Нанокомпозитные органоминеральные гибридные материалы. Часть 1
Автор: Kudryavtsev Pavel Gennadievich, Figovsky Oleg Lvovich
Журнал: Нанотехнологии в строительстве: научный интернет-журнал @nanobuild
Рубрика: Международный опыт
Статья в выпуске: 1 т.8, 2016 года.
Бесплатный доступ
The paper addresses the issues of alkoxide method of solgel synthesis and non-hydrolytic method of solgel synthesis and colloidal method of sol-gel synthesis. The authors also consider an alternative approach based on the use of soluble silicates as precursors in the solgel technology, of nanocomposites. It was shown that nanocomposites can be produced through aerogels. The paper also analyzes the mixing technologies of nanocomposites preparation. It has been demonstrated the possibility to change the types of nano-phase which is used for obtaining nanocomposites in different approaches. Various models of packaging spherical, fibrous and layered nanoparticles, introduced into the structure of the nanocomposite, in the preparation thereof were examined.
Nanocomposites, sol-gel synthesis, soluble silicates, gels, aerogels, packing of spherical nanoparticles, packing of fibrous nanoparticles, metal alkoxide, sols
Короткий адрес: https://sciup.org/14265790
IDR: 14265790 | УДК: 69.001.5 | DOI: 10.15828/2075-8545-2016-8-1-16-56
Текст научной статьи Нанокомпозитные органоминеральные гибридные материалы. Часть 1
The composite material, the composite – is inhomogeneous solid material consisting of two or more components with a clear boundary between them, which is created artificially. Most composites (except layered) components can be divided into a matrix or binder, and included in it reinforcing elements or fillers. In composites constructional purposes, reinforcing elements normally provide the necessary mechanical characteristics of the material (strength, stiffness etc.), and the matrix, in turn, enables collaboration reinforcing elements, and protecting them from mechanical damage and corrosive chemical environment. Also, composites are called multicomponent systems which consist of a polymer, metal, carbon, ceramic or other substrate (matrix) reinforced with fillers of fibers, whiskers, particles, etc. [1, 2]. The use of composite materials in various engineering applications has become almost an art.
Inorganic non-metallic materials such as glass or ceramics, people receive thousands of years from the solids using high temperatures. As raw materials, natural minerals used and the processing of these materials usually includes shredded of solid raw materials, and sintering a mixture thereof at temperatures exceeding 700оС. In particular, oxide ceramics and glass have attracted attention in the last century due to their thermal longevity and chemical inertness. They are generally is obtained from oxide minerals, by mixing with different additives to obtain specified compositions. High temperatures are usually necessary for carrying out of these solid-state reactions. This is due to the fact that the feedstock used in the form of powders, and they react in the solid state or in the melt to form the
INTERNATIONAL EXPERIENCE final product. Product formation in the solid state is possible only if ionic components of reagents diffuse through the grains of material. For this purpose they have to overcome the relatively high binding forces in crystals, and this requires an increase in temperature. Many modern electronic and optical devices require special forms or application procedures bonding of ceramic parts, and it is inadmissible to use of high-temperature treatment. Furthermore, for some ceramic products is not available application of powder technology, for example, of thin oxide films. Therefore, classic solid reactions have the following disadvantages:
-
• high temperatures and long reaction time associated with the necessity
of ions movement through the solid phase or the formation of melts,
-
• the reaction conditions and product quality are largely dependent on the conditions of raw material preparation (grinding, pressing, etc.),
-
• specific morphology, in many cases not available through classical techniques (thin films, porous materials, etc.)
-
• a combination of organic or biological materials is impossible due to the extreme conditions of materials manufacture.
These problems have been solved with the use of composite materials. A special place in this group of materials is occupied by nanomaterials and nanocomposites [2].
Nanomaterials – materials made by using nanoparticles and / or by means of nanotechnology, and have some unique properties due to the presence of these particles in the material. To nanomaterials belong objects that have one of the typical dimensions is between 1 and 100 nm [3]. There are two basic ways to create of nanoobjects:
-
1. Reduce the size of macroscopic objects (dispersing, disintegrating, grinding to the cluster level using a ball mills or using the mechano-chem-ical synthesis);
-
2. Creating nanostructures from atoms and molecules (crystallization) clustering, nanostructuring, nucleation, condensation, coagulation, polymerization, etc.
In the group of nano-materials are the following types:
-
• nanoporous structure;
-
• nanoparticles;
-
• nanotubes and nanofibers;
-
• nanodispersions (colloids);
-
• nanostructured surfaces and films;
INTERNATIONAL EXPERIENCE
-
• nanocrystals and nanoclusters;
-
• nanocomposites.
Nanocomposite – multicomponent material consisting of a base (matrix) and filler – nanomaterial surface modified and having a new and improved complex of properties. In some cases there may be an inversion nanodimension at the binder and filler.
Nanomaterials themselves are divided on the appointment to the: functional, compositional, and constructional.
By the number of measurements, they are divided into:
-
• zero-dimensional / quasi-zero-dimensional (quantum dots, spheroid nanoparticles);
-
• one-dimensional / quasi-one-dimensional (quantum wires, nanotubes);
-
• two-dimensional / quasi-two-dimensional (thin films, phase boundary);
-
• three-dimensional / quasi-three-dimensional (multilayer structures with nanoscale dislocations, superlattices, nanoclusters, nanocomposites, supramolecular compounds).
Properties of nanomaterials are usually differing from similar materials in the bulk state. For example, nanomaterials may be observed change of the optical, magnetic, thermal and conductive properties. For very fine materials can observe the change in melting temperature towards its reduction.
In this review, we discuss a particular group of nanocomposites – organic-hybrid composites. In practice, the nanocomposite materials contain reinforcing elements with an extremely high specific surface area, immersed, for example, in a polymer matrix. In this case, the organic and inorganic components form independent phases, so the contact is achieved at the phase boundary [4].
Promising modern composite materials are those in which the organic and inorganic components interact at the molecular level. They were called «polymer hybrids» [5, 6]; the concept of «hybrid» was made in order to emphasize the nature of the molecular interaction between the components.
Hybrid materials – materials produced due to the interaction of components with different chemical properties. Most often it is the organic and inorganic substances which form a certain spatial structure. These structures differ from that of the initial reagents, but often inherit certain motives and functions of the original structures.

INTERNATIONAL EXPERIENCE
Feature of the new composite materials is the fact that they have nanometer parameters of their structural elements. The size of at least one of the directions is not more than 100 nm . This is either nanometer distances between the lattices and the layers which formed by polymer and inorganic ingredients or nanometer size formed of particles including particles containing metals [7].
As inorganic compounds – precursor – typically used: oxides of silicon, aluminum, titanium, zirconium, vanadium, molybdenum, clays, layered silicates and zeolites, phosphates, and metal chalcogenides, iron oxychloride, graphite, various metals, etc. As the polymer component used carbochain and organometallic polymers, usually silicone polymers.
From an environmental point of view are optimal drainless methods of obtaining composite materials, in particular, sol-gel or spin-on-glass process. This method is allows to exclude multiple washing steps, as used as starting material a compound without introducing impurities into the final product composition [8].
Sol – a colloidal dispersion of solid particles in a liquid. Colloids – this is a suspension in which the dispersed phase is so small (1 : 1000 nm ), that the gravitational forces may be neglected. Here are dominant short-range forces, such as van der Waals, and also the Coulomb forces, attraction and repulsion between the surface charges. The inertia of the dispersed phase is small, so there is a Brownian motion of the particles (Brownian diffusion), ie random jumps caused by the kinetic energy imparted by the collision of the sol particles with each other and with the molecules of the dispersion medium. The important factor is that the dispersed particles are not molecules that are aggregates consisting of a plurality of molecules [10].
Colloidal gel formation occurs by a different mechanism. The particles of the dispersed phase (micelles) under the influence of attraction dispersion forces interact with each other to form a skeleton of the inorganic polymer.
The gel obtained from a polymeric sol formed during polymerization of the monomers and the polymers are in sol. In this process, gradually from polymerizable branched oligomers is formed a gigantic cluster. When the cluster reaches macroscopic size and will spread to the entire volume of the sol, said that there was a sol-gel transition. In this case, the gel will comprise, on the one hand, of a continuous structural grid – solid skeleton (core) and on the other – of a continuous liquid phase.
INTERNATIONAL EXPERIENCE
Colloidal gel formation occurs by a different mechanism. The particles of the dispersed phase (micelles) under the influence of attraction dispersion forces interact with each other to form a skeleton of the inorganic polymer. The gel comprises continuous solid and fluid phases which are of colloidal size (1 to 1000 nm ). These phases are continuous interpenetrating systems.
1. SOL-GEL TECHNOLOGY
The most studied of the sol-gel chemistry, certainly system based on silica that also appeared historic starting chemistry of sol-gel processes [21]. For the first time in 1845 Ebelmen transparent material received by the slow hydrolysis of the ester of silicic acid. In this case the formation of silica gel in the acidification of alkali metal silicates has been known to scientist’s chemists earlier, but the practical value of this process, no one gave. At the first stage of the sol-gel process, pure silica is mainly formed ceramic. In the early stages of the sol-gel process study, of pure silicon dioxide was mostly formed ceramics. However, it soon became clear that the process may also be used for the formation of other metal oxides [22]. Furthermore, it was shown that the mixture of several starting materials, allows obtaining the materials of a more complex composition. However, in such complicated systems for achieving material homogeneity, it is necessary to know the properties and behavior of each individual component in the conditions of the synthesis implementation.
With respect to other methods for synthesis of inorganic oxide materials, including nanoparticles [9, 10], the sol-gel process have a number of significant advantages [7], in particular, these include:
-
• Ensuring high purity as the starting material, and the resulting product (especially in the case of alkoxides);
-
• The homogeneity of the distribution of components, including the small modifying additives;
-
• The possibility of achieving homogeneity of the resulting compounds, which can go down to the molecular and ionic levels of the material structure;
-
• The possibility of obtaining new crystalline and amorphous phases, materials with cations in unusual oxidation States, the synthesis of which traditional methods is difficult or impossible;
INTERNATIONAL EXPERIENCE
-
• Regulation of the rheological properties of sols and nanoparticle dispersions, which allows obtaining a wide range of products ranging from coatings to monoliths.
Typically, for the implementation a sol-gel processes use two traditional approaches [10], which, however, have a number of branches:
-
• Colloidal method — hydrosols gelation occurring due to the association of particles in water suspension (for example, through hydrogen bonds between groups belonging to different particles). A variation of this method is the direct deposition and polymerization of the hydrated oxides of chemical elements from solutions of their salts, such as soluble silicates;
-
• Alkoxide method — hydrolytic polycondensation of the starting compounds in aqueous-organic media. The starting materials for this process may be alkoxides, nitrates, etc. Removal of the liquid phase from the obtained products structures is carried out either under atmospheric, or under supercritical conditions. In recent years, began to use – non-hydrolytic method. This is an alternative way which consists in the interaction a metal halide with oxygen donors – the metal alkoxide in an anhydrous medium.
-
1.1. Alkoxide method of sol-gel synthesis
There are alternative reaction scheme when forming the oxide material by precipitation [11], the hydrothermal treatment [12, 13] or using a solgel process [14, 15]. The Sol-gel process is the most interesting process, due to high technology applications in such advanced areas as thin films in electronic or optical devices [16–19]. It begins with a molecular precursors and the formation of oxide grid occurs at rather low temperatures [20]. In contrast to classical solid-phase reactions, the material formation is usually carried out in solution. Thus, reactive reagents are dispersed at the molecular level, which provides a low diffusion length of reacting substances and thus high reaction rates under mild conditions. In addition, the molecular precursors show the advantage that they can be purified by conventional methods such as rectification and chromatography. Consequently, for the formation of materials, are available very pure starting substances, which are very important in application areas such as electronics, optics, or biomedical devices.
INTERNATIONAL EXPERIENCE
One of the bases of nanotechnology is that the primary size, initial structural elements formed in a sol-gel process, is in the nanometer size range. There are several technologies, where the sol-gel process is the most advanced state of the art, for example, wear resistant or anti-reflective coatings [23, 24]. At present, this process is widely used in the production of nanoparticles [25, 26].
The sol-gel process provides control of the structure of various length scales and thus enables to form hierarchically structured materials [27]. The advantages of the sol-gel process with respect to the production of nanocomposite materials are the ability to control the mechanism and kinetics of the existing reaction steps. This allows to form the hierarchical materials, for example, to control the properties of materials ranging from the macroscopic and ending, the molecular level. Moreover, because this process takes place under mild conditions, it is possible to make modifications of materials that are not possible in case of the classical high-temperature ceramic synthesis. For example, due to the low temperature and the presence of solvent, may be included in the material structure, organic or biological components and groups. This makes it possible to carry out the formation organomineral hybrid materials or nanocomposites those exhibit properties that are completely different from conventional materials [28]. Thus, the sol-gel process is more similar to the polymerization process leading to formation of a three-dimensional ceramic structure, as in the case of formation of the polymer network. In this it differs from the classical high-temperature inorganic solid-phase process. Due to this similarity, the sol-gel process is ideally suited for the formation of nanocomposites, which contain both inorganic and organic polymer structures.
The sol-gel process is a chemical reaction which starts from an ion or molecular compound, and allows forming a three-dimensional polymeric network, through the occurrence of bridging oxo-bonds between the ions (see Fig. 1), and the release of water or other small molecules. Thus, this process is the polycondensation reaction, which leads to a three-dimensional polymer network.
When applying sol-gel process in an aqueous solution, a special kind of a radical is formed in the first stage, M–OH bond which is unstable and reacts with other types of radicals. This first step is hydrolysis. In the second step, the labile group M–OH condenses with other M–OH or M–OR (when the initial product of the sol-gel process was used alkoxides of elements) groups
INTERNATIONAL EXPERIENCE to form M–O–M bonds and elimination of water or alcohol. Thus is formed a three-dimensional lattice. Typically, the obtained intermediate not completely condensed in the process, as a consequence of steric and kinetic difficulties. They include into its structure water or OH-groups. Therefore, the products obtained correctly be classified as hydrated oxides [29, 30].
Hydrolysis:
R
R
O
OR
R
O
olMlW
R
O
„иОПOH
+ ROH
O
R
Condensation:
O
R
Or
R
O
R
O
..mmOH
O
R
R
O
R
O
иПЙ^ OH
O
R
+
+
R
O
O
HOит,п
R
R
O
O
Rm"i*T
R
R
R
HO
HO \
OH
HO
R
RR
O < O^
RR
R
O O^
HO OH
R OR
O + H2O
R
R
OR
O + ROH
R
HO
HO
Si
HO^ I
HO HO
Si
HO
Si HO
O O OSi
O HO Si HO OH
i OH
O Si Ox/OS
/ Si-Hill O
\ HO
OOH
O^ SiSi
O O "O OH OH
O Si Si O
O Si O Si
Si / OOH
O
O V
Si
O^
Si HO
O
HO Si O O
HO OH \ Si OH Si OH
HO OH
Fig. 1. The main chemical reactions occurring during the sol-gel process in aqueous solution

INTERNATIONAL EXPERIENCE
The progress of hydrolysis and condensation, leads at first to the formation of solid particles which are suspended in a liquid, the so-called sol. Particles on the condensation stages contain at their surface active groups and, therefore, they are crosslinked to gel. The gel is formed as a solid openwork net and framework which contain the liquid phase in the pores.
As a rule, the hydrolysis of silicon alkoxides is a pretty slow process. Thus, typically to accelerate the sol-gel processes are used as catalysts, acid or base. The catalysts have a significant impact on the final structure of the resulting network. Furthermore, there is also a different reactivity, with no condensed or partially condensed intermediate particles of silicic acid, which leads to the formation of various silicate structures. Stage of network forming is statistical in nature. In the result, the formed silicate structure, which is best, described using fractal geometry.
The term «fractal» was introduced by Benoit Mandelbrot in 1975, and it became widely known with the release in 1977 of his book «The Fractal Geometry of Nature» [120]. The word «fractal» is used not only as a mathematical term. Fractal is called an object that has at least one of the following properties:
-
• It has a non-trivial structure in all scales. This differs from regular geometric figures such as a circle, an ellipse, a graph of a smooth function. If we consider a small fragment of a regular figure in a very large scale, it will be like a fragment of the line. For fractal, zoom in, does not lead to the simplification of the structure, that is, at all scales, we can see the same complicated picture.
-
• It is self-similar or approximately self-similar.
-
• It has a fractional metric dimension or metric dimension that exceeds the topological dimension.
Typically, the acids as catalysts lead to extended structure similar polymers, while the base leads to a structure consisting of separate interconnected particles.
In the case of gels based on the alkoxysilanes, size, structure and crosslinking formed polymer chains depends on the ratio of SiOR in SiOH, and the rates of hydrolysis and condensation.
For understanding mechanisms of these processes, it should be considered the electronic structure of the silicon atom, and silanol and siloxane
INTERNATIONAL EXPERIENCE bonds, formed by the silicon atom. In general, there are two important differences between organic derivatives of elements of of carbon subgroups from similar derivatives of boron subgroups. Thus, the elements of carbon subgroup have low polarity of the bond the E–C (where E = Si, Ge, Sn, Pb) and octet stable configuration, at the central atom in the compounds of the binary type ER4. An important issue that has a direct relation to the mechanism of the substitution reactions, to the nature of multiple bonds, and the explanation at these elements, the presence of hypervalent compounds of the type [SiX6]2–.
Traditionally believed, that 3d-orbitals of the silicon atom are involved in hybridization (sp3d and sp3d2). However, more recent studies have shown that the d-orbitals of silicon are placed too high in energy, and do not contribute significantly to the formation of bonds. An alternative to this idea is the concept of negative hyper-conjugation [124] (Fig. 2). In the formation of «multiple» Si–O bonds in the silanolsё, antibonding orbitals σ *(Si–C) can be act as an electron acceptors.
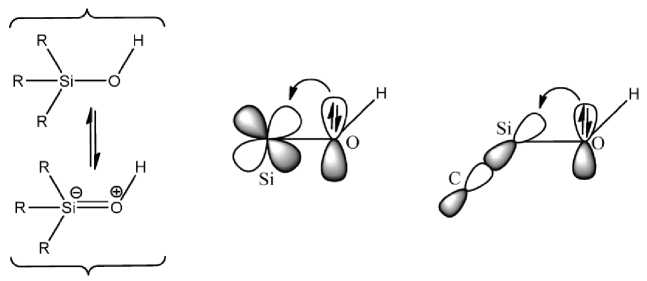
Resonance d ϖ (Si) Ђ p ϖ (O) σ *(C-Si) Ђ p ϖ (O)
Fig. 2. Negative hyper-conjugation, for example, the structure of silanol.
Increased acidity of silanol compounds compared with conventional alcohols, explains the presence of a partial π -character of the chemical bond Si–O. Another important property of the elements, starting from silicon is – hypervalency. Hypervalency is an increase of the coordination number > 4 for non-transition elements, or, in a general form, the violation of the octet rule. This phenomenon is not necessarily connected with the par-
INTERNATIONAL EXPERIENCE ticipation in the binding of nd-orbitals of the central atom. The structure of these compounds can be explained on the basis of three-center interactions, for example, three 4e3c-bonds X (o) -E (p) -X (a), in octahedral complexes EX6. The need to use d-orbitals as basis functions in the quantum chemical calculations, as hypervalent, and «ordinary» non-transition elements compounds, due to the fact that they allow taking into account the polarization of the electrons.
In the sol-gel process, an acid catalyzed in the first stage there is a rapid protonation of alkoxide group. This reaction is a nucleophilic substitution reaction. In it, the attack is carried out, by a nucleophile – reagent carrying a lone electron pair. Alkoxyl group substituted with a water molecule by the reaction scheme with the mechanism SN2. SN2 reaction mechanism or bimolecular nucleophilic substitution reaction, takes place in one step without formation of an intermediate. In this case, the nucleophilic attack and cleavage of the leaving group occurs simultaneously. SN2 reaction rate depends on both the concentration of the nucleophile and the concentration of the substrate [31]:
Га = ka X [Si(OR)J X [HO*];Гь = kb X [Si(OR)J X [OH-]. (1)
Since the reaction with a nucleophile attack, hydronium ions H3O+ or OH– ions, may occur on only one side, the result of the reaction is the inversion of the stereochemistry of the resulting product. This phenomenon may be useful in the preparation of biologically active nanocomposites, using the methods of stereoselective synthesis.
Stereoselective synthesis is also called – chiral synthesis, asymmetric synthesis, enantioselective synthesis. This is a chemical reaction in which stereoisomeric products are formed in unequal amounts. Methodology for the stereoselective synthesis plays a role in the pharmacology, because different enantiomers and diastereomers of one molecule often have a different biological activity.
Thus, the acid-catalyzed hydrolytic reactions of nucleophilic substitution are more likely occurs at the ends of the resulting oligomers with the preferred formation of linear polymers (see Fig. 3).
INTERNATIONAL EXPERIENCE
Acid catalysis:
R
OR
O— sluiO + H 3 O +
RO
R
H
R
R OR
H OR
O Si O
HO H
R
R
HR O O
O Si
O
R
+ H+
Basic catalysis:
R
g-sLG + OH -
R
HO O R
O Si O
RO
R
R
O g—sLgh + R—G
RO
R
Fig. 3. Mechanism of formation of silanol groups, depending on the catalyst which is used.
From the foregoing, it becomes clear why there is no nucleophilic substitution at the silicon atom by a dissociative mechanism (D, SN1), which was to take place through the formation of intermediate silyl-cations. Instead, it is assumed associative mechanism participation (A, SN2). This assumption is confirmed, the dependence of the rate of substitution, the nature of the attacking nucleophile, slowing the reaction in the presence of electron-donating substituents R, while also often observed, inversion of configuration, at the silicon atom. The direct use of the criterion of inversion of symmetry in silicon chemistry is not possible, because of the possible increase in the coordination number of the central atom and the rearrangement of trigonal bipyramidal intermediates, through pseudorotation. Such a frontal attack is possible, due to the presence of free silicon atom d-orbitals and low-lying antibonding σ* orbitals, which stabilize the coordination number 5. Often observed in nucleophilic substitution reactions, racemization, can lead to an erroneous conclusion about the dissociative mechanism. Actually, however, the racemization is no evidence for the formation of intermediate silyl-cation as hypercoordinate intermediates can undergo rearrangements (pseudorotation), which may lead
INTERNATIONAL EXPERIENCE to loss of chirality in the resulting inversion or preservation of chirality from a statistical probability.
In an alkaline medium, polycondensation occurs much faster, and the reactivity increases with the decreasing number of alkoxy-groups associated with, a silicon atom. Mechanism, in this case, based on the interactions of the nucleophilic hydroxyl anion, with, silicon atom, which belongs to the alkoxysilanes. The hydrolysis reaction occurs through the formation of negatively charged, an intermediate product with a coordination number of 5. The condensation of silanol groups preferably occurs not at the chain ends, and the internal centers of oligomers, which leads to highly branched, dense structure. Thus, the small spherical particles are formed.
Usually, as the catalyst used simple mineral acids or metal hydroxides, but also can also be used fluoride ions F–.
Sol-gel transition depends upon the concentration of the starting reactant, the amount of water, catalyst, temperature and pH. The final solid material has a plurality of surface OH groups, which can be stabilized by hydrogen bonds with the solvent and residual water. Furthermore, after completion of gelation in a large amount in the obtained material are present residual alkoxide groups and free OH groups that are not participating in the condensation reaction. In the aging step, these groups react with each other to form additional quantities of water and alcohols. Furthermore, in the aging process is observed substance transfer of gel particles from the outer zone to the contact zone between the particles and thus increase the size of the particles that formed a gel. The aging time has great influence on textural properties of the material. Further condensation step leads to compaction of the material and compression of the gel. The aging can be accelerated by increasing the temperature. But it can lead to crack formation in pure gels.
For subsequent applications, the gel should be dried. Removing the liquid from a gel is a sharp compression of the gel structure; as a result, a product gets most shrinkage as compared original form. Compression of the gel structure is known as syneresis. Shrinkage of the gel at syneresis can be up to 50–70% of its original size. During the syneresis occur two types of processes. First – substance transfer from the outside of the gel particles which form a gel in the inner part of their zone of contact between them. Thus, in the contact area of the particles takes place the formation of bridges between particles, which are formed out of gel material. The sec-
INTERNATIONAL EXPERIENCE ond process is determined by the movement of the particles relative to each other, with a gradual decrease in the pore spaces in the gel. This process is also caused by the transfer of substances of the gel, and certain fluidity, of the gel material.
Upon receipt of a nanocomposite volatile components must be removed out of the final material, before its application in the respective products. This process is also important to obtain a high quality material. When removing the liquid phase from the gel structure in the gel pores have a free liquid surface, and thus the capillary forces arise, they tend to destroy the gel structure. If the resulting capillary forces exceed the strength of the gel structure, is a phenomenon of decryptation – the destruction of the gel structure, due to its cracking. In some cases, even the formation of a powdered material. Therefore, the proper conduct of the operation of aging the gel, and the proper carrying out of syneresis and drying processes, provides high-quality products, in the implementation of the sol-gel process in the preparation of nanocomposites.
Typically, the feedstock for the implementation of the sol-gel process is used alkoxides of corresponding chemical elements. In the case of silicon, the most known alkoxides of following: tetra -methoxysilane Si(OCH3)4 (TMOS) and tetra -ethoxysilane Si(OCH2CH3)4 (TEOS). TMOS hydrolysis rate is much higher compared to TEOS. Thus, as a result of the reaction, methanol is obtained. It is not always acceptable alcohol in the sol-gel technology, because of his toxicity. Both agents are liquid at standard conditions, and may be purified by rectification. Typically, the substitution pattern, and hence, the organic radicals in the precursors have a large influence on the kinetics of the sol-gel process. As shown above, the use of TMOS or TEOS as precursors in the sol-gel process, with an average thermal treatment leads to a three-dimensional lattice of silica. However, the sol-gel process is well known for the production of hybrid materials that include organic functional groups which are attached to the inorganic lattice. This requires different starting materials which contain Si–OR groups and can be hydrolyzed and Si-C bonds which are stable to hydrolysis. The result of the use of such intermediates for sol-gel reaction is the introduction of the organic group into the final material. The application of this methodology makes it easy to incorporate organic functional groups into the resulting inorganic network. The result is a final material, which may bear certain organic functional groups. These groups can give to obtain materials, cer-
INTERNATIONAL EXPERIENCE tain optical or electronic properties, and modify chemical reactivity and polarity of the silica lattice. Formation of the lattice, may be possible only in a case where the precursor is used, having at least three possible locations for crosslinking. Both tetra-alkoxysilanes Si(OR)4 and tri-alkoxysi-lanes (RO)3SiR°, possess this ability (see Fig. 4). Other alkoxides of type (RO)2SiR°2 or (RO)SiR°3 can also react by hydrolysis and condensation, but the bis-alkoxides may form only chained molecules and mono-alkoxides form only dimers. If they are used in ordinary sol-gel process, only allow to modify the inorganic network. For example, if mono-alkoxysilane is connected to the surface of the silica lattice, then the result will be a certain amount of functional groups attached to the surface of inorganic substance. Although the mono-alkoxysilanes are not used in ordinary sol-gel process, they can be used for surface modification of the inorganic component by surface reactions.
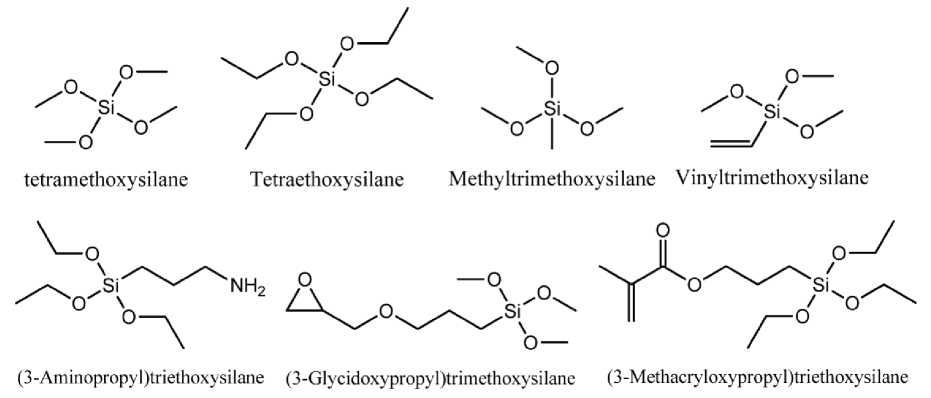
Fig. 4. Selection of commonly used alkoxysilane compounds.
Molecules that contain more than one silicon alkoxide group, for example, a system containing two or more alkoxy groups ( tri -alkoxide (RO)3Si– R°–Si(OR)3) is also used in sol-gel processes [34]. These starting substances allow comprise organic functional group directly in the lattice of solid material. This means that the organic functional groups are part of the lattice. Thus molecules of the type (RO)3SiR°, attached functional group R°, to the network formed (see Fig. 5).
INTERNATIONAL EXPERIENCE
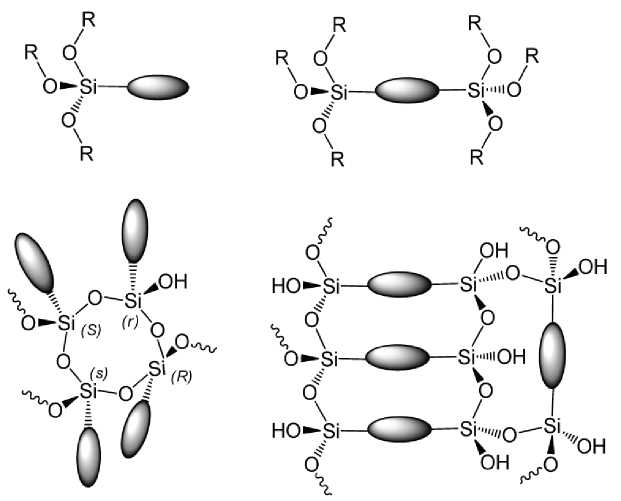
Fig. 5. The difference between the organo-silanes of the type (RO)3SiR° and (RO)3Si–R°–Si(OR)3, in the reaction of formation of siloxane-organic network.
On the one hand, in the sol-gel processes, use a mixture of tetra -alk-oxysilanes and tri -alkoxysilanes for obtain a dense of the silica lattice by their hydrolysis and condensation. On the other hand, the introduction of tri -alkoxysilanes in the reaction mixture is used to include organic functional groups into the silicate lattice and for the formation of hybrid materials.
An important parameter for optimization of the sol-gel process, are ratios of the various components, for example, the ratio of water to alkoxide C = H2O/M(OR)n; the use of catalysts; and the nature of the alkoxide precursor. The kinetics of the process can change significantly, depending on the type of the alkoxy-group used in the precursor [32].
Materials based on silicon oxide, obtained by the sol-gel process, are often porous, in connection with that the final material is a gel. Its pores are filled with solvent, water and alcohol, formed from the initial silicon alkoxide. Furthermore, the formation of gel in the process of gelation of the sol does not mean that the hydrolysis and condensation reactions are stopped in the reaction vessel. Gelation point determines only the time
INTERNATIONAL EXPERIENCE point when there is a sharp increase in viscosity of the reaction mixture due to the three-dimensional crosslinking of the sol particles, and formation an infinite cluster of them. Therefore, as a rule for obtained materials is carried out aging procedure, over time at ambient or elevated temperature. During the aging, there is a further sealing of material which is conditioned by the continuing hydrolysis and condensation reactions. As a result, the gel is shrunk. Removal of the solvent from the unmodified gel, for example by its evaporation at elevated temperature, generally results in destruction of the gel structure, and ends with the formation of the powder. The reason for this is the high capillary forces that arise during the evaporation process of the liquid phase, which destroy the filigree gel network. To eliminate or reduce this phenomenon, the liquid in the pores of the gel can be replaced by a solvent which provides a low capillary force. One such process is the exchange of solvent in the composition of the gel to a substance that is in its supercritical condition and thus may be introduced into the material directly in the gas phase. This technique is called drying in the supercritical region. Application of this method leads to the preservation of the gel structure. The thus obtained light materials called aerogels. In addition to above described morphology for the gel network, depending on the treatment conditions, can also be prepared different particles, fibers, and thin films.
If tetra -alkoxysilanes are the only ones precursors used in the formation of the structure of the silica, the obtained materials have a hydrophilic surface. Thus, these materials can actively interact with water and atmospheric moisture. This is particularly the case when the materials have high porosity, such as aerogels [35]. The hydrophilic properties of the surface can be changed, if the silanol groups on the surface are replaced with hydrophobic organic groups. This process can occur after the preparation of the material as well as the process of obtaining of the material. In the latter case it is possible, if the material is prepared by co-condensation in the presence of a second functional organic substance.
Adding the gel structure of certain functional properties, using different ways, is also an important step in the formation of nanocomposites. This is because in these materials the interfacial interaction between the inorganic and organic components plays an important role in determining what kind of material is formed – the homogeneous or heterogeneous.
INTERNATIONAL EXPERIENCE
-
1.2. Non-hydrolytic method of sol-gel synthesis
Another direction of obtaining organo-mineral hybrid materials is a non-hydrolytic method. This method is based on a non-hydrolytic reactions of hydroxylation, or aprotic condensation reactions (see Fig. 6) [4, 33]. In a particular case, this method is based on the reaction of a metal halide (MHaln) in an anhydrous medium with an oxygen donor, such as a metal alkoxide, ether, alcohol, etc. As a byproduct of, this reaction produces an alkyl halide compound.
RR
R O OR
O Si Cl + O Si O
OR O
RR
Cl R
O Si O
RO O SiO R
RR O O R
RRR
OOO
O Si Si + R Cl
ROO O
Fig. 6. Example of one of the non-hydrolytic reaction mechanisms for the sol-gel process of obtaining of inorganic oxides.
In the preparation of nanocomposites, the method is rarely used in connection with temperature limitations and consequently available only for certain types of polymers.
However, this method has several advantages:
-
• The absence of solvents;
-
• Reducing or eliminating the formation of silanol groups in the final product owing another reaction mechanism in comparison with a hydrolytic sol-gel method synthesis;
-
• Easy to achieve homogeneity of the mixture of starting substances, in particular for non-polar molecules.
At the same time it should be borne in mind that:
-
• It is necessary to take extreme caution when dealing with some highly reactive reagents, which are used in a non-hydrolytic method;
-
• The interaction of oxygen-containing molecules can be complicated by
their participation in other reactions as oxygen donor.
Nonhydrolytic method has been the subject of research in a number of studies [38–44], which were carried out to identify its advantages in order to obtain inorganic oxides. However, it extremely small used for the synthe-
INTERNATIONAL EXPERIENCE sis of organo-mineral hybrids. In 1955 has been described the synthesis of several alkyl-and aryl-modified silicates (and linear polyorganosiloxanes) by various combinations of di-methyl-di-chlorosilane, methylphenyldichlorosilane, phenyltrichlorosilane, phenyltriethoxysilane and feniletildietok-sisilana in the presence of iron chloride (III), or aluminum chloride (III), at T = 95: 100oC [36]. Checking the obtained results showed that these reactions occur by the mechanism of hetero functional, stepwise polycondensation, to form an insoluble branched organo-modified silicate. Such passage of process observed in the case when di- and tri-functional alkoxysilanes are used as the silicon-containing precursor. But additional studies of the samples were not conducted.
Nonhydrolytic sol-gel method of synthesis was studied in the formation of the organo-modified silicates (called ORMOSIL) with various organic radicals [45]. For the formation of the silica lattice were used mono- and di- substituted alkoxy- precursors with alkyl groups of various lengths, from –CH3 to –С10Н21. Although similar hybrids can be obtained, including, and hydrolytic method nevertheless nonhydrolytic approach has some advantages, especially in the synthesis of hydrophobic hybrids. For example, there is a limitation to the introduction of the compound containing –С8Н17 groups during the synthesis of the hybrid by hydrolysis, this is due to the fact that with increasing in their concentration, the observed phase separation of the mixture in the system [46]. Such problems do not arise in the process of non-hydrolytic synthesis of silicon-con-taining compounds having as substituents even С10Н21– groups. The only restriction such interactions the bulky substituents is a steric effect that can affect the rate of the condensation reaction, and the overall degree of condensation.
For example, hybrids of SiO2 – polydimethylsiloxane can be obtained as a hydrolytic or non-hydrolytic sol-gel method. Using the method of hydrolytic sol-gel synthesis, can be obtained materials which exhibit a different degrees of hardness [47]. Properties of the resulting materials depend on the ratio of precursors, and may vary, ranging from solid and up to rubber-products [48]. With non-hydrolytic process may be synthesized hybrid materials based on silicon-containing compound and polydimethylsiloxane, using as catalyst iron chloride (III). Thus, reaction products do not have elastic properties, even when the content of siloxane of 50%.
INTERNATIONAL EXPERIENCE
Benefits of colloidal method in comparison with an alkoxide method are as follows:
-
• The use of ready-made, aggregately stable sols of polysilicic acid with different particle sizes from 5 to 100 nm ;
-
• Low cost of silicon-containing precursor;
-
• The ability to use various modifying agents that promote changes in: adhesion, strength, electrical and other properties of the resulting material.
The term «colloidal silica» refers to stable dispersions of discrete particles of amorphous silica (SiO2). It is usually considered hydrophilic sol because particles stabilized by «solvation» or «hydration». Such a definition excludes from this group, the solutions of polysilicic acids in which the polymer molecules or particles are so small that they are unstable. In aqueous solution, the silica at t = 25 oC and pH 7 exists as Si (OH)4 and its solubility is about 0.001 wt. %. At pH 2, it is increased by 1.5 times, and at pH 10 – nearly 10-fold [49]. When the monomer concentration in solution exceeds the value corresponding to the equilibrium solubility, and there is no solid phase on which soluble silica might be precipitate, then the monomer is polymerized by polycondensation [50]. As a result, the polycondensation of low molecular weight silicic acid sol the germinal is formed, and takes place growth of its particles. Aggregation of the particles does not occur if the electrolyte concentration is less than 0.1 ^ 0.2 N, depending on the silica concentration. In silica sols, the free energy of interfacial interaction amorphous silica-water is 50 erg/cm2 [50].
Silica gels are synthesized from molecular silicon-containing precursors. Two general methods are used to initiate of gelation water glass solution:
-
1. Acidification or partial neutralization of a sodium silicate solution by adding Bronsted acids.
-
2. Replacing the sodium ions Na+ on hydroxonium ions H3O+ using an ion exchange resin in acid form, forming thereby a solution of silicic acid
INTERNATIONAL EXPERIENCE and initiating gelation by addition of a Lewis base (F ) or Bronsted base (OH–).
The method (1) is a so-called single stage process. Adjusting the pH to a value between 5 and 9, is equivalent to the partial neutralization of sodium silicate. Typically, for the description of this process use the term acid catalysis. Strictly speaking, this is only partially true, because the addition of an acid serves as the primary purpose partial neutralization of the alkaline solution of sodium silicate and a decrease in pH. The method (2) is a classic two-stage process. The terms used in describing the various steps of preparing of the sol and the gel formation is often found in the literature for systems based on liquid glass. Subsequently, let us consider the formation of silica gel from a liquid glass. The two main steps in this process are neutralization and condensation. Figure 7 shows the neutralization of the silicate with the formation of silicic acid H2SiO3. In the second stage, respectively, is shown as are formed of dimeric particles by reaction with one equivalent of the silicic acid (A) or sodium silicate (B).
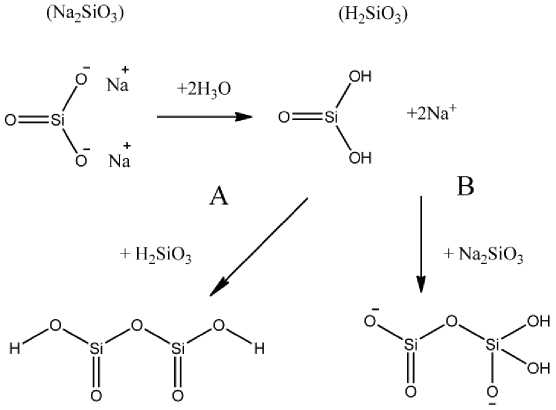
Fig. 7. Acidification of the sodium silicate molecule to produce a silicic acid and reaction with another molecule (A) of silicic acid or (B) of sodium silicate [76].
The main step in the formation of a gel – is the collision of two silica particles having a relatively low surface charge. When the particles come into mutual contact between them are formed siloxane bonds, which irre-
INTERNATIONAL EXPERIENCE versibly hold the particles together. For formation of such connection, it is necessary, or the catalytic action of hydroxyl ions, or dehydration particle surface at higher pH values. This is confirmed by the fact that the rate of the gel formation at pH 3.5 increases with pH and is proportional to the concentration of hydroxyl ions. At pH < 6, the lack of hydroxyl ions is not anymore a factor that limits the rate of gelation. However, aggregation rate is reduced due to reduction in the number of collisions between particles, due to the increased amount of charge on their surface. The overall result of the simultaneous action of these two effects is the highest rate of the gelation at pH 5. As soon as between the particles forming siloxane bonds begins further deposition of silica at the contact point due to a negative radius of curvature of the surface, the resulting particles [51]. This process goes fast above pH 5 and slowly at pH 1.5. The rate of gelation, appears proportional to the total surface area of the silica present in a given volume of the sol, and increases with increasing temperature. Substantial data relating to the activation energy of the particle aggregation can only be obtained when the particles have already completed their growth, and stabilized at a higher temperature than provided for in the experiments. Below pH 3.5, the presence of salts of weakly affects the rate of gelation, whereas water-miscible organic liquids like alcohols slow this process.
Once the sol turns into a gel, at first, increases viscosity of the system, since the particles bonded together to form branched chains that fill the whole volume, and then, the gel solidifies. In this case, you must always bear in mind that such a network, by capillary structure can hold a significant amount of fluid.
Liquid glass can be subdivided by type of alkali cations on the sodium, potassium, lithium, organic bases. By mass or molar ratio in the glass: SiO2 and M2O, where M – is K, Na, Li, or an organic base. In this case, the molar
INTERNATIONAL EXPERIENCE ratio SiO2/M2O called – «silicate module» of liquid glass – n. Secondary characteristic of liquid glass is the content of SiO2 and M2O in wt.%; content of impurity components: Al2O3, Fe2O3, CaO, MgO, SO42–, etc., and its density (g/cm3). The chemical composition of the liquid glasses is characterized by the content of silica and other oxides, regardless of the specific form of their existence in the solution. In some countries in characteristic of liquid glasses also include the solutions viscosity.
Sodium liquid glasses typically produce within the silicate modulus values of from 2.0 to 3.5, with the density of the solutions from 1.3 up to 1.6 g/cm3 . Liquid glasses based of potassium have the silicate modulus values in the range of 2.8 : 4.0 with a density of 1.25 : 1.40 g/cm 3 [52-55].
Acid resistant building materials based on liquid glass are widely used in construction as a silicate polymer concretes, putties, fillers, etc. Soluble sodium silicates (liquid glass) are used as binders for the production of heat-resistant and chemically resistant materials. Liquid glass have high cohesive strength, easy and safe, has a low cost, does not are subject to corrosion, not inflammable volatile components were evaporated and do not adversely affect in the environment of use.
A new trend in the technology of ceramics and inorganic composites, in recent years, intensive development has received, is the use of sol-gel processes to form materials directly from solutions sols. Naturally, in the first row of such materials are the products based on the silica sol, which in this case are a continuation of a number of liquid glasses while striving to infinity silicate module [56].
Practical use of liquid glasses is realized in the following directions. The first direction is the manifestation in the liquid glass binding properties – the ability to self-hardening to form an artificial of silicate rock. The unique ability of the liquid glass is its high adhesive properties to substrates of different chemical nature. In these cases, the liquid glass acting as a binder for a chemical gluing different materials used in coatings and production of the composite materials of wide application.
The second direction involves the use of liquid glasses as a soluble source of silica, i.e. raw source component for the synthesis of various siliceous materials – of silica gel, white carbon, zeolites, catalysts and carriers for them, silica sol, etc.
The third area relates to the use of alkali metal silicates, as chemical components in various substances. This direction provides for the use liq-
INTERNATIONAL EXPERIENCE uid glass in the manufacture of synthetic detergents, for bleaching and cloth dying, in papermaking, etc.
Liquid Glass – alkaline solutions of sodium and potassium silicates are representatives of a wider class of water-soluble silicates and liquid glasses produced on an industrial scale. The group of water-soluble silicates includes crystalline anhydrous sodium and potassium silicates, and crystalline and amorphous sodium and potassium hydrosilicates in the form of powders, etc. Amorphous powders hydrosilicates of alkali metals [57], characterized by compositions within the SiO 2 /M 2 O = 2.0 : 3.5, when the content of bound water is 15 : 20%. Such powders are usually obtained by spray drying the concentrated liquid glasses and high hydration of glassy silicates. They are loose, quickly dissolved in hot and cold water. Crystalline hydrosilicates manufacturing, usually represented crystalline hydrate disubstituted sodium orthosilicate Na2H2SiO4, containing from 4 to 9 molecules of crystalline hydrate water. This is also known as hydrated metasilicate formulas Na2O · SiO2· 5H2O and Na2O · SiO2· 9H2O.
The above products – liquid glass, glassy silicates, hydro silicates in crystalline and amorphous state – are so-called low-modulus silicates with a molar ratio SiO2/M 2 O = l : 4. The need to improve certain properties of composite materials based on them, such as water resistance and thermal properties have led to the development of «high-modulus liquid glass» – polysilicates of alkali metals. Polysilicates group includes alkali metal silicates (silicate module 4 to 25), representing the transition region of compositions from liquid glass to silica sol stabilized by alkali [50]. Polysilicates have a wide range of polymerization degree of the anions and they are colloidal silica dispersions in an aqueous solution of alkali metal silicate. Synthesis and practical application of polysilicates as the binder allowed filling the space existing among the alkali silicate binders are thus three groups represented by decreasing alkalinity: soluble (liquid), glass, polysilicates, silica sols.
Relatively new field of water-soluble silicates, which found currently considerable practical output amounted silicates organic bases. The synthesis of this class of compounds is based on the ability to dissolve silica, at a pH above 11.5, in the organic bases of different nature, above all in the quaternary ammonium bases. Quaternary ammonium bases – are sufficiently strong bases for dissolution of silica in their solutions. Watersoluble silicates of this class – quaternary ammonium silicate – are char-
INTERNATIONAL EXPERIENCE acterized by the general formula [N(R1, R2, R3, R4)]2O1–nSiO2, where R1, R2, R3, R4– H, alkyl-, aryl-, or alkanolgroups [70,71].
Quaternary ammonium silicate solutions – it is usually highly siliceous lipophilic stable dispersion systems in which the silica is present in colloidal forms, and forms specific to true solutions. They often produce in those cases when the sodium or potassium analogues of such systems are not sufficiently stable [50]. The dissolved silica in such systems is an oligomer with a polymerization degree of 10 : 25, the particle size of the colloidal silica increases from 2 to 100 nm depending on the value of the silicate modulus in the range n = 2 : 12. Greatest practical application was found lower alkyl- and alkanolderivatives – tetrabutylammonium silicate, tetraethyl silicate, tetraethanolammonium silicate. Absence of alkali metal ions in this group of water-soluble silicates, and the ability to control a wide composition of organic bases, have opened up new areas of application such water-soluble silicates that differ significantly from traditional applications.
Thus, the group of liquid glasses – alkali silicate solutions is very extensive. Included in this group of silicate systems are classified by the following features.
By degree of polymerization ( l ) silica – average number of silicon atoms forming the siloxane bonds continuous system = Si-O-Si = during polymerization. In the polymerization of silica occurs increase of its molecular weight ( M ), and at high degrees of polymerization of – increasing the size ( d ) of colloidal silica particles. At a certain degree of polymerization (l) in the alkali silicate systems appears colloidal silica as a sol or as highly dispersed hydrated silica:
|
Lower Monomers Ђ oligomers |
Colloidal silica, Ђ Higher oligomers Ђ sols |
|
(l = 1) (l = 1 : 25) |
(polysilicic acids, (М > 105 or, М < 105) d > 2 nm) |
According to chemical composition with increasing alkalinity, alkali silicate system characterized by a certain molar ratio SiO2/M2O (silicate system module n ), and form a series corresponding to the four previously listed forms of silica:
INTERNATIONAL EXPERIENCE
|
Overbased Ђ systems |
qu Ђ Polysilicates Ђ Sols glasses |
|
(n < 2) |
(n = 2 : 4) (n = 4 : 25) (n > 25) |
The type of cation liquid glass is divided into potassium, sodium, lithium silicate and silicates of organic bases. Synthesize mixed liquid glass inside these four groups [53].
Processes that occur during the curing are complex and diverse. Modern view of the general idea of hardening liquid glass itself and in the various homogeneous and heterogeneous systems, the most commonly encountered in practice, presented in several reviews [50, 53, 58]. System based on liquid glass, acting as adhesive or binding material changes from a liquid to a solid state, in many ways. They can be divided into three types:
-
1) The loss of moisture by evaporation at ordinary temperatures;
-
2) Loss of moisture from the system, followed by heating above 100 o C ;
-
3) The transition to the solid state by introducing specific reagents, which are called curing agents. Naturally, these three types are used in combination.
In solution the degree of polymerization of silicate anions is known to depend on two factors – the silica modulus and the solution concentration. Each solution has a distribution of degree of polymerization of at anion. These two factors determine the distribution of the degree of polymerization of the anions and imposed on him the charge distribution of the anions.
One method of curing of liquid glasses is a process of curing under ordinary temperatures due to the removal of moisture. Processes occurring in the silicate solution are regulated by two reversible reactions:
= SiOH+OH- о = SiO-+H 2 O;
= SiOH + = SiO- о = Si-O-Si = + OH-. (2)
Polymers formed by the second reaction are preferably spherical structure, and are formed during polymerization as colloidal particles charged negatively [50, 54]. Therefore, they do not come together to interact, if not created the conditions for coagulation. Dimensions of colloidal particles and thus their concentrations are regulated by internal distillation process.
INTERNATIONAL EXPERIENCE
It lies in the fact that the solubility of small silica particles in the solution depends on the particle size, and if the particle size is increased, solubility is decreased. During the internal distillation process, the large particles grow due to the dissolution of the smaller particles. For larger particles, their solubility is not dependent on size. Therefore, internal distillation, at a certain point, slowing down, and further, stops absolutely. This leads to some particle size distribution. This phenomenon is especially characteristic for cases where the formation a silicate solution started from monomeric particles. If a silicate solution is formed by dissolving large polymeric forms of silica, the internal distillation process may not be developed, or as a secondary process, to obtain a solution and the polymer distribution of the anions other than the first case. The internal distillation process, especially in the later stages, proceeds rather sluggishly, so old and freshly prepared solutions may be very different from each other, although the module and the concentration of the solutions are the same. Sharp dilutions of solutions or temperature change also lead to changes in the anionic composition.
If evaporate dilute solution having a large silicate unit, the liquid phase is represented only by ionic forms of silica. However, because of the hydrolysis caused by a lower concentration of hydroxyl ions in the first reaction, there will be a greater amount of ions of the type HSiO43–, but in much smaller quantities ions HSiO42–. During the evaporation of the solution will start to change in the direction of reducing the module, since the module is a solid phase are higher than the module source solution (Figure 8). Concentration of HSiO43– will be smaller, and ions SiO44– more, as it evaporates, will the emergence of new solid phases and, ultimately, would be to fall phase Na4SiO4 · m H2O.
At some concentration of hydroxide ions in a solution of ionic forms of silica hydrolysis goes so far that there are completely hydrolyzed forms have reached uncharged molecular state Si(ОН)4. If the interaction between the two ions, the second type of reaction, it is unlikely due to electrostatic repulsion, then between the molecular and ionic forms, it is possible. So may be obtained polymeric forms of silica. They are already in the early stages can take a three-dimensional structure where the silicon atoms are connected inside the Si–О–Si, and the outer atoms have at least one bond Si–OH. The latter may also exist in the ionic form SiO–. With a length of chain, equal to 4 ^ 5, there is a formation of ring structures, which subsequently acquire a three-dimensional structure.
INTERNATIONAL EXPERIENCE
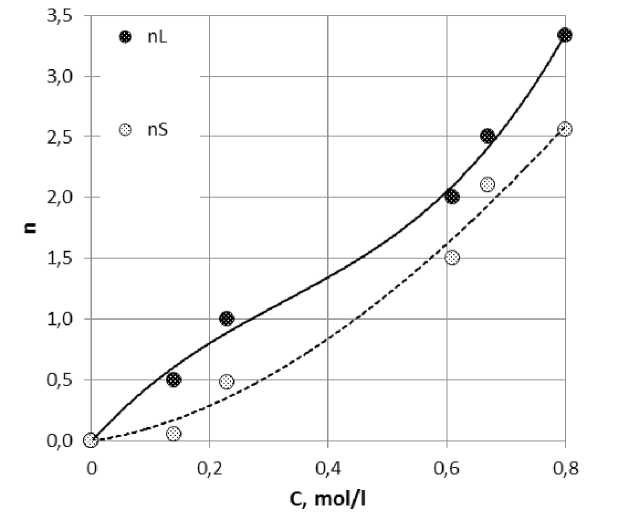
Fig. 8. The initial solution concentration of tetra -butylammonium silicate and anionic composition of crystals obtained from it [59, 60].
nL – composition of the liquid phase;
nS – composition of the solid phase.
Slow evaporation at elevated temperature increases the degree of polymerization of silicates. Therefore, for obtaining readily soluble alkali silicate powders from the viewpoint of product quality, the process is advantageously carried out at a low temperature rapidly using not very concentrated solutions. Further conversion to the hardened silicate system associated with the slow loss of hydration water in the atmospheric conditions and the absorption of carbon dioxide
CO2+OH– → HCO3–,
that causes the migration of sodium ions to the surface to form the crystalline carbonate structure, and forming a silica frame with a low water content. This leads to an increase of water resistance.
Another way of hardening of liquid glasses is a process of solidification by means of reagents. A special place among hardeners that increase module of liquid glass, take alkali metal hexafluorosilicates. Their pecu-
INTERNATIONAL EXPERIENCE liarity lies in the fact that they not only interact with the alkali, reducing its content, but also to form the silicic acid with its decomposition, which significantly plumping a hardening system, lowering its porosity. The reaction takes place between hexafluorosilicate ion and hydroxide ions according to the following conditional scheme:
SiF 6 2-+4OH- о SiO2 • 2H 2 O+6F-. (4)
This is a typical reaction of the ligand substitution in the complexes, but it is accompanied by a change in the coordination number of the silicon atom and, as often happens in such cases, complexes with mixed ligands are very unstable. The reaction is reversible and takes place in acidic media in the opposite direction. Introduction Na2SiF6 powder in sodium liquid glass, as in other cases, mixing with solid acidic hardeners immediately causes coagulation of silicate and then gelation occurs around the hardener grain surface. Therefore, usually sodium hexafluorosilicate powder is premixed with filler and then a liquid glass.
Upon receipt acid resistant concretes and putties, hexafluorosilicate sodium administered in an amount greater than needed to neutralize all the alkali of liquid glass [61]. For example, to neutralize all the alkali contained in the sodium liquid glass ( n = 3, p = l.45 g/cm 3 ), sodium hexafluorosilicate requires slightly less than 16% by weight of the glass, when n = 2, and p = 1.40 g/cm3 need 18 wt. % of Sodium hexafluorosilicate. Featured recipes offer 25 ^ 0 wt. % Na 2 SiF 6 for acid putties [50, 52-54, 61-63]. After neutralization all the alkali entering the liquid glass composition, decomposition of sodium hexafluorosilicate is completely stopped, and it is probably that the hardened system practicable simultaneous presence of Na2SiF6, and silica. It is also important to note that in an acidic environment, this reaction goes in the opposite direction when NaF, formed during the manufacture of putties, will be present in sufficient concentration in the system. Therefore washing of NaF after solidification will increase acid resistance for three reasons, firstly because of the removal of NaF, available moisture secondly because Na2SiF6, which remained in the system and enters into the reaction and thirdly due to plugging pores in the material, with the help of the resulting of silica gel. To hardener of liquid glass relate esters of light organic acids, and esters of carbonic and silicic acids are saponification by alkali of liquid glass:
INTERNATIONAL EXPERIENCE
RCOOR°+OH– → RCOO–+R°OH.
Different esters have their rate constants for the reaction. However, most of the ester hardener used, is very limited solubility in water and form a separate phase that is formed in the form of emulsion droplets. Around these droplets is formed silicate semipermeable membranes which are broken off under the action of osmotic pressure and mechanism of action of such hardeners are quite complicated. Hardener composition for each technological object must be selected by mixing various esters that slow down or speed up the process, as well as the need to experimentally select hardener dosage.
The preferred hardener is aluminum triphosphate which is a kind of solid acid having the formula:
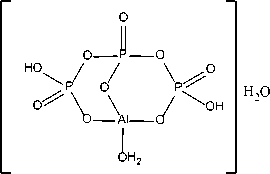
This material has been found to have no oral toxicity and no skin irritation. Aluminum triphosphate reacts with soluble sodium silicate as follows:
Na2O · xSiO2+H2AlP3O10 → Na2AlP3O10+H2O · xSiO2. (6)
The time necessary to initially form a gel after addition of the hardener to the soluble alkali silicate decreases as the amount of hardener used is increased. When aluminum triphosphate is used as the hardener, amounts of from about 3 parts by weight to about 8 parts by weight of triphosphate per 100 parts by weight of soluble alkali silicate will give initial gelling times of from 12 to 1 hours. The higher the content of hardener the shorter the useful working life of the composition will become. The amount of hardener included should therefore be chosen to provide a convenient initial gelling time consistent with the circumstances under which the composition is to be used [167].
Hardening of liquid glass may also effected by its interaction with neutral electrolytes and water soluble organic compounds [52–55]. This pro-
INTERNATIONAL EXPERIENCE cess is widely described in the technology of silica gels, but not directly used in binding systems. There are many technologies that produced structures with very different porosity, strength in the hardened state. In carrying out these processes, is controlled by their temperature change of the process, the type and concentration of added salt concentration and silica modulus liquid glass solution, the exposure time of the system at a pH in the range of weakly alkaline solutions. These studies are described in detail in the review article [50, 54].
As hardeners for of liquid glass are the compounds of calcium and other divalent metals [64–66]. Interaction of silicate solutions with calcium compounds is important in applied chemistry. Calcium silicates, which are precipitated using calcium salts from liquid glass solutions, at ordinary temperature, are amorphous substances. The crystalline products may be formed at elevated pressure and temperature in autoclaves or very dilute solutions of low-alkalinity, as well as in aging. Deposition of silicates of alkaline earth, polyvalent and heavy metals is possible, as a rule, at a pH slightly lower than the pH of precipitation of the corresponding hydroxides. Therefore, when mixing the two solutions, besides metal silicate, there is always the formation of metal hydroxides and the silica gel. Their formation always occurs to a greater or lesser amount depending on the mixing intensity. Procedure for their formation depends on the nature of the reactants. The result of the interaction of solutions of divalent and trivalent metal salts with a solution of liquid glass is silicate solution coagulation [67]. Composition of precipitated amorphous oxide flocks depends substantially on the order of draining reagents from the mixing intensity, the concentration of the solution used, and pH of the resulting reaction mixture. It may include hydroxides of silicon and corresponding metal and its silicates, with the captured anions. Such nature of the interaction observed with the majority of salts of divalent and trivalent metals. This process is called co-precipitation or co-crystallization of the hydrated metal oxide and silica, or metal hydroxide adsorption on colloidal silica, or conversely, the deposition of silica on metal oxides and hydroxides. Such interactions are widely used in hydrometallurgy and radiochemistry, for the isolation and separation of radioactive elements [68].
INTERNATIONAL EXPERIENCE
In addition to these methods of synthesis and processing, it should be emphasized that the flexibility of the sol-gel processes can increase the variety of aerogels, except silicon dioxide, aero-gels based materials such that are at the moment still available. Architecture of bulk materials can be adapted by using the template method [72]. Chemistry of gel may be modified by grafting, either before or during [73] or after gelation [74].
Composites and nanocomposites can be created by impregnating the foams or fibrous meshes, dispersing particles [75], powder [76], the polymers [77], or by synthesis of mixed oxides based on silica [78, 79], or other metal oxides [54, 56]. Organic silica hybrids [80] can also be produced using plurality techniques such as co-gelling and crosslinking [81] or by reaction with functionalized particles [82].
For the recent years has been a large body of research in the field of preparation of energetic materials. Work was carried out for application of aerogels and sol-gel derivatives, for the preparation of nanostructured composites of energetic (e.g., explosives, propellants and pyrotechnics) and studied their characteristics. Aerogels are a unique density, composition, porosity and particle size, and low temperature and mild conditions chemical synthesis techniques, all of which makes them attractive candidates for creating energy nanomaterials.
Using these materials and methods in this field of technology has led to three principal types of energy sol-gel materials [87]:
-
1) Pyrotechnics – inorganic sol-gel oxidants/metallic fuel (thermite composites);
-
2) A sol-gel derivatives of porous pyrophoric metal powders and films;
-
3) An organic sol-gel fuel/inorganic nanocomposite oxidants (composite solid propellants and explosives).
INTERNATIONAL EXPERIENCE
The behavior of all sol-gel nano energy materials to a large extent depends on several factors including the surface area, the degree of mixing between phases, the type of mixing (a sol-gel or physical mixing of solids) ways of loading solids, and presence of impurities. Sol-gel methods are attractive for the field, preparation of nanostructured energetic materials. These methods offer many options for the form, of the obtained materials, such as monoliths, powders, and films and have a wide compositional flexibility. These attributes, combined with the severity of the synthetic control of microstructural properties of sol-gel matrix, ensure the preparation of energetic nanocomposites with reconfigures characteristics.
Energetic materials are divided into three classes [88]:
-
1) Explosives;
-
2) Solid rocket propellants;
-
3) Pyrotechnic materials.
Thus, materials may be classified based on their speed of interfacial interaction of reactants and the type of the energy output. Explosives are materials that react to a supersonic velocity (detonation), and whose reaction products primarily are gaseous substances. Rocket propellants are also reacting quickly and give mainly gaseous reaction products, but react unlike explosives at subsonic speeds. Pyrotechnic materials tend to react most slowly from all three types of energetic materials and generate high-temperature, solid reaction products and few gas, and thereby generating an intense visible light output.
At least the past two decades, the field of nano researches was one of the most active areas of research in various scientific disciplines, and energetic materials were not an exception to this [88, 96]. Nano energy composites were synthesized through the use of nano-materials and advanced manufacturing techniques, which are promising opportunities. Nano Energy composites are defined as a mixture of oxidizer and fuel particles which have dimensions or at least one critical dimension of less than 100 nm [97]. Reducing the size increases the surface area of contact between the phases of reactants. This has been achieved using a variety of methods, including vapor condensation [98], micellar synthesis, chemical reduction, ultrasonic mixing [99], as well as mechanical mixing methods [100]. Have been received very good results [101]. For example, for the pyrotechnic nanocomposites Al/MoO3 were fixed burning rates by almost three orders of magnitude higher than conventional mixtures [102]. Such properties of energetic materials as sensitivity to im-
INTERNATIONAL EXPERIENCE pact or shock, depends on particle size. Energetic materials with smaller particle sizes may be less sensitive to ignition and thus have better properties in terms of safety [103]. These examples provide a good stimulus for the use of nanomaterials, and technologies in the energy fields. With this in mind, aerogels and other gelatinous materials obtained from sols were investigated in the last decade as a nanostructured of energetic materials.
Along with good miscibility energy nanocomposites have extremely high surface area interface. The sol-gel method of obtaining these materials enables more large interfacial contact areas. All these favorable attributes have led to active research on the use of sol-gel chemistry to research and development of energetic materials.
Organo-mineral nanocomposites based on silica aerogels possess a complex of unique optical properties. The refractive index of the airgel modified with tri -methylsilyl groups may be in the range of 1.008-1.06, depending on their densities. Figure 9 shows the relationship between the density and the refractive index TMSA aero-silica-gel. The relative value of the index of refraction n , is almost proportional to the density of airgel material in a range of high porosity. This result corresponds to the theoretical ratio of the Maxwell-Granat, as applied to nanocomposites formed organically modified silica and air [89].
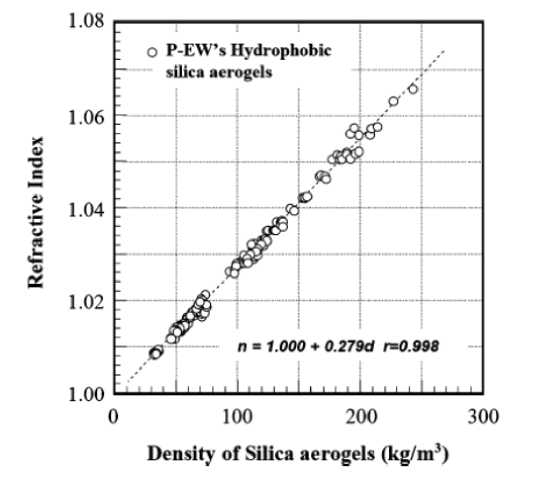
Fig. 9. The correlation between the density and the refractive index of the hydrophobic silica aerogels [35].
INTERNATIONAL EXPERIENCE
Since modified aerogels have excellent optical properties, transparency, extremely low index refractive index and moisture resistance, they are often used as media in Cerenkov counting. When a charged particle passes through a transparent medium at a speed faster than the speed of light in the material, there is a glow of Cherenkov radiation [90]. Although monolithic blocks of silica airgel produced by supercritical drying methods are quite expensive for industrial applications, they have greatly contributed to progress in such scientific fields as high-energy physics. The progress of science has always contributed to the improvement of research and development in the industrial world, so we can expect that the airgel can be a pioneer of new technologies, such as nanocomposites, optics, space exploration, energy devices, and so on [87, 91, 92].
Active work is being done in the field of nanoscale engineering of composites based on silica to create a variety of sensors [85, 93, 94]. In [93], they are described in «Composites silica – modified silica», prepared by modification of the silica gel after gelling, base-catalyzed, with another silica sol, this time prepared using acid catalysis. This base-catalyzed, acid-modified gel is then treated with carbon dioxide supercritical extraction method to obtain the airgel. Airgel monoliths obtained as a result of this process are the bulk properties of silica aerogels prepared base catalysis, including a high level of transparency, however, at the same time with the surface properties are more typical of the airgel obtained by acid catalysis. Consequently, it is possible to catch various kinds of strongly polar molecules, including acid-base indicators, and use them as an interface to the respective sensors.
In [85], reported the composite aerogels silica containing colloidal metal particles (gold or platinum) and which have optical transparency of silica aerogels, combined with the surface and optical properties of the metallic colloid. Metal colloidal particles are uniformly distributed throughout, the volume of the mixture and hence are isolated from each other. At the same time, the porosity of the silica matrix makes these metal colloid particles available for the particles that pass through the matrix. The surface of the metal colloid may be modified, either before or after gelling, in order to adapt it to the optical properties of the material.
Subsequently, this method was applied to the preparation of airgel monoliths doped protein cytochrome c [94]. In the buffer, the protein forms a superstructure containing thousands of individual protein mole-
INTERNATIONAL EXPERIENCE cules around a colloidal gold particle. The modified particle of gold is reacted with TMOS catalyzed base, sol to obtain a composite material prepared as described in [85]. Despite the fact that fragments of cytochrome c in the outer part of the superstructure are damaged during the process of exchange and solvent extraction, most of the internal proteins that survived the extraction process without change, in environment such as a buffer around the gold particles. These monoliths airgel retained some reactivity of cytochrome c, as shown by their response to the presence of NO in the gas phase, the presence of which was monitored by a change in optical density over time.
It should be noted that the relatively low temperature process using carbon dioxide supercritical extraction is of great importance for the conservation of protein function in this application. When using a fast process, supercritical extraction, one should not expect comparable results, because this protein is not withstand the higher temperatures required for the implementation of such a drying process.
In [86] described the preliminary results, which demonstrate that the inclusion of nanofibers of polyaniline in silica aerogels obtained on the basis of TMOS and carbon dioxide supercritical extraction, leads to an increase in strength of materials. Thus there is the possibility of their potential application for the detection of gaseous acids and bases. It has been found that including in the introduction, only about 6% of polyaniline by weight of the material, was increased strength airgel three times, in obtaining a material such as low density (0.088 g/cm3 ). When using a gold electrode on the surface of the airgel composite was a strong decrease in resistance when the airgel is exposed to a vapor HCl.
After carrying out all stages of the synthesis process, airgel is a solid, amorphous, but it is extremely porous (75–99% porosity) material. The last step in the transformation is its densification by thermal treatment. It is often necessary to convert the material by the sintering of the airgel, a solid glass devoid of porosity, i.e., having a relative density equal to 1. Relative density – the ratio between the bulk density of the airgel, and the density of quartz glass (2.2 g/cm3 ). Fig. 10 shows a typical evolution of the relative density and the specific surface area during sintering by heat treatment. These curves are strongly dependent on the temperature of heat treatment and the content of hydroxyl groups in the airgel structure, which affect the viscosity of the airgel [93].
INTERNATIONAL EXPERIENCE
Fig. 10. Change in relative density ρ r (1) and the specific surface area S (2) for the airgel, depending on the time of sintering at 1000оС [35, 93].
Gels which are initially non-crystalline may crystallize during subsequent heat treatment. The successful formation of a glass is the result of competition between the processes which lead to densification of the material, and those which promote the crystallization [93, 94].
Follows from these data the importance of the use of nanocomposites based on silica aerogels, which is the sealing of radioactive waste from nuclear power plants. The actinides and other radioactive nuclides generated in the nuclear fuel cycle, present as salts in aqueous solutions. Using a fully open pore structure of the airgel can be filled with solutions of these salts, the entire volume of the airgel. Then, the liquid phase was removed by evaporation and the porous composite material (airgel+salt) completely sintered, leading to the synthesis of multicomponent material. The porous structure of the airgel is used as the receiving vessel. In accordance with the small pore size of the airgel, the preparation of such nanocomposites is a very simple process. The size of the domains being formed will depend on the size of the pores in the airgel, and the content of actinides and other radionuclides in the liquid phase.
However, if trying to fill an airgel with a liquid such as water, capillary forces may cause destruction of the airgel [95]. Due to the complexity
INTERNATIONAL EXPERIENCE of the texture of the airgel, a detailed calculation of the local stresses at filling it with liquid to make difficult, it depends on the surface energy of the liquid-vapor and pore size.
Thus, to avoid cracking of the material during filling, can be offered different strategies:
-
1) Synthesis of airgel with large pores that reduce the magnitude of capillary forces;
-
2) Increase the mechanical strength of the airgel due to aging and its partial sintering;
-
3) Surface functionalization, due to the imparting surface of the airgel of chelating groups.
The continuation of the paper is to be published in the issue 2/2016. The references are to be published in the issue 3/2016.
D ear colleagues !
T he reference to this paper has the following citation format :

МЕЖДУНАРОДНЫЙ ОПЫТ
МЕЖДУНАРОДНЫЙ ОПЫТ
М ашиночитаемая информация о CC- лицензии в метаданных статьи (HTML- код ):
, публикуется на условиях ...
У важаемые коллеги !
П ри использовании материала данной статьи просим делать библиографическую ссылку на неё :
D ear colleagues !
T he reference to this paper has the following citation format :


