloroplasts of bundle sheath cells and participates in providing CO2 donor for the carbon concentrating mechanism (Hatch, 1987). Activity of the NADP-MDH in the cells is regulated by a substrate concentration and light intensity (Johnson and Hatch, 1970; Buchanan, 1980; Ashton and Hatch, 1983; Luchetta et al., 1990, 1991; Miginiac-Maslow and Issakidis, 1997).
The NADP-MDH activity is several times lower in C 3 plants compared with C 4 species and higher in young leaves than in old ones (Merlo et al ., 1993). Total amount of the enzyme is 10 times higher in maize compared with spinach (Ferte et al ., 1986).
In this paper NADP-MDH activity, its isoenzyme spectrum, subcellular localization, physicochemical and kinetic properties have been comparatively studied in leaves of high-productive wheat genotypes with contrasting drought tolerance under water-stressed conditions.
MATERIALS AND METHODS
Plant materials. Field experiments have been carried out at the experimental station of the Research Institute of Crop Husbandry situated in Absheron peninsula during 2012-2014 seasons. The objects of the investigation were two local genotypes of Triticum durum Desf. with distinct drought tolerance: high-productive (75-85 cwt/ha) and drought tolerant Barakatli-95 (genotype I), and high-productive (70-80 cwt/ha) and drought sensitive Garagilchig-2 (genotype II). Plants were grown in 20 m2 plots (2x10 m), in triplicate. During May-June, when field experiments were conducted temperature was between 20-30°C and there was no virtually precipitation. Watering stopped at the end of the leaf tube formation stage thereby creating soil drought condition, while control plants were watered till the end of the vegetation. Leaf samples were taken in the morning after 2-3 hours of the natural illumination. The data presented in the article are results of the latest (2014 season) experiments.
Enzyme extracts and subcellular fractions. Flag leaves were separated from stems, washed in distilled water, and dried. The medium of 100 mM Tris-HCl buffer (pH 8.0) containing 1 mM ethylendiaminetetraacetic acid (EDTA), 5 mM dithiothreitol (DTT), 10 mM MgCl2, 5% glycerol and 1% polyvinilpyrrolidone (1:7 ratio, w/v; 4°C) was added and leaves were homogenized for 2 min. The homogenate was filtered through 2 layers of a capron cloth and filtrate was centrifugated for 5 min at 200 g to precipitate cell debris. Supernatant was centrifuged for 10 min at 1000 g. Resulting precipitate and supernatant obtained after centrifugation consist of chloroplast and cytosolic fractions, respectively. Each subcellular fraction was kept in the homogenization medium containing 0.5% Triton X-100 for an hour and formed enzyme extracts were used for further studies.
NADP-MDH activity . NADP-MDH activity was measured spectrophotometrically (Ultrospec 3300 pro, Amersham) at λ=340 nm. Before its determination NADP-MDH was activated in the medium of 1.0 M Tris-HCl (pH 8.0), 10 mg/ml BSA and 100 mM DTT for 15 min. The reaction mixture (final 1 ml) contained 100 mM Tris-HCl buffer (pH 8.0), 10 mg/ml bovine serum albumin (BSA), 1 mM EDTA, 20 mM MgCl 2 , 0.2 mM NADPH and 30 μl of the activated enzyme extract. The reaction was initiated by adding 10 μl of the oxaloacetate (OAA, initial 1 mM). The determination of the enzyme activity was based on the decrease of the optic density of the medium for 1 min (Scheibe and Stitt, 1988). The extinction coefficient of 6.22 mM cm-1 was used for NADH and NADPH.
Native gel electrophoresis. Separation of proteins and investigation of the isoenzyme spectra, including specific revelation of enzymes were performed using native gel electrophoresis method (Davis, 1964). 150 µg of protein samples in 30-40 µl aliquots were applied on each well of the gel. Electrophoresis was performed in 7.5% polyacrylamide gel (PAAG) (pH 8.0) in a cold room at 4°С. The visualization of separated marker proteins was done in 0.025% bromophenol blue solution. Residual bromophenol blue was washed out by 7% (v/v) acetic acid at room temperature with regular agitation (vizualization of isoenzymes was done as below).
Specific revelation of enzymes. Molecular isoforms of NADP-MDH separated by PAAG electrophoresis was revealed using tetrazole (Fieldes, 1992). The native gel was transferred to the appropriate reaction medium and incubated in the dark at 37°С, for 35-40 min. Electrophoresis running conditions were the same as described above. Freshly prepared 100 mM Tris-HCl buffer (pH 8.0), containing 50 mM malate, 20 mM NADPH, 10 mM phenazine methosulfate and 10 mM nitroblue tetrazolium was used as the reaction medium. Proteins were visualized after 40-60 min staining at 37°С. Diformazan formed in this reaction shows an exact localization point of NADP-MDH on the gel.
Michaelis-Menten constant . The investigations of kinetic parameters were carried out with purified enzyme preparations. The standard assay system consisted of 100 mM Tris-HCl buffer (pH 8.0), 1 mM EDTA, 1 mg/ml BSA, 0.2 mM NADPH and 1 mM OAA. The reaction rate constant K m and maximum rate (Vmax) of the reaction were calculated using the Lineweaver-Burk procedure. The substrate concentrations ranged from 1 to 20 mM.
Molecular weight determination . Molecular weights (Mr) of NADP-malate dehydrogenase, numbers and Mr of its subunits were determined using the electrophoretic method of Laemmli (Laemmli, 1970). β-galactosidase (116 kDa), BSA (66.2 kDa), ovalbumin (45 kDa), lactate dehydrogenase (35 kDa), restriction endonuclease BSP981 (25 kDa), α-lactoglobulin (18.4 kDa) and lysozyme (14.4 kDa) (Sigma, USA) were used as protein markers.
Proline. To determine free proline, 0.3 g of the plant material was extracted twice with 3% sulfosalicylic acid. The homogenate was centrifuged at 1000 g, for 15 min. Proline was determined quantitatively in supernatant using Bates method (Bates et al., 1973). The medium containing 1 ml acetic acid and 1 ml ninhydrin (1.25 g of ninhydrin dissolved in a mixture of 30 ml acetic acid and 20 ml of 6.0 M phosphoric acid) was added to 1 ml of supernatant. Samples were incubated for 1 hour in a hot water bath. After rapid cooling and adding 3 ml toluene, samples were intensively shaken. Optical density of the reddish orange toluene fraction was measured at 520 nm. Calibration curves made with standard L-proline (Sigma, USA) were used for quantitative determination of proline.
Malone dialdehide . Malone dialdehide (MDA) was determined quantitatively according to its reaction with tiobarbituric acid (TBA) (Heath, Pasker, 1968). Plant material (1 g) was homogenized in 20 ml of 0.1% (w/v) 3-chloroacetic acid. The homogenate was centrifuged at 12000 g for 10 min. A mixture (4 ml) of TBA (0.5%) and 3-chloroacetic acid (20%) was added to supernatant, kept in a water bath at 95°С for 30 min and rapidly cooled with ice, then centrifugated at 12000 g for 10 min. Optical density of the supernatant was measured at 532 and 600 nm, and nonspecific absorption was subtracted. Extinction coefficient of 155 mМ-1∙cm-1 at 600 nm was used for the calculation of MDA quantity.
Chlorophyll concentration . Chlorophyll was quantified spectrophotometrically (Ultraspec 3300 pro, Amersham, USA) using its 80% acetone extract (Sims, Gamon, 2002).
Protein concentration. Total protein content was determined according to Sedmak and Grossberg (1977). BSA was used as a standard protein marker for constructing calibration curve.
Relative water content . Leaf relative water content was determined in every phase of ontogenesis using the method described in (Tambussi, 2005) and the following formula for calculations:
RWC = 100% (MF ‒ MD) / (MT ‒ MD), where MF is leaf fresh mass, MD - leaf dry mass and MT – mass after saturation with water.
All values presented in the tables and figures are mean of three replicates. The data were processed statistically: mean arithmetic values and their standard deviations were determined.
RESULTS
Subcellular fractions (cytosolic and chloroplast) of mesophyll cells were isolated from flag leaves of two durum wheat genotypes with distinct drought tolerance, and a highly purified NADP-MDH preparation was obtained. The total activity of the enzyme was found to be localized in chloroplasts (7075%) and cytosol (25-30%) fractions of both genotypes.
Using PAAG electrophoresis the isoenzyme content of NADP-MDH, their localization and molecular weights were determined. As seen in figure 1 (see also Tab.1) three isoforms of 42, 66 and 74 kDa were revealed in cytosol and three isoforms of 66, 74 and 86 kDa in chloroplast fractions of both genotypes after the tube formation stage before the drought. The highest protein amounts were observed for 86 kDa in genotype I and for 42 kDa in genotype II.
The number of the isoforms did not change during anthesis phase in the mentioned fractions of both genotypes when watering. In the cytosolic fraction molecular weights of the isoforms also remained as they were in the leaf tube formation stage (three isoforms of 42, 66 and 74 kDa). While in the chloroplast fraction of drought-exposed plants of each genotype, besides constitutive 66, 74 and 86 kDa isoforms a new inductive isoform of 90 kDa appeared. At this stage the protein amount and the enzyme activity significantly increased in genotype I compared with genotype II (Fig. 1 and Tab. 1).
At the wax ripeness stage in the cytosolic fractions of both genotypes in control as well as drought-exposed plants the number of the enzyme isoforms did not change. However, their activities and protein content decreased markedly. A new 90 kDa isoform almost disappeared at this stage in the chloroplast fraction of genotype II under drought, whereas it existed in genotype I (Fig. 1).
Due to localization of NADP-MDH in chloroplasts, we examined the chlorophyll content in leaves of watered and drought exposed plants. Results of this study show that despite the high amount of chlorophyll in leaves during early stages of ontogenesis, there were no marked decrease in chlorophyll content due to destructive changes in leaves of both genotypes to the end of the vegetation, however, chlorophyll amount was always higher in drought resistant genotype I than in drought sensitive genotype II (Tab. 2). Pronounced differences were not detected in chlorophyll a/b ratio of studied genotypes I and II. However, drought caused a decrease in this parameter in both genotypes.
Data presented in table 2 indicate that increase in proline synthesis occurred to the end of the vegetation of drought tolerant and sensitive genotypes. These data was approx. 2-fold for anthesis stage, while it increased more during seed formation. However, in seed formation stage genotype II showed decreased synthesis of the proline under drought, while genotype I demonstrated almost the same results for watered and drought-exposed plants. Similar 2-fold increase was observed also in the formation of MDA - the main product of lipid peroxidation in plant tissues at the early stages of active development. Further, in seed formation stage of ontogenesis, in both variants synthesis of MDA slows down to initial values observed in leaf-tube formation stage.
During all stages of the plant ontogenesis NADP-MDH activity was always high in the chloroplast fraction of genotype I (Fig. 2). The same behaviour was also observed under drought conditions. The enzyme activity significantly decreased in both control and drought-exposed plants during maturation phase. The results are consistent with protein data obtained from gel-electrophoresis (Tab. 2).
The enzyme activity vs. substrate OAA and cofactor NADPH was examined in watered and drought exposed plants. As seen in table 3 and figure 3 (A) K m (OAA) and V max for the enzyme activity in control plants were found to be 3.03 mM and 1.8 EU∙mg-1, respectively, in the chloroplast fraction of the genotype I. These results obtained for cofactor
NADPH were 0.75 mM and 6.25 EU∙mg-1. In drought exposed genotype I, K m and V max for OAA increased almost twice (6.67 mM and 4.0 EU∙mg-1, respectively). For NADPH these values (1.0 mM and 5.26 EU∙mg-1) were very close to the data obtained in control plants (Tab. 3). The comparison of the obtained data indicates a non-concurrent relations between substrate OAA and cofactor NADPH, and thereby the presence of sufficient concentrations of NADPH in the reaction medium positively affected the course of the reaction.
In the cytosolic fraction isolated from genotype I leaves, K m (OAA) decreased from 8.3 (control) to 6.7 mM (drought) leading to 2-fold increase in the reaction rate from 0.77 to 1.41 EU∙mg-1 under drought conditions. For NADPH the results for K m and V max were 7.69 mM and 4.2 EU∙mg-1 in watered and 4.0 mM and 1.8 EU∙mg-1 in drought exposed plants, respectively.
Genotype II demonstrates the following values (Tab. 3, Fig. 3B) of the enzyme activity. In the chloroplast fractions K m (OAA) was 5.0 mM in control plants and this value decreased to 4.0 mM for drought exposed plants, with the adequate decrease in V max (from 2.0 to 1.33 EU∙mg-1). These results match the data obtained for K m and V max for the enzyme cofactor-NADPH (0.75 mM and 10.0 EU∙mg-1 for control, and 0.61 mM and 5.0 EU∙mg-1 under drought).
However, in the cytosolic fraction of genotype II (Tab. 3) drought caused the increase in K m from 16.7 mM (control) to 33.3 mM (OAA) and from 3.7 mM (control) to 5.0 mM (NADPH), while V max in drought exposed plants changed differently for OAA
(decreased from 2.78 (control) to 2.38 EU∙mg-1) and NADPH (increased from 1.0 (control) to 5.0 EU∙mg-1).
Studying the reaction catalyzed by NADP-MDH we observed synchronic changes of kinetic parameters in chloroplast fractions in flag leaves of genotype I and II and we established that the rate of the reaction catalyzed by NADP-MDH increased according to the chemical kinetics until the temperature of the medium reached 40-50°C (Tab. 3). A gradual increase in the temperature up-to the optimum (40-50°C) and increase in the kinetic energy of the enzyme molecule led to the increase of the reaction rate. Exceeding the optimum temperature gradually increased the partial denaturation of the enzyme, which completely lost activity at 70°C.
The activities of the enzymes are known also to be dependent on pH. They usually express high activities in narrow pH range. However, in our study NADP-MDH was found (Tab. 3) to have larger pH optimum interval (pH 7.5-8.8) at normal physiological state (watered plants) as well as in drought-exposed plants and the initial reaction rate was strongly dependent on the substrate concentration.
In our study experiments were carried out to clarify the role of some activators (Mg2+) and inhibitors (ATP) in the regulation of the enzyme activity. Figure 4 shows that values of Km were close in chloroplast fractions of mesophyll cells from flag leaves of genotype I in both watered and drought-exposed plants (3.33 mM and 3.03 mM, respectively) under the influence of Mg2+ (MgCl 2 ) ions, whereas ~1.4-fold increase in V max occurred under drought (9.09 EU∙mg-1) compared to the watered plants (6.45
EU∙mg-1). In the chloroplasts of the drought sensitive genotype II, ~1.5-fold decrease in V max was observed under drought (K m =3.12 mM; V max =0.43 EU∙mg-1) compared with the watered plants (K m = 2.78 mM; V max = 0.67 EU∙mg-1). In the cytosolic fraction of genotype I, K m and V max was 7.69 mM and 0.77 EU∙mg-1 for watered, and 5.56 mM and 1.43 EU∙mg-1 for drought exposed plants, respectively. For genotype II these values were 7.69 mM and and 0.83 EU∙mg-1 for control, and 8.0 mM and 0.63 EU∙mg-1 for drought exposed plants.
We found useful to verify inhibitory effects of ATP in the chloroplast fraction of genotypes I and II grown under different conditions. In the chloroplast fractions obtained from watered and drought exposed genotype I, K m values were 0.85 mM and 0.77 mM, respectively (Fig. 5). While V max decreased ~3.6 times under drought (1.82 EU∙mg-1) compared with the well watered plants (6.67 EU∙mg-1). Similar behaviour was also observed in the drought sensitive genotype II. Thus, the K m and V max values of the chloroplast fractions in control and drought variants were 0.94 mM and 4.0 EU∙mg-1, and 0.78 mM and 2.0 EU∙mg-1, respectively. K m and V max values were 0.91 mM and 2.44 EU∙mg-1 for control and 0.7 mM and 1.25 EU∙mg-1 for drought-exposed plants obtained for the cytosolic fraction of genotype I. For genotype II these data were 0.95 mM and EU∙mg-1 in control plants and 1.2 mM and 5.0 EU∙mg-1 for drought exposed plants.
Thus these data confirm the inhibitory effect of Mg2+ and ATP on NADP-MDH in both wheat varieties. However, effects of inhibitors were differently manifested for genotypes I and II.
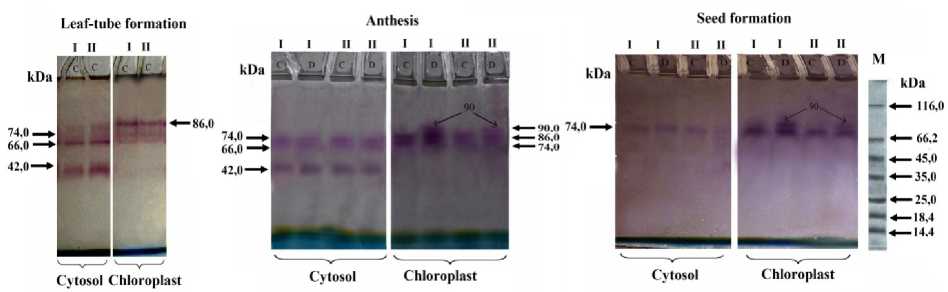
Figure 1. Determination of NADP-MDH isoforms using gel-electrophoresis in cytosol and chloroplasts of mesophyll cells at different stages of ontogenesis in genotypes I and II. There is no drought at the leaf tube formation stage. C – control (watered), D – drought; M – marker proteins.
Table 1. Isoforms and subunit content of NADP-MDH in leaves of genotypes I and II at anthesis
|
Genotypes |
Variants |
Subcellular fraction |
Number of isoforms |
Mr of isoforms, kDa |
Number of subunits |
Mr of subunits, kDa |
|
C |
Cyt |
3 |
42, 66, 74 |
2 |
21, 33, 37 |
|
|
Chp |
3 |
66, 74, 86 |
2 |
33, 37, 43 |
||
|
I |
D |
Cyt |
3 |
42, 66, 74 |
2 |
21, 33, 37 |
|
Chp |
3+1 |
66, 74, 86, +90 |
2 |
33, 37, 43, +45 |
||
|
C |
Cyt |
3 |
42, 66, 74 |
2 |
21, 33, 37 |
|
|
Chp |
3 |
66, 74, 86 |
2 |
33, 37, 43 |
||
|
II |
D |
Cyt |
3 |
42, 66, 74 |
2 |
21, 33, 37 |
|
Chp |
3+1 |
66, 74, 86, +90 |
2 |
33, 37, 43, +45 |
Note: (+90)-molecular weight of the inductive isoform, (+45)-molecular weight of the monomer of inductive izoform. C ‒ control (watered), D ‒ drought
The numbers and M r of subunits were determined according to Laemmli (1970).
Table 2 . Biochemical and physiological parameters of wheat genotypes I and II under drought
|
Gen |
Var |
Protein mg/g FW |
RWC, % |
Chl a/b |
Proline, (µmol (g FW)‒1 |
MDA, nmol (g FW)‒1 |
|
Leaf-tube formation |
||||||
|
I |
C |
84.00±1.57 |
82.00±0.62 |
2.22±0.09 |
12.50±1.53 |
0.093±0.007 |
|
D |
- |
- |
- |
- |
- |
|
|
II |
C |
64.00±0.91 |
79.40±0.56 |
2.560±0.084 |
14.00±1.23 |
0.094±0.005 |
|
D |
- |
- |
- |
- |
- |
|
|
Anthesis |
||||||
|
I |
C |
110.0±3.5 |
80.0±1.5 |
2.330±0.087 |
26.00±0.85 |
0.177±0.057 |
|
D |
127.5±2.8 |
64.0±1.2 |
2.030±0.056 |
27.50±0.82 |
0.181±0.068 |
|
|
II |
C |
61.0±1.1 |
79.70±1.25 |
2.59±0.08 |
24.30±0.51 |
0.260±0.070 |
|
D |
72.5±0.9 |
63.0±1.0 |
2.130±0.098 |
19.00±0.20 |
0.230±0.078 |
|
|
Seed formation |
||||||
|
I |
C |
66.00±0.87 |
69.20±0.64 |
2.040±0.069 |
40.00±0.72 |
0.076±0.005 |
|
D |
48.0±1.0 |
31.30±0.30 |
1.980±0.032 |
41.50±0.70 |
0.096±0.004 |
|
|
II |
C |
40.00±0.85 |
67.60±0.50 |
1.930±0.011 |
80.00±1.02 |
0.076±0.001 |
|
D |
38.00±0.80 |
27.40±0.46 |
1.820±0.010 |
62.00±0.80 |
0.149±0.006 |
|
|
Note: Gen – |
genotypes, Var – |
variants, RWC – relative water content, Chl |
– chlorophyll, MDA – |
malone dialdehyde. |
||
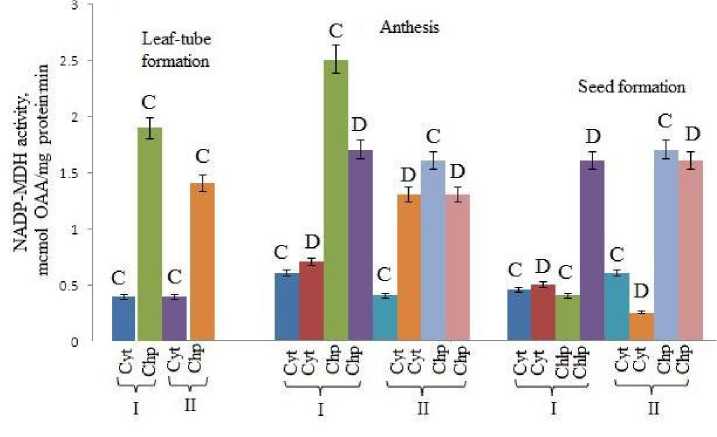
Figure 2. Dynamics of changes of NADP-MDH activity in cytosolic and chloroplast fractions of mesophyll cells in flag leaves during ontogenesis of genotypes I and II under drought: Cyt ‒ cytosol, Chp ‒ chloroplast. Other denotes as in figure 1.
Table 3. Some kinetic parameters of NADP-MDH in leaves of genotypes I and II at anthesis.
|
Variants |
Subcellular fractions |
pH opt |
T opt, (°C) |
OAA |
NADPH |
|
|
K m , mM |
V max , EU.mg-1 |
K m , mM |
||||
|
Genotype I |
||||||
|
C |
Cytosol |
7.5-8.5 |
40-50 |
8.3 |
0.77 |
7.69 |
|
Chloroplast |
7.5-8.8 |
3.03 |
1.8 |
0.75 |
||
|
Cytosol |
7.5-8.5 |
40-50 |
6.7 |
1.41 |
4.0 |
|
|
D |
7.5-8.8 |
6.67 |
4.0 |
1.0 |
||
|
Chloroplast |
||||||
|
Genotype II |
||||||
|
C |
Cytosol |
7.5-8.5 |
40-50 |
16.7 |
2.78 |
3.7 |
|
Chloroplast |
7.5-8.8 |
5.0 |
2.0 |
0.75 |
||
|
Cytosol |
7.5-8.5 |
40-50 |
33.3 |
2.38 |
5.0 |
|
|
D |
7.5-8.8 |
4.0 |
1.33 |
0.61 |
||
|
Chloroplast |
||||||
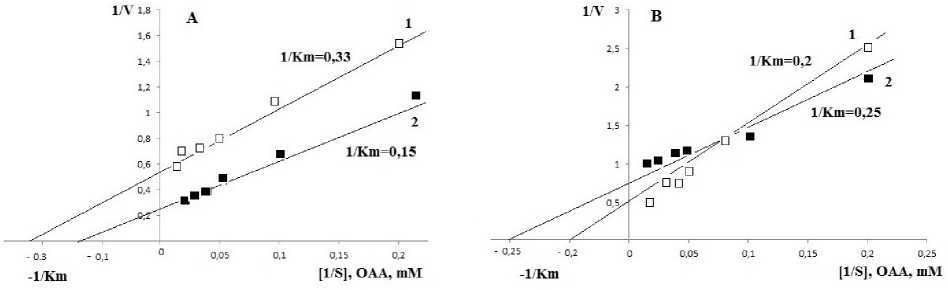
Figure 3. Kinetics of the alterations of the reaction rate catalyzed by NADP-MDH relative to the concentration of OAA in chloroplast fractions of genotype I (A) and II (B): 1 ‒ watered, 2 ‒ drought
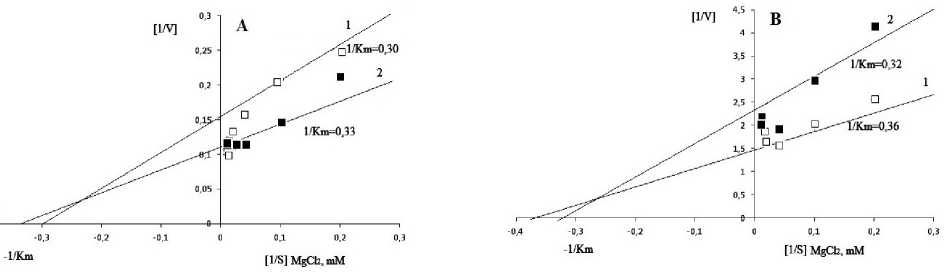
Figure 4. Kinetics of the activation effect of MgCl 2 on NADP-MDH activity in chloroplast fractions of flag leaf mesophyll cells of genotype I (A) and II (B): 1 ‒ watered, 2 ‒ drought.
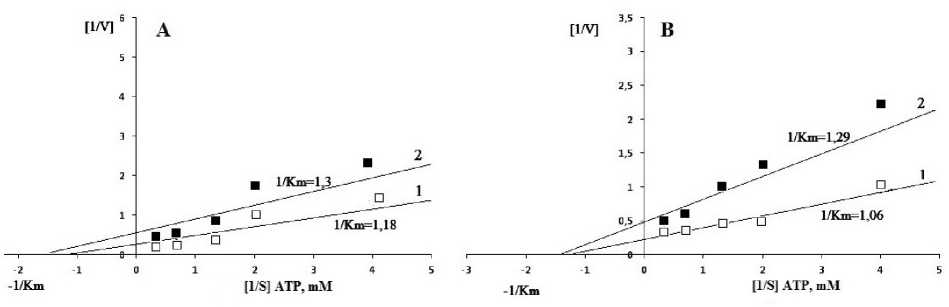
Figure 5. Effect of ATP different concentrations on NADP-MDH activity in chloroplast fractions of flag leaf mesophyll cells of genotypes I (A) and II (B): 1 ‒ watered, 2 ‒ drought.
DISCUSSION
Water deficit is considered as one of the main factors forming natural distribution of plants, and their growth, evolution, development and physiology (Dudley, 1996). In the present study we have carried out a comparative analysis of sub-cellular localization, isoenzyme spectrum and kinetic characteristics of NADP-malate dehydrogenase of two durum wheat genotypes with different drought tolerance.
The results show that, in spite of the negative effect of water stress on photosynthesis native structure of the enzyme and its molecular weight, with the exception of appearence of the 90 kDa inductive izoform in both drought-resistant and -sensitive genotypes at anthesis stage, did not change significantly. However, NADP-MDH demonstrates a high activity in chloroplast fractions of leaves. Moreover, at anthesis stage the drought resistant genotype shows a higher activity of the enzyme than the activity compared with the drought sensitive one. Furthermore, despite some fluctuations observed in the enzyme activity of the cytosolic fraction at different stages of development, its activity was significantly lower in this fraction than in the chloroplast fraction. Therefore, it can be assumed that drought responses are regulated by chloroplast reactions.
The obtained results showed the pronounced differences between drought tolerant genotype I and drought sensitive genotype II in rate parameters of the reaction catalyzed by NADP-MDH. The reaction rate catalyzed by NADP-MDH is higher in droughtresistant genotype I than in genotype II in both subcellular fractions. Due to low Km and high Vmax values the rate of the catalytic reaction in the chloroplast fraction of genotype I was higher than that of genotype II. These parameters equally increased further under drought. A slight decrease in Km and Vmax in the chloroplast fraction of drought-exposed genotype II showed different responses of these genotypes to drought. This suggests that because of the low adaptive capacities genotype II completes its vegetation faster than genotype I under water deficiency. Oxaloacetate and malate, which are the substrates of the enzyme in direct and reverse reactions, are competitive inhibitors for each other. The reaction is feedback inhibited by malate. Inhibitory effects of malate were more pronounced in plants exposed to prolonged soil drought.
Thus, a wide isoenzyme spectrum of NADP-MDHs differing in their subcellular distributions, mobilities, catalytic activities and protein amounts was appeared in leaves of two Triticum durum Desf. genotypes with distinct drought tolerance. It demonstrates a functional ability of the genotypes to survive in the adverse conditions. We can conclude that plant responses to environmental changes are related to specific mechanisms, including the formation of new isoforms of MDH-system enzymes which are different in chloroplast and cytosolic fractions depending on environmental conditions.
ACKNOWLEDGEMENTS
This work was supported by the Science Development Foundation under the President of the
Republic of Azerbaijan – Grant EIF-2012-2(6)-39/19/3.
Список литературы NADP-malate dehydrogenase isoforms of wheat leaves under drought: their localization, and some physicochemical and kinetic properties
- Agostino A, Jeffrey P, Hatch MD. (1992) Amino acid sequence and molecular weight of native NADP malate dehydrogenase from the C4 plant Zea mays. Plant Physiol., 98: 1506-1510
- Ashton A., Hatch M. (1983) Regulation of C4 photosynthesis: physical and kinetic properties of active (dithiol) and inactive (disulfide) NADP-malate dehydrogenase from Zea mays. Arch. Biochem. Biophys., 227: 406-415
- Bates L.S., Waldren R.P., Teare I.D. (1973) Rapid determination of free prоline for water stress studies. Plant Soil., 39: 205-207
- Buchanan B.B. (1980) Role of light in the regulation of chloroplasts enzymes. Annu. Rev. Plant Physiol., 31: 341-374
- Chaves M.M., Oliveira M.M. (2004) Mechanisms underlying plant resilience to water deficits: prospects for water-saving agriculture. J. Exp. Bot., 55: 2365-2384
- Chaves M.M., Pereira J.S. et al. (2002) How plants cope with water stress in the field. Photosynthesis and growth. Ann.Bot., 89: 907-916
- Cornic G., Massacci A. (1996) Leaf photosynthesis under drought stress. In: Baker N.R., ed. Photosyntesis and the environment, Kluwer, Dordrecht, The Netherlands. p. 347-366
- Davis B.J. (1964) Disk electrophoresis. II. Method and application to human serum proteins Ann. N.Y. Acad. Sci., 121: 404-427
- Dudley S.A. (1996) Differing selection on plant physiological traits in response to environmental water availability: a test of adaptive hypotheses. Evolution, 50: 96-102
- Edwards G.E., Andreo C.S. (1992) NADP-malic enzyme from plants. Phytochemistry, 31: 1845-1857
- Ferte N., Jacquet J.P., Meunier J.C. (1986) Structural, immunological and kinetic comparisons of NADP-dependent malate dehydrogenase from spinach (C3) and corn (C4) chloroplasts. Eur. J. Biochem., 154: 587-595
- Fickenscher K., Scheibe R. (1988) Limited proteolysis of inactive tetrameric chloroplast NADP-malate dehydrogenase produces active dimers. Arch. Biochem. Biophys., 260: 771-779
- Fieldes M.A. (1992) Explanation of the achromatic bands produced by peroxidase isozymes in polyacrilamide Electrophoresis gels stained for malate dehydrogenase. Electrophoresis, 13: 82-86
- Gietl C. (1992) MDH isoenzymes. Cellular localization and role in the flow of metabolites between the cytoplasm and cell organelles. Biochim. Biophys. Acta, 1100: 217-234
- Goward C., Nicholls D. (1994) Malate dehydrogenase: a model for structural, evolution and catalysis. Protein Sci., 3: 1883-1888
- Hatch M.D. (1987) C4 photosynthesis: a unique blend of modified biochemistry, anatomy and ultrastructure. Biochim. Biophys. Acta, 895: 81-106
- Häusler R.E., Hirsch H.J. et al. (2002) Overexpression of C4-cycle enzymes in transgenic C3 plants: a biotechnological approach to improve C3-photosynthesis. J. Exp. Bot. 53: 591-607
- Heath R., Pasker L. (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichometry of fatty acid peroxidation. Arch. Biochem. Biophys., 125: 189-198
- Jacquot L.P., Buchanan B.B. et al. (1981) Enzyme regulation in C4 photosynthesis: purification and properties of thioredoxin-linked NADP-malate dehydrogenase from corn leaves. Plant Physiol., 68: 300-304
- Johansson K., Ramaswamy S. et al. (1999) Structural basis for light activation of a chloroplast enzyme: the structural of sorghum NADP-malate dehydrogenase in its oxidized form. Biochemistry, 38: 4319-4326
- Johnson H.S., Hatch M.D. (1970) Properties and regulation of leaf nicotinamide-adenine dinucleotide phosphate-malate dehydrogenase and «malic»-enzyme in plant with the C4-dicarboxylic acid pathway of photosynthesis. Biochem. J., 119: 273-280
- Kagawa T., Bruno P.L. (1988) NADP-malate dehydrogenase from leaves of Zea mays: purification and physical, chemical and kinetic properties. Arch. Biochem. Biophys., 260: 674-695
- Laemmli U.K. (1970) Cleavage of structural proteins during assembly of the head of bacteriophage. Nature, 77: 680-683
- Lizana C., Wentworth M. et al. (2006) Differential adaptation of two varieties of common bean to abiotic stress. I. Effects of drought on yield and photosynthesis. J. Exp. Bot., 57: 685-697
- Luchetta P., Cretin C., Gadal P. (1990) Structure and characterization of the Sorghum vulgare Gene encoding NADP-malate dehydrogenase. Gene, 89: 171-177
- Luchetta P., Cretin C., Gadal P. (1991) Organization and expression of the two homologous genes encoding the NADP-malate dehydrogenase in Sorghum vulgare leaves. Mol. Gen. Genetics, 228: 473-481
- Merlo L., Ferretti M. et al. (1993) Developmental changes of enzymes of malate metabolism in relation to respiration, photosynthesis and nitrate assimilation in peach leaves. Physiol. Plant., 89: 71-76
- Miginiac-Maslow M., Issakidis E. et al. (1997) Light-dependent activation of NADP-malate dehydrogenase: a complex process. Aust. J. Plant Physiol., 24: 529-542
- Passioura J. (2007) The drought environment: physical, biological and agricultural perspectives. J. Exp. Bot., 58: 113-117
- Quartacci M.F., Pinzino C. et al. (1995) Lipid composition and protein dynamics in thylakoids of two wheat cultivars differently sensitive to drought. Plant Physiol., 108: 191-197
- Rampino P., Pataleo S. et al. (2006) Drought stress response in wheat: physiological and molecular analysis of resistant and sensitive genotypes. Plant, Cell Environ., 29: 2143-2152
- Scheibe R. (1990) Light-dark modulation regulation of chloroplast metabolism in a new light. Bot. Acta, 103: 327-334
- Scheibe R., Fickenscher К. (1985) The dark (oxidized) from the light-activatabie NADP malate dehydrogenase from pea chloroplasts in catalytically active in presence of guanidine HCl. FEBS Lett., 80: 317-320
- Scheibe R., Stitt M. (1988) Comparison of NADP-malate dehydrogenase activation, QA Reduction and O2 evolution in spinach leaves. Plant Physiol. Biochem. 26: 473-481
- Sedmak J.J., Grossberg S.E. (1977) A rapid, sensitive, and versatile assay for protein using Coomassie Brilliant Blue G-250. Anal. Biochem. 79: 544-552
- Sims D.A., Gamon J.A. (2002) Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ., 81: 337-354
- Tambussi E.A., Nogues S., Araus J.L. (2005) Ear of durum wheat under water stress: water relations and photosynthetic metabolism. Planta, 19: 1-25


