Нейронально-глиальные мембранные контакты при пессимальной электростимуляции
Автор: Сотников Олег Семенович, Сергеева Светлана Сергеевна, Васягина Татьяна Ивановна
Журнал: Морфологические ведомости @morpholetter
Рубрика: Оригинальные исследования
Статья в выпуске: 3 т.28, 2020 года.
Бесплатный доступ
После создания способа получения межнейронных щелевых контактов в нервной системе, лишенной глии, целесообразным является воспроизведение щелевых нейронально-глиальные контактов на модели, содержащей также гибридные нейронально-глиальные щелевые контакты, которые, как известно, функционально принципиально отличаются от межнейронных контактов. Эксперименты проведены на ганглиях truncus sympathicus лабораторных крыс с помощью пессимальной электростимуляции и трансмиссионной электронной микроскопии. При электрической активации ганглиев с частотой до 100 Гц обнаружены местные и распространенные варианты разнообразных нейронально-глиальных связей (контактов, мостиков), покрытых бахромой околомембранных филаментозных белков. Они имели размытую форму пелены, маскирующую двухслойные нейромембраны. Часть контактов напоминала щелевые или плотные 5-слойные структуры без видимой межнейронной щели, но с предельным уменьшением толщины щели контактов. Главным результатом экспериментов оказалось образование, помимо щелевых, множественных септированных (лестничных) контактов. Относительно самостоятельные агрегаты электронно-плотного вещества септ располагались внутри межклеточных щелей, пересекая обе смежные мембраны, и, возможно, прободая их. Формировались и примембранные слабо очерченные пирамидо-подобные белковые конусы, связанные с обеими клеточными оболочками. Такие мембраны казались пунктирно-штриховидными, то есть не сплошными. Значительное количество септированных контактных мембран имело эндоцитозные впячивания (инвагинации), обращенные в сторону нейроплазмы с пирамидо-подобными краевыми выступами. Все реактивные измененные структуры, возникшие de novo, рассматриваются авторами как развившиеся под воздействием частотной электростимуляции денатурации и агрегации собственных и околомембранных белков.
Нейрональноглиальные контакты, пессимальная электростимуляция, септированные контакты, щелевые контакты, эндоцитоз
Короткий адрес: https://sciup.org/143177405
IDR: 143177405 | DOI: 10.20340/mv-mn.2020.28(3)35-50
Текст научной статьи Нейронально-глиальные мембранные контакты при пессимальной электростимуляции
Сотников О.С., Сергеева С.С., Васягина Т.И. Нейронально-глиальные мембранные контакты при пессимальной электростимуляции// Морфологические ведомости.- 2020.- Том 28.- № 3.- С. 35-50. mn.2020.28(3):35-50
Sotnikov OS, Sergeeva SS, Vasyagina TI. Neuronal-glial membrane contacts during pessimal electrical stimulation. Morfologicheskie Vedomosti – Morphological Newsletter. 2020;28(3):35-50. (3):35-50
Введение. В 2019 году нами был создан метод массового экспериментального образования электрических синапсов при удалении глиальных оболочек. Он был с успехом использован в физиологических опытах для обоснования механизмов частотной активации мозга и создания ее импульсной реверберации с участием только электрических синапсов [1]. Однако возникла необходимость получить щелевые аксонально-глиальные контакты с сохраненной глиальной оболочкой аксонов. Известно, что электрическая активность усиливает регенерацию поврежденных ядер мозга [2-3]. Подкорковая стимуляция волоконных сетей из премоторных отделов применяется для изучения кортикоподкорковых связей [4]. Используется также чрезкожная активация и прямая электрическая стимуляция нервов для посттравматического ускорения их роста и жизнедеятельности [5-6]. Широко используются различные модификации электростимуляции при лечении эпилепсии [7-10]. К сожалению, никаких данных о формировании нейронально-глиальных синапсов при обычной или пессимальной электростимуляции нам найти не удалось. Большинство исследований влияния нейростимуляций на нервную систему состоят в изучении химических синапсов, размеров бутонов и сом, распределения синаптических пузырьков, теории синаптического кругооборота медиаторных пузырьков. Однако, важно отметить, что во всех электрически активных зонах нормальной нейрональной мембраны, где формируются потенциалы действия (далее - ПД), непременно появляются, подобно бахроме аксоплазмы, примембранные белковые ассоциаты (рис. 1, [1]). Это и начальный сегмент любого аксона (рис. 1а) и активная зона химического синапса (рис. 1б), и обширные, покрытые рыхлой фибриллярной белковой бахромой (наш термин) контакты кардиомиоцитов, непрерывно проводящих импульсы (рис. 1д), септы перехватов Ранвье (рис. 1г). В организме, как известно, почти все тканевые клетки контактируют друг с другом. Поэтому, по-видимому, и их смежные мембраны обязательно должны быть взаимосвязанными химически и физиологически. Следовательно, множественные детальные описания одиночных мембран клеток во многом неполноценны [11]. Учитывая такое уникальное совпадение мембранных ассоциатов (агрегатов) и зоны активной генерации ПД, мы посчитали возможным попытаться получить сходные контактные структуры при одновременной частотной генерации ПД нейритов с оболочками леммоцитов в комплексе у естественного нервного волокна вне обычных активных участков мембраны, постоянно генерирующих электрические спайки. Предполагалось, что при повторной активации сходные изменения можно вызвать и во многих других участках нейритов. Фактически, была предпринята попытка экспериментального получения различных нейронально-глиальных мембранных контактов. Данные о белковом окружении смежных мембран практически отсутствуют. Соответственно, нет и достаточных данных о межклеточных отношениях между нейронами и глией. Тем не менее, морфология нервных структур, непосредственно связанных с генерацией потенциала действия четко отличается от морфологии других нервных проводников.
Многими мембранологами нервной системы помимо четких билипидных мембран неоднократно, в разных условиях демонстрировались выраженные разнообразные около-мембранные структуры нейролеммы, внешне здоровых контрольных животных, функциональное значение которых все еще остается малоизученным [12], хотя такие структуры, как дополнительные рыхлые пирамидальные выпячивания, мостики, септы и «бахрома» могут повлиять на возбудимость нейрона [13]. Возможно, описанная изменчивость нервных и глиальных мембран связана с тем, что они не бывают полностью пассивными ни в контроле, ни в эксперименте, так что электрические ионно-мембранные процессы оказываются морфологически весьма чувствительными и достаточно быстрыми, чем это часто предполагается. При электростимуляции описанные ниже структуры, возможно, весьма важны для функции нервной системы. К ним следует отнести следующие, перечисленные ниже, структуры.
Начальный сегмент аксона . Как известно, сома нейронов играет менее важную роль в формировании ПД по сравнению с начальным сегментом нейрона (рис. 1а). Наружный примембранный слой аксона имеет необычно большую толщину и в 35-50 раз более высокую плотность Na+ каналов [14]. Они прободают аксолемму и прикрепляются к цитоскелету [15-17]. Выявляется и цитолеммальная подложка, состоящая из плотного материала. Описывается сходство химического состава начального сегмента и перехватов
Ранвье [18-19]. Возможно, существенное утолщение мембраны вызывают и множественные белки [12-14]. Эти околомембранные структуры мы будем именовать термином «бахрома». В пределах бахромы из видимой в некоторых участках двухслойной мембраны иногда выявляются развивающиеся эндоцитосомы. Белковые агрегаты здесь часто образуются в цитоплазме изнутри нейролеммы [16, 20] и могут иметь обволокненную пирамидоподобную форму, содержать молекулы клеточной адгезии [13]. Выявлена продольная дистальная двигательная подвижность начального сегмента аксона [21-22].
Активная зона химического синапса . Терминальным отделом аксона, как известно, часто служит химический синапс. Здесь рождается новый спайк и здесь же, вдоль обеих активных зон мембран, располагаются субмембранные слои фибриллярных белков (рис. 1б) Внеклеточный матрикс синапса часто напоминает бахрому внутренней поверхности мембраны начального сегмента аксона [23]. Он также часто является более толстым (плотным) у внутренней поверхности постсинаптической мембраны. Длина активных зон аксодендритных синапсов может изменяться [24]. В синаптической щели отмечается содержимое, которое является более электронно-плотным, чем у нейроплазмы пре- и постсинапсов. Возможно, это остаточные белки экзоцитоза синаптических пузырьков. Около активной зоны синаптические пузырьки обладают более высокой плотностью размещения, а вдалеке от мембраны они рассредоточены. Основные работы по морфологии синапсов посвящены процессу рециркуляции эндо-экзоцитоза [25]. Так как нервная система даже, в в условяих физиологической нормы постоянно испытывает спонтанную активность, то возникает предположение, что такие повторяющиеся различия микроструктуры синапсов могут быть связаны с ее периодической импульсной активностью.
Десмосомы . При описании примембранных белков синапса нельзя не отметить строение важной белковой межклеточной мембранной структуры – десмосомы (macula adherens, рис. 1в), обладающей богатой двухсторонней примембранной бахромой. Десмосомы имеют небольшую длину, как у активных зон синапсов и поддерживаются микротрубочками [26], адгезионными белками и кадгеринами [27]. Десмосомы часто путают с активными зонами синапсов [28]. Кроме двухсторонних широких околомембранных слоев бахромы липидный бислой внутри десмосомы на 10% толще, чем соседние участки плазматической мембраны [29]. Внутридесмосомная щель может быть расширена до 220-350 ангстрем по сравнению с обычной межклеточной щелью [27]. Их структура способна собираться и разбираться [30]. Благодаря наличию густой бахромы мембраны десмосомы часто напоминают фрагменты межклеточных контактов кардиомиоцитов (рис. 1в, д). Роль таких филаментозных околомембранных окружений окончательно не выяснена.
Перехваты Ранвье . Непременные околомембранные белковые агрегаты (рис. 1г) отмечены и в области активации ПД в перехвате Ранвье [18, 31]. Безмиелиновый активный участок аксона может быть покрыт отростками глиоцитов. Авторы отмечают сходство белков компонентов перехвата и начального сегмента аксона [18-19].
Эндоцитосомы . Часто выглядят как одномембранные впячивания нейроплазмы. Эндоцитоз в нервной системе, судя по литературе, исследован на основании экзо-эндоцитоза синаптичсеких пузырьков [25, 32]. Показано, что кривизну мембраны пузырьков фактически регулирует пресинаптический белок синкулин. Важную роль играет также дефект упаковки липидов, при встраивании протеинов [33]. Предполагается также и участие в ретракции миозина-II [34]. Бахрома эндоцитов обычно располагается на цитоплазматической стороне мембраны, как и бахрома начального сегмента аксона.
Септальные контакты . У септ различных клеток имеются некоторые морфологические сходства мембран с начальным сегментом аксона [12, 18], химическими синапсами, десмосомами и контактами между кардиомиоцитами и перехватами Ранвье. Они могут распространяться вдоль межклеточной щели на значительное расстояние [35]. В септированных (лестничных) контактах так же, как и в начальных сегментах аксонов сосредоточены Na+ и K+- каналы [36]. Предполагается, что септы в норме резко снижают утечку тока по пери-аксональному пути [37]. Мембранные контакты аксона и глиолеммы
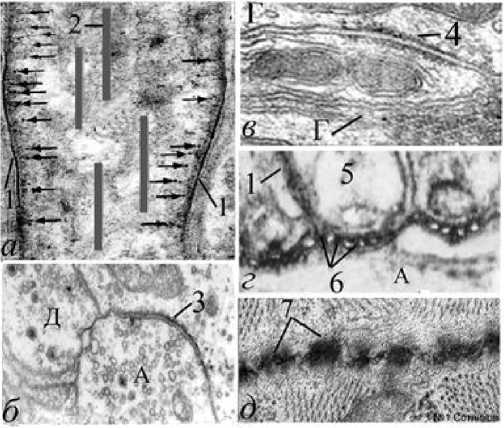
Рис. 1. Парамембранная бахрома и зоны электрической активности позвоночных. Обозначения: а - начальный сегмент аксона, генерирующий ПД (схема); б - электрически активная зона химического синапса; в –
десмосома; г - септированные нейрональноглиальные контакты перехвата Ранвье в зоне генерации ПД; д - нейронально-глиальная бахрома на границе электрической передачи двух кардиомиоцитов; 1 - толстая мембрана; 2 - микротрубочки; 3 - активная зона химического синапса (специализация); 4 – бахрома десмосомы; 5 - паранодальная глиальная петля; 6 - аксонально-глиальные септы (мостики); 7 - контакты между кардиомиоцитами (поперечный срез); стрелки - белковые субмембранные конические выступы; А - аксон; Г - отросток глиоцита; Д - дендрит. Электронная микроскопия. Ув.: б - х2000, в - х23000, г -х50000, д - х25000.
появляется только тогда, когда в мембрану
перехватов Ранвье непосредственно влияют на натриевую и калиевую проводимость волокна [38]. Белковые септы могут внедряться в толстые мембраны смежных клеток [39]. Однако, они принципиально отличаются от истинных межклеточных цитоплазматических мостиков (коллатералей) которые имеют синцитиальную связь и абсолютную проницаемость обеих контактирующих мембран [40]. У септированного контакта нет локального уменьшения ширины межклеточной щели. Описаны поперечные белковые «мостики», пересекающие нексусы, и септы сердечной трубки у дрозофил [4142] и органов других животных.
Протеины мостиков. Считается, что септы обеспечивают барьеры электрического тока [43-44]. У мутантных мышей, у которых отсутствуют септы перехватов Ранвье, снижается скорость проведения импульсов [45-46], но все-таки их функция не может считаться достаточно изученной. Роль протеинов как мостиков–септ между смежными мембранами обсуждалась неоднократно. Альберто Политов [47] сформулировал гипотезу полупроводника. Интерпретация щелевого мостика, как скопления белка с высокой специфической проводимостью, полностью совместима с тем, что известно об ультраструктуре щелевых и септированных мостиков и согласуется с точкой зрения Робертсона [47].
Минимальная проницаемость фосфолипидных искусственных мембран вообще из внешнего раствора вводятся некоторые
пептиды или белки [48]. Давно известно, что некоторые белки дестабилизируют мембраны, образуя мелкие и крупные поры [49]. Молекулы белка мембран могут внедряться в липидный слой мембран и перемещаться в нем довольно свободно [50]. Однако, о
межмембранном транзите белков судить, возможно, еще преждевременно. Анализ литературы выявляет много общего в морфологии и физиологии локусов генерации ПД. Представляется целесообразным сопоставить эти признаки с ультраструктурными особенностями мембранных нейронально-глиальных контактов, перенесших пессимальную
электростимуляцию, а также выявить возможные значимые критерии, указывающие на увеличение межмембранной проницаемости.
Цель исследования : установить микроструктурные эффекты прямой электрофизиологической стимуляции элементов нейронов и нейроглии и возможности нейронально-глиальной проницаемости контактов смежных мембран при пессимальной стимуляции с учетом того факта, что одна из контактных структур является электрически возбудимой, а другая (глиальный компонент) - невозбудимой.
Материалы и методы исследования . Объектом исследования были 35 аутбредных белых крыс линии Vistar, выращенных в стандартных условиях вивария. Эксперименты проводились в соответствии с требованиями Совета Европейского сообщества (86/609/ЕЕС) 1986 г., Решением об использовании лабораторных животных этической комиссии Института физиологии им. И.П. Павлова Российской академии наук по гуманному обращению с животными №26/12 от 26 декабря 2019 г. После препарирования симпатического ствола (truncus sympathicus) выделялся одиночный поясничный ганглий. Обе его межганглионарные ветви помещали в противоположно закрепленные стеклянные пипетки, заполненные раствором Рингера. У девяти животных раздражение осуществляли электростимулятором ЭСУ-1. Отведение тока осуществляли от коннективов с помощью изолированных стеклом серебряных электродов и усилителя УБП1-02. Ответ регистрировали на осциллографе С1-19. Препарат стимулировали прямоугольными импульсами ортодромного тока, добиваясь пессимума Н.Е Введенского [51]. Такое электрическое раздражение, как известно, приводит к деполяризации нейромембран [48]. Стимуляцию препарата осуществляли электростимулятором ЭСУ-1 в течение 10-15 минут с частотой 80-100 Гц (1-2 В, 0,2 мс). После электрофизиологического эксперимента поясничный ганглий фиксировали в течение 1 часа в 2,5 % растворе глютарового альдегида (glutaraldehyde, Acros Organics, США), приготовленного на 0,1 М какодилатном буфере pH 7,4 (Сacodilate buffer, Sigma, Израиль) при 40˚С. Далее фиксацию проводили в 1% растворе охлажденной четырехокиси осмия (Osmium tetroxide, Sigma-Aldrich, Германия). После дегидратации в растворах этилового спирта восходящей концентрации материал заливали в смесь аралдитов. Ультратонкие срезы готовили на ультратоме Leica Microsystems (Австрия) и окрашивали методом тройного контрастирования по Рейнольдсу. Общеизвестно, что при электронно-микроскопическом исследовании нервной системы четко импрегнируются и хорошо контрастируются только фосфолипидные двухслойные мембраны и протеины [52], образующие внутренние и околомембранные структуры. Это позволяет интерпретировать некоторые морфологические изменения как перестройку соответствующих химических белковых и липидных образований. Около мембранные филаментозные скопления протеинов, формирующие в экспериментах новые морфологические элементы, в последующем тексте будут именоваться авторами «бахромой». Авторы обращают также внимание на тот факт, что, используя термин «парабиоз» (т.е. в том значении, в котором он используется в настоящем исследовании) Н.Е. Введенский имел ввиду существенные, но еще прижизненные изменения нейрона (от греческого термина «пара» – около). В англоязычных же журналах, авторы единодушно используют термин «парабиоз», как экспериментальное хирургическое соединение сосудистых систем двух животных [53].
Результаты исследования и обсуждение . В контрольных препаратах симпатического ганглия осевые цилиндры, перехваты Ранвье, насечки Шмидта-Лантермана и химические синапсы имели вполне нормальное общепринятое классическое строение. В контроле, в области смежных нейрональных и глиальных мембран обнаружены только три нечетких щелевых нейронально-глиальных контакта. Это не позволяет провести их статистическую обработку. Принципиальных отличий в препаратах, раздражаемых с различной частотой, не выявлено. Однако, после электрического раздражения ганглиев, было обнаружено появление множества разнообразных нейронально-глиальных мембранных контактов. То есть, кроме нейронально-нейрональных щелевых контактов нам впервые удалось получить совершенно другим способом и разнообразные гетеромембранные контакты. В условиях электростимуляции обнаружены щелевые и плотные аксонально-глиальные контакты (рис. 2). Это были и одиночные, и серийные межмембранные связи (рис. 2б, 2е). У аксональных и глиальных мембран возникали агрегаты, связывающие их с цитоплазматическими органеллами (рис. 2г). Встречались пяти-и семислойные мембранные контакты (рис. 2д). Щелевые контакты нередко трудно отличить от плотных контактов (рис. 2е), так как межклеточные щели могут быть заполнены полностью электронно-плотным белковым материалом (рис. 2г).
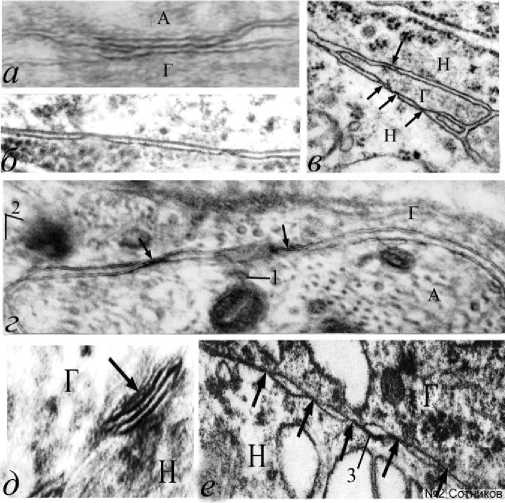
Рис. 2. Различные варианты щелевых и плотных аксональноглиальных контактов при частотной ортодромной стимуляции вегетативного ганглия крысы. Обозначения: а – комплексный щелевой и плотный контакт, расширение межклеточной щели по бокам от контакта; б – парные межклеточные контакты;
На некоторых одиночных аксонах можно встретить до 5 разновидностей нейронально-глиальных связей (рис. 3). В препаратах образуются и сравнительно длинные, и локальные контакты, напоминающие септы (рис. 3). Между ними нередко были четко видны обычные двуслойные аксональные и глиальные мембраны.
Однако нередко развивались и мембранные уплотнения, утолщающие нейрональную мембрану, которую можно называть толстой мембраной (рис. 4). По-видимому, это изменившие под влиянием электрического раздражения свою конформацию молекулы около мембранных белков. Толстая мембрана нейрита после стимуляции подобна толстой мембране электрически активного начального сегмента аксона (рис. 1а), где обычно возникает спайк, но отсутствует у мембран глиоцитов, даже если они являются смежными (рис. 4). Такие аксолеммы часто имеют нежные, плохо заметные пирамидо-подобные белковые в – множественные щелевые контакты малого глиального отростка; г – нейрональноглиальная адгезия мембраны и митохондрии; д – семислойный щелевой контакт; е – пять нейрональноглиальных контактов и вакуолизированная цитоплазма; стрелки – нейрональноглиальные мембранные контакты; 1 – белковый агрегат между митохондрией и аксолеммой; 2 – белковые агрегаты конической формы, соединенные с глиолеммой; 3 – толстая мембрана А, Г – аксон, глиоцит. Н – нейрон. Электронная микроскопия. Ув.: а – х45000; б, в, е – х23000; г – х30000; д – х50000.
агрегаты, направленные внутрь нейроплазмы (рис. 4а), а также формирующие белковые перемычки через межмембранную щель. По-видимому, это белки, денатурированные при электростимуляции.
Толстые мембраны аксона встречаются и в области перехвата Ранвье между двумя миелиновыми сегментами (рис. 4б, 4в). На внутренней стороне аксолеммы видны рыхлые конусовидные агрегаты и, связанные с ними, «тени» одиночных рыхлых эндоцитозных пузырьков (указаны стрелками), которые
также бывают заметны на внутренней поверхности аксолеммы начального сегмента нейрона (рис. 1а). Толстая мембрана аксона может формироваться дополнительными
агрегатами внутренней поверхности цистерн ЭПР, мембрана которого сливается с аксолеммой (рис. 4в). Щелевые контакты иногда располагаются между синаптическими
аксоном и дендритом, как смешанные химические и электрические аксо-дендритный синапсы (рис. 5а). Они могут быть дополнены и глиальными мостиками. Такие структуры можно назвать смешанными трехкомпонентными контактами. На препаратах отмечалось резко выраженное увеличение площади фибриллярной около аксолеммальной агрегации (бахромы) особой пирамидальной (конической) конфигурации (рис. 5а, стрелки), несколько напоминающей десмосому (рис. 1в), или межклеточные контакты ритмически возбудимых кардиомиоцитов (рис. 1д). Особенностью таких синаптических контактов является то, что у них резко увеличена плотность обеих мембран активной зоны, а пресинаптическая аксональная мембрана оказывается существенно толще, чем постсинаптическая (рис. 5а),
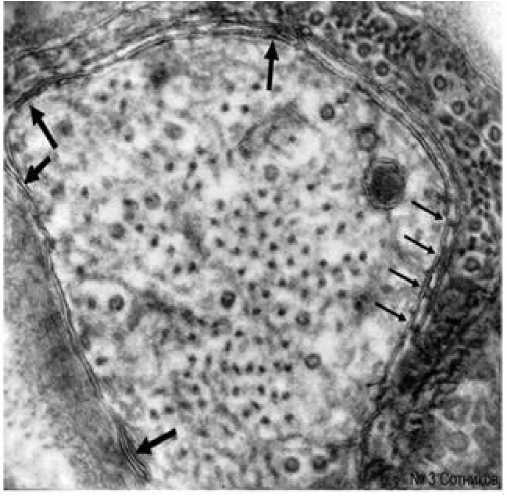
Рис. 3. Множественные гетерогенные нейронально-глиальные контакты одного аксона. Тостые стрелки - щелевые и плотные
контакты; тонкие стрелки - септы лестничного контакта. А, Г - аксон, глиоцит. Электронная микроскопия. Ув.: х 46000.
которая в норме обычно тоньше пресинаптической. Утолщение аксолемы здесь (рис. 5а, 5б) имеет слабоконтрастные, но четкие конические суб-мембранные утолщения, подобные мембране начального сегмента нейрона (рис. 1а).
Пирамидоподобные агрегаты всегда имеют в основании толстые мембраны. Поэтому их и можно считать исходным, неотъемлемым ультраструктур-ным признаком возбуждения нервных проводников. Интересно, что субмембранные фибриллярные, конические выступы всегда направлены острием к цитоплазме. Утолщение и конические формы около мембранных белков пресинапса в определенной степени объясняются агрегацией экзоцитозных синаптических пузырьков во время стимуляции, но, по-видимому, не только этим. Рядом с десмосомой, как и около химического синапса, могут встречаться точечные щелевые контакты (рис. 5а, 5в), а также септы (рис. 5а) и напоминающие по форме греческий знак «омега» - «омега»-подобные эндоциты с коническими агрегатами на мембране (рис. 5б). Появление крупных белковых межклеточных агрегатов-септ характерно для перехватов Ранвье и в норме (рис. 1г).
В наших экспериментах впервые было показано, что они являются специфическими контактными структурами и для любых смежных аксонально-глиальных мембран при частотной электростимуляции. Эти структуры нередко называют септальными лестничными контактами или мостиками (рис. 6). При электростимуляции одиночные септы нередко формируются на небольших расстояниях друг от друга, часто имеют почти одинаковые расстояния и сходные размеры. Однако их промежутки и ширина могут и значительно колебаться. Септы имеют ровные и перпендикулярно к мембранам направленные перемычки одной нейрональной или обеих нейрональной и глиальной мембран.
Появление септ - это процесс, который, по-видимому, начинается с образования бугорка агрегата, денатурированного при электростимуляции белка, на одной или двух поверхностях мембран (рис. 6в). Затем, на этот белковый бугорок-агрегат постепенно наслаиваются другие коагулирующие примембранные белки, формируя мостик. Такие четвертичные протеиновые структуры прободают обе мембраны и создают пирамидоподобную форму над предыдущими агрегатами. Этот сложный процесс мы называем аккумуляцией септ и прободением мембран. Такое ультрамикроскопическое образование четко ориентированных парных мембранных «шапочек» на молекулярном уровне, по-видимому, объясняется первоочередной денатурацией рыхлых гидрофильных апекальных спиралей, выступающих на торцах вертикальных «твердых», параллельных, гидрофобных доменов внутренних мембранных белков.
Белковые агрегаты внутри межмембранных щелей не сужают их, в отличие от щелевых контактов, которые также имеют внутри щелевые агрегаты, по-видимому, вызывающие уменьшение ее просвета (рис. 2г; рис. 5а, 5в). Необходимо отметить, что возникающие при электростимуляции септы подобны септированным контактам перехватов Ранвье, в которых также в норме возникают ПД. Мостики пересекают все мембраны, как нейролеммы, так и глиолеммы и также мембраны цистерн ЭПР, контактиру-
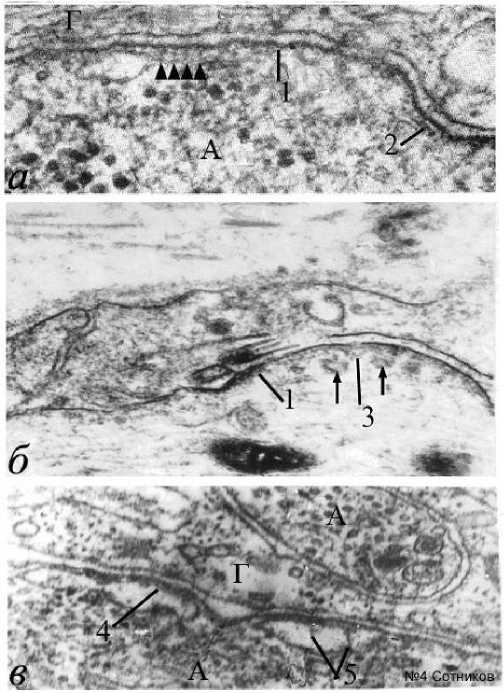
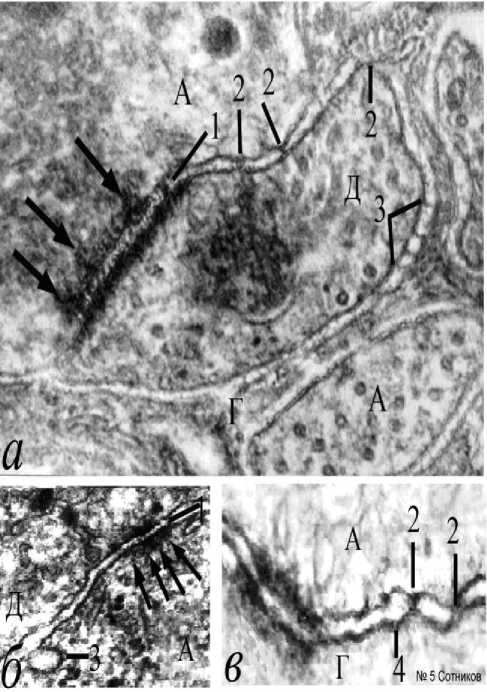
Рис. 4. Формирование толстых мембран высокой электронной плотности под влиянием электростимуляции. Обозначе-ния: а – толстая мембрана аксона; б – толстая мембрана аксона с формированием эндоцитосомных пузырьков между сегментами перехвата Ранвье; в – формирование мембраны под влиянием агрегации белка цистерн ЭПР; 1 – толстая мембрана; 2 – адгезия белковых агрегатов у мембраны; 3 – конические агрегаты под мембраной; 4 – белковые агрегаты, соединяющие аксо-лемму и мембрану цистерны ЭПР; 5 – конусовидные белковые агрегаты внутри цистерны эндоплазматического ретикулума. Стрелки – эндоцитозные пузырьки – производные мембраны; Наконечники стрелок – конусовидные агрегаты под толстой мембраной. Электронная микроскопия. Ув.: а – х50000; б – х40000; в – х60000.
Рис. 5. Десмосомоподобные белковые агрегаты (бахрома) и другие изменения нейронных и глиальных мембранных контактов при частотной электростимуляции ганглия. Обозначения: а – смешанный аксо-дендрит-но-глиальный контакт; б – совместное разра-стание бахромы обеих мембран синапса и появление эндоцитоза; в – десмосомный ак-сональноглиальный контакт, щелевые контакты и толстые мембраны. 1 – белковые перемычки (мостики) внутри синаптической щели; 2 – множественные точечные щелевые контакты; 3 – омегоподобное эндоцитозное впячивание толстой мембраны; 4 – расширение межклеточной щели у щелевого контакта; 5 – нейрональноглиальный лестничный контакт; стрелки – апексы конусовидных агрегатов аксолеммы; А – аксон; Г – глиоцит; Д – дендрит. Электронная микроскопия. Ув.: а - х50000, б – х25000, в – х55000.
ющие с аксоном (рис. 6б). Судя по препаратам, белки, по-видимому, способны не только располагаться внутри межмембранных щелей, но и прободать мембраны нарушая изоляционную структуру билипидных слоев цитолеммы. Создается впечатление, что у тонких глиальных отростков встречаются мембранные агрегаты, которые, пересекают всю глиоплазму клетки и соединяют обе собственные мембраны. В данном случае, речь идет не о вариантах структур, а о процессе постепенного роста степени агрегации дополнительных белков и перфорации мембран. При этом обязательным условием построения таких структур является дополнительное образование ими едва заметной конической формы (рис. 6д, 6е).
Одиночные самостоятельные выпячивания концов мостиков представляют собой апикальные домены агрегатов сверхдлинных базисных протеинов и, адгезированных с ними, фибриллярных примембранных белков (рис. 6г-6е). Непременным признаком электрической стимуляции следует признать появление эндоцитоза. Общеизвестно, что эндоцитоз и экзоцитоз участвуют в циклическом медиаторном процессе. К сожалению, механизм появления эндоцитозных пузырьков остается неокончательно выясненным. Однако, увеличение бахромы, особенно пресинапса, не вызывает сомнения. При электростимуляции образуются и совершенно иные крупные внесинаптические эндоцитозные «омега»-подобные впячивания нейролеммы, (рис. 7). Они часто содержат в качестве включений фрагменты глиоплазмы (рис. 7а-7в). Имея в виду это наблюдение, можно предположить, что при образовании шейки «омеги» и замыкании эндоцитозного пузырька, включения глиоплазмы могут оказаться в центре
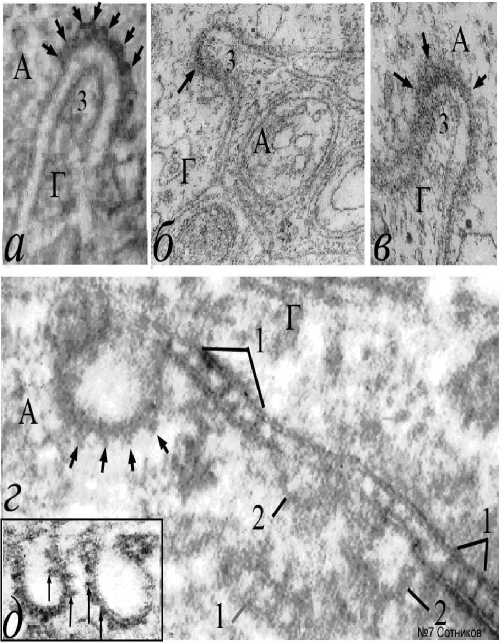
Рис. 7. Макроэндоцитоз толстых мембран в сочетании с конусовидной агрегацией мембран эндоцитосом и множественными септами протеиновых мостиков. Обозначения: а-в – варианты эндоцитоза нейритов с деформацией глиоцитов; г – септированные белковые агрегаты значительной длины, окружающие аксон; д – серийная агрегация пирамидальных белков на внутренней поверхности аксолеммы; 1 – септальные аксональноглиальные мостики; 2
эндоцитосом с плотной сердцевиной. Однако, такие завершенные стадии эндоцитоза в нашем материале не встречались. По-видимому, для объяснения механизма образования такого эндоцитоза важно отметить три структурных фактора. Во–первых, в наших опытах эндоцитоз возникает на основе толстых мембран и всегда путем
– разрушающиеся эндоцитозные пузырьки, окруженные конусовидной бахромой, примембранные агрегаты эндоцитосом; 3 – отростки глиоцитов, втянутые в дугу эндоцитосомы; стрелки – пирамидальные белковые агрегаты аксональной поверхности эндоцитосом. А – аксон; Г – глия. Электронная микроскопия. Ув.: а, в – х40000; б, г – х60000; д – х25000.
впячивания внутрь нейроплазмы, а не глиоплазмы. Во-вторых, примембранные белковые агрегаты всегда наслаиваются только на внутреннюю поверхность мембраны волокна. В– третьих, апексы белковых пирамид эндоцитозного пузырька всегда направлены радиально. Поэтому предполагается, что искривление аксолеммы, формирование «омега»-подобных впячиваний и образование эндоцитозных пузырьков вне синаптической мембраны существенно отличается от медиаторного эндоцитоза своими размерами и белковой короноподобной аксолеммальной оболочкой (рис. 7д). Можно предположить, что известные по другим препаратам образования конических выпячиваний субмембранных белковых агрегатов (рис. 4а, 4в; рис. 5а; рис. 6д, 6е), как и любая агрегация вызывает сокращение объе-
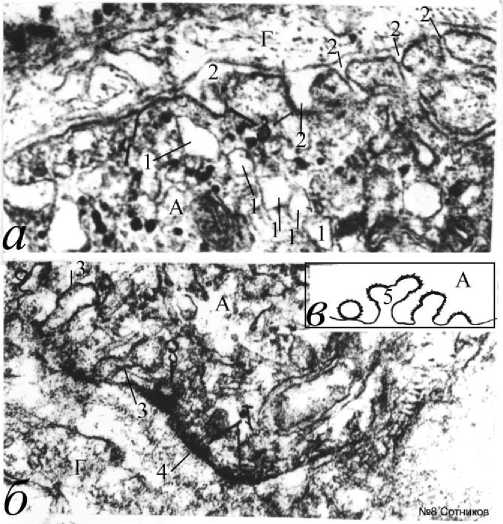
Рис. 8. Последовательный серийный эндоцитоз на основе толстой мембраны с образованием мембранных пузырьков при
элекростимуляции ганглия.
а – серийные аксолеммальные “омеги” и эндоцитозные пузырьки; б – формирование многоэтажных серийных пузырьков; в – эффект вторичных эндоцитосом (схема); 1 –
множественные последовательные пузырьки нейроплазмы, сформированные во время повторного эндоцитоза; 2 – эндоцитоз толстых мембран во время электростимуляции; 3 – повторное формирование эндоцитозных пузырьков; 4 – толстая мембрана. А – аксон; Г – глиоцит. Электронная микроскопия. Ув.: а, б – 45000.
ма и ретракцию миозина около мембранной цитоплазмы. Это способствует изгибу и образованию локальной эндоцитозной мембранной дуги, «омега»-подобной структуры с мембранной шейкой и формированием крупного пузырька. Важно, что эндоцитоз часто сопровождается появлением длинных лестнично-подобных септальных структур (рис. 7г). В некоторых участках ганглия на поверхности аксонов из толстой мембраны образуются множественные эндоцитозные впячивания в нейроплазму и непокрытые пузырьки. Они часто внутри себя не содержат впячиваний глиоплазмы (рис. 8). Эндоцитосомы могут располагаться в ряд вдоль аксолеммы. Иногда наблюдается повторный процесс формирования эндоцитосомы из мембраны уже образованного эндоцитозного пузырька (рис. 8а, 8б). В этом случае, пузырьки могут располагаться в один ряд из предшествующих пузырьков (рис. 8а). Формирование вторичного «омега»-подобного эндоцитоза из толстых мембран первичных эндоцитосом при электростимуляции, по-видимому, описано нами впервые и нуждается в специальном анализе механизма (рис. 8в).
Проведенные эксперименты по частотной пессимальной электростимуляции вегетативных ганглиев выявили несколько новых нейробиологических фактов. Так, впервые в эксперименте удалось получить щелевые контакты,
соединяющие нейрональную и глиальную мембраны, существенно отличающихся по функции, хотя и имеющих одинаковые названия. Впервые показано, что септированные (лестничные) контакты, то есть белковые ассоциаты, возможны не только в перехватах Ранвье, в которых генерируется ПД сальтаторного проведения, но и во многих других участках мембран под влиянием частотной электростимуляции. Причем, в отличие от физиологической нормы, бахрома образуется более интенсивно на стороне аксоплазмы, а не дендрита. У аксолеммы возникает несколько пирамидо-подобных протеиновых неконтрастных коагулятов. Важным признаком раздраженных мембран является также формирование множества эндоцитосом только из аксолеммы с белковыми агрегатами-септами. Основным морфологическим признаком электрически вызванной перестройки мы считаем денатурацию и агрегацию нативных внутренних и примембранных протеинов четвертичной структуры. Белковые наслоения толстых мембран с выступами конической формы непременно присутствуют на всех электрически возбудимых мембранах аксонов, напоминая сходные толстые мембраны электрически активной мембраны первого сегмента каждого аксона. Почему это присуще только для аксонов, но не для глии, пока остается неясным. Такая белковая оккупация липидных мембран и массовое агрегатное состояние белков, по-видимому, должно сказываться на электрических свойствах нейрона. Массовое прободение аксона и глиоцита ассоциированными комплексами измененных собственных центральных белков, несомненно, нарушают стройную геометрическую структуру фосфолипидных бислоев, уменьшая их значительное естественное сопротивление. Белки септ, как и другие протеины, являются полупроводниками, и пересекая обе смежные мембраны глиоцита, возможно, могут служить мостиками-шунтами между активной нейромембраной и неактивной глиолеммой ингибируя или погашая вызванную частотную импульсацию. Возможно, эта структурная перестройка является одним из факторов, отвечающих за развитие пессимума частоты в наших экспериментах.
В литературе описано большое количество химических высоко специфических центральных и около мембранных белков, участвующих в мембранных превращениях. Но в наших опытах, как и в естественных процессах (рис. 1 [1]), мы скорее наблюдаем изменения их физических свойств: коагуляцию и агрегацию дентатурированных протеинов и изменение степени дисперсности коллоидов. При этом невольно напрашивается мало цитируемая ныне физическая теория паранекроза, денатурационная теория повреждения и раздражения [54-56]. По-видимому, при электростимуляции мы имеем дело с неспецифическим свойством свертывания и развертывания структурных элементов белка [57-59]. «Сходные явления предполагаются в живой клетке, например, в процессе транспорта белков через мембраны» (цит. по [11], с. 229). Щелевые контакты считаются основным типом межклеточных электропроницаемых каналов у всех многоклеточных животных. Однако, недавно было обнаружено, что у ряда животных электрически проницаемые мембраны не имеют ни коннексинов, ни паннексинов, формирующих, как известно, такие каналы [60]. Это возможно означает, что в клеточных мембранах помимо постоянных ионных каналов могут существовать и другие проницаемые межклеточные связи. Возможно, могут существовать и спонтанные или образованные из деградирующих щелевых контактов синцитиальные поры [1] или белковые септированные (лестничные) мостики, во многих других тканях животных, образованные, как в нашем случае, множественным прободением обеих смежных билипидных мембран собственными центральными мембранными белками. Суммарная электрическая проницаемость таких полупроводников может оказаться достаточно высокой. Еще в 1972 году была продемонстрирована модель прободения липидных мембран полипептидом аламецитином с молекулярной массой 1700. Этот полипептид мог образовывать поры величиной около 6 молекул [61]. Существование зон проводимости в белковых структурах предположил еще Сент-Дьерди [62]. Обилие белковых межклеточных соединений наводит на мысль о том, что коннексиновые протеины являются неисключительными проницаемыми белками. Естественно, окончательное решение вопроса относительно электрической проводимости парамембранных белковых адгезионных ассоциаций (налипаний) толстых мембран и септированных трансмембранных аксональноглиальных образований при электростимуляции можно получить только при непосредственном экспериментальном измерении сопротивления спаренных мембран.
В настоящее время показано, что появление щелевых контактов вызывает любая травма нервной системы [1, 63-64]. Не исключено, что и частотная ортодромная электрическая активация вегетативных ганглиев в наших экспериментах также явилась причиной инициации гетерогенных аксонально-глиальных массовых контактов. Щелевой контакт, синцитиальная перфорация и любая новая электрическая связь нейролеммы с электрически неактивной глиолеммой, очевидно, должна как-то ингибировать нервные эффекты. Показано, что синцитий астроглиоцитов и олигодендроцитов ограничивает электрическую активность нервных структур, особенно быстрое периаксональное накопление ионов К+ во время высокочастотной активации [65-66]. Так коннексиновые нейрональноглиальные электрические синапсы олигодендроцитов, способствуя рециркуляции ионов К+, помогают его буферизации [67]. Астроциты, являясь третьим элементом химического синапса, активно участвуют в нейротрансмиссии ритмической активности центральной нервной системы [68] путем изменения концентрации ионов Са++. Их астроглиальные щелевые контакты участвуют в ритмической активности центральной
Морфологические ведомости – Morphological Newsletter: 2020 Том 28 Выпуск 3 нервной системы. Существует множество других примеров скоординированной взаимосвязи мембран нейрона с различными глиоцитами. Обнаружен также взаимный двунаправленный перенос липидов с помощью липид-ассоциированных белков глиальных клеток в нервные и из нейронов в глиоциты [69-70]. Так как этот процесс осуществляется, по мнению авторов, с помощью протеинов, наши данные позволяют предположить, что он может быть активирован при формировании белковых мостиков во время частотной импульсации в результате образования прямых септальных контактов, пересекающих три смежных мембраны, как показано на наших препаратах.
Известно, что агрегация белков способствует сокращению их субмембранных отделов, и, соответственно, длины мембраны. Относительно субмембранных белков это может свидетельствовать о локальном сокращении длины мембраны, то есть ее внутреннему искривлении и образования эндоцитосомы. На наших препаратах одиночные макроэндоцитосомы закономерно всегда вогнуты в сторону септальных структур аксоплазмы и конусовидных примембранных структур. В этом, возможно, состоит отличие возбудимой и невозбудимой мембран. Только у аксолеммы отмечено явление серийного эндоцитоза и возможно выявить серийные, многоэтажные эндоциты и прямолинейное расположение следующих друг за другом пузырьков. Не менее важной остается проблема нейронально-глиальной проницаемости контактов. В экспериментах было показано, что через поры щелевых контактов, помимо небольших красителей, типа процеонового желтого, в некоторых случаях проникают и Fura-2, и Calcium Green, слишком большие, чтобы преодолеть щелевой контакт [71]. При этом, как будто, коннексиновые поры, расширившись, превращаются в синцитиальные поры, как показано [1]. Отмечено также, что специфические коннексиновые блокаторы электрических синапсов не могут полностью предотвратить эффекты присутствия щелевого контакта [72]. При исследовании нокаутных животных, дефектных по коннексину-36, а, следовательно, и по щелевым контактам, показано, что способность гиппокампа к синхронизации спайков сохраняется [73-74]. Следовательно, помимо пор щелевых контактов в гиппокампе, возможно, существуют еще и синцитиальные поры. Достаточно убедительно показано, что в области так называемых высокопроницаемых контактов контактирующих мембран, встречается проницаемость через обе смежные мембраны молекул, содержащих до 1000 дальтонов [75]. Это напоминает результаты наших исследований. Некоторые около мембранные белки способны дестабилизировать структуру мембраны, образуя мелкие и крупные поры [49]. Появились и новые данные о нейронально-глиальной цитоплазматической взаимосвязи. Эксперименты свидетельствуют о возможности переноса из шванновской клетки в аксон рибосом (27 молекул белка), а также мРНК [31]. Условная связь швановская клетка–аксон названа функциональным синцитием [76-77]. Как показано, белки являются полупроводниками и в этом, возможно, состоит функция биологического импорта [67]. По нашему мнению, такой глионейрональный транзит морфологически показан через полноценные нейрональноглиальные синцитиальные перфорации [1]. Второй способ нейронально-глиального транзита можно предположить в условиях фрагментирования смежных мембран во время их прободения собственными агрегированными белковыми комплексами при электростимуляции, как показано в наших иллюстрациях. Однако, эти теоретические предположения нуждаются в дополнительных электрофизиологических исследованиях. В полученных результатах интригующим кажется прямое совпадение результатов электрофизиологических экспериментов Н.Е. Введенского и морфологических результатов соответствующих опытов цитологов школы Д.Н. Насонова. Мы, с помощью пессимальной электростимуляции, воспроизвели явление парабиоза Н.Е. Введенского [51] и, одновременно на тех же препаратах исследовали важнейшие признаки паранекроза Д.Н. Насонова [55]. Речь идет не о всех общих свойствах паранекроза протоплазмы, а только о конкретных микроскопических денатурационных признаках белков нейромембран, где снижение дисперсности сопровождается их агрегацией. В заключение, следует упомянуть о прежней дискуссии мембранологов и сторонников молекулярных конформационных белковых перестроек протоплазмы.
Заключение . Полученные данные, как и вся современная нейрофизиология основаны на принципах мембранно-ионной проводимости, но авторам, на основании результатов собственных экспериментов, вряд ли можно отрешиться от мысли, что возбудимость и нервное проведение непосредственно связаны и с состоянием конформационных изменений мембранных протеинов.
Список литературы Нейронально-глиальные мембранные контакты при пессимальной электростимуляции
- Sotnikov OS. Ob’edinyonnaya nejronno - retikulyarnaya teoriya. SPb: Nauka. 2019. 239s.
- Nekhendzy V, Davies MF, Lemmens HJ, Maze M. The role of the craniospinal nerves in mediating the antinociceptive effect of transcranial electrostimulation in the rat. Anesth Analg. 2006;102(6):1775-1780. https://doi.org/10.1213/01.ANE.0000219588.25375.36.
- Jiang F, Yin H, Qin X. Fastigial nucleus electrostimulation reduces the expression of repulsive guidance molecule, improves axonal growth following focal cerebral ischemia. Neurochem Res. 2012;7(9):1906-1914. https://doi.org/10.1007/s11064-012-0809-y.
- Schucht P, Moritz-Gasser S, Herbet G, Raabe A, Duffau H. Subcortical electrostimulation to identify network subserving motor control. Hum Brain Mapp. 2013;34(11):3023-3030. https://doi.org/10.1002/hbm.22122.
- Shchudlo NA, Borisova IV, Shchudlo MM. Morphometric evaluation of the effectiveness of post-traumatic peripheral nerve regeneration after a single and repeated courses of electrostimulation. Morfologiia. 2012;142(6):30-35.
- Cavalcante Miranda de Assis D, Martins Lima Ê, Teixeira Goes B. The parameters of transcutaneous electrical nerve stimulation are critical to its regenerative effects when applied just after a sciatic crush lesion in mice. Biomed Res Int. 2014; Article ID 2014:572949. https://doi.org/10.1155/2014/572949.
- Xu Z, Wang Y, Chen B, Xu C, Wu X, Wang Y, Zhang S, Hu W, Wang S, Guo Y, Zhang X, Luo J, Duan S, Chen Z. Entorhinal principal neurons mediate brain-stimulation treatments for epilepsy. EBioMedicine. 2016;14():148-160. https://doi.org/10.1016/j.ebiom.2016.11.027.
- Cota VR, Drabowski BM, de Oliveira JC. The epileptic amygdala: Toward the development of a neural prosthesis by temporally coded electrical stimulation. J Neurosci Res. 2016;94(6):463-485. https://doi.org/10.1002/jnr.23741.
- Inman CS, Manns JR, Bijanki KR. Direct electrical stimulation of the amygdala enhances declarative memory in humans. Proc Natl Acad Sci USA. 2018;115(1):98-103. https://doi.org/10.1073/pnas.1714058114.
- Mardani P, Oryan S, Sarihi A, Alireza K, Amir S, Dehghan S, Mirnajafi-Zadeh J. ERK activation is required for the antiepileptogenic effect of low frequency electrical stimulation in kindled rats. Rain Res Bull. 2018;14:132-139. https://doi.org/10.1016/j.brainresbull.2018.04.013.
- Finkelshtejn AV, Pticyn OB. Fizika belka: kurs lekcij s cvetnymi i stereoskopicheskimi illyustraciyami i zadachami. Moskva: KDU. 2012:456.
- Brückner G, Szeöke S, Pavlica S, Grosche J, Kacza J. Axon initial segment ensheathed by extracellular matrix in perineuronal nets. Neuroscience. 2006;138(2):365-375.
- https://doi.org/10.1016/j.neuroscience.2005.11.068.
- Ogawa Y, Rasband MN. The functional organization and assembly of the axon initial segment. Curr Opin Neurobiol. 2008;18(3):307-313. https://doi.org/10.1016/j.conb.2008.08.008.
- Bakkum DJ, Obien MEJ, Radivojevic M, Jäckel D, Frey U, Takahashi H, Hierlemann A. 2019. The Axon Initial Segment is the Dominant Contributor to the Neuron's Extracellular Electrical Potential Landscape. Adv Biosyst. 2019;3(2):pii:1800308. https://doi.org/10.1002/adbi.201800308.
- Kole MH, Ilschner SU, Kampa BM, Williams SR, Ruben PC, Stuart GJ. Action potential generation requires a high sodium channel density in the axon initial segment. Nat Neurosci. 2008;11(2):178-186. https://doi.org/10.1038/nn2040.
- Sotelo C. Development of "Pinceaux" formations and dendritic translocation of climbing fibers during the acquisition of the balance between glutamatergic and gamma-aminobutyric acidergic inputs in developing Purkinje cells. J Comp Neurol. 2008;506(2):240-262. https://doi.org/10.1002/cne.21501.
- Kole MH, Stuart GJ. Signal processing in the axon initial segment. Neuron. 2012;73(2):235-247. https://doi.org/10.1016/j.neuron.2012.01.007.
- Hedstrom KL, Rasband MN. Intrinsic and extrinsic determinants of ion channel localization in neurons. J Neurochem. 2006;98(5):1345-1352.
- Pillai AM, Thaxton C, Pribisko AL, Cheng J, Dupree JL, Bhat MA. Spatiotemporal ablation of myelinating glia-specific neurofascin (Nfasc NF155) in mice reveals gradual loss of paranodal axoglial junctions and concomitant disorganization of axonal domains. J Neurosci Res. 2009;87(8):1773-1793. https://doi.org/10.1002/jnr.22015.
- Iwakura A, Uchigashima M, Miyazaki T, Yamasaki M, Watanabe M. 2012. Lack of molecular-anatomical evidence for GABAergic influence on axon initial segment of cerebellar Purkinje cells by the pinceau formation. J Neurosci. 2012;32(27):9438-9448. https://doi.org/10.1523/JNEUROSCI.1651-12.2012.
- Grubb MS, Burrone J. Activity-dependent relocation of the axon initial segment fine-tunes neuronal excitability. Nature. 2010;465(7301):1070-1074. https://doi.org/10.1038/nature09160.
- Wefelmeyer W, Cattaert D, Burrone J. Activity-dependent mismatch between axo-axonic synapses and the axon initial segment controls neuronal output. Proc Natl Acad Sci USA. 2015;112(31):9757-9762. https://doi.org/10.1073/pnas.1502902112.
- Song I, Dityatev A. Crosstalk between glia, extracellular matrix and neurons. Brain Res Bull. 2018;136():101-108. https://doi.org/10.1016/j.brainresbull.2017.03.003.
- Mikheeva IB, Shtanchaev RS, Pen'kova NA, Pavlik LL. Structure of Interneuronal Contacts in the Neuropil of the Oculomotor Nuclei in Mouse Brain under Conditions of Long-Term Microgravity. Bull Exp Biol Med. 2018;165(4):457-460. https://doi.org/10.1007/s10517-018-4193-8.
- Gormal R, Valmas N, Fath T, Meunier F. A role for tropomyosins in activity-dependent bulk endocytosis? Mol Cell Neurosci. 2017;84:112-118. https://doi.org/10.1016/j.mcn.2017.04.003.
- Simard-Bisson C, Bidoggia J, Larouche D. A Role for DLK in Microtubule Reorganization to the Cell Periphery and in the Maintenance of Desmosomal and Tight Junction Integrity. J Invest Dermatol. 2017;137(1):132-141. https://doi.org/10.1016/j.jid.2016.07.035.
- Buck VU, Hodecker M, Eisner S, Leube RE, Krusche CA, Classen-Linke I. Ultrastructural changes in endometrial desmosomes of desmoglein 2 mutant mice. Cell Tissue Res. 2018;374(2):317-327. https://doi.org/10.1007/s00441-018-2869-z.
- Moshkov DA, Tiras NR, Pavlik LL, Dzeban DA, Mikheeva IB, Mukhtasimova NF. Structural differences between desmosome-like contacts in afferent chemical and mixed synapses of Mauthner neurons in the goldfish. Neurosci Behav Physiol. 2002;32(5):471-476. https://doi.org/10.1023/a:1019899320714.
- Lewis JD, Caldara AL, Zimmer SE, Stahley SN, Seybold A, Strong NL, Frangakis AS, Levental I, Wahl JK 3rd, Mattheyses AL, Sasaki T, Nakabayashi K, Hata K, Matsubara Y, Ishida-Yamamoto A, Amagai M, Kubo A, Kowalczyk AP. The desmosome is a mesoscale lipid raft-like membrane domain. Mol Biol Cell. 2019;30(12):1390-1405. https://doi.org/10.1091/mbc.E18-10-0649.
- Kitajima Y. Mechanisms of desmosome assembly and disassembly. Clin Exp Dermatol. 2002;27(8): 684-690. https://doi.org/10.1046/j.1365-2230.2002.01116.x.
- López-Leal R, Alvarez J, Court FA. Origin of axonal proteins: Is the axon-schwann cell unit a functional syncytium? Cytoskeleton (Hoboken). 2016;73(10):629-639. https://doi.org/10.1002/cm.21319.
- Villarreal S, Lee SH, Wu LG. Measuring Synaptic Vesicle Endocytosis in Cultured Hippocampal Neurons. J Vis Exp. 2017;127:55862. https://doi.org/10.3791/55862.
- Smurova K, Podbilewicz B. Endocytosis regulates membrane localization and function of the fusogen EFF-1. Small GTPases. 2017;8(3):177-180. https://doi.org/10.1080/21541248.2016.1211399.
- Maritzen T, Haucke V. Coupling of exocytosis and endocytosis at the presynaptic active zone. Neurosci Res. 2018;127:45-52. https://doi.org/10.1016/j.neures.2017.09.013.
- Kolosov D, Jonusaite S, Donini A, Kelly SP, O'Donnell MJ. Septate junction in the distal ileac plexus of larval lepidopteran Trichoplusia ni: alterations in paracellular permeability during ion transport reversal. Exp Biol. 2019;222(11):pii: jeb204750. https://doi.org/10.1242/jeb.204750.
- Li J. Molecular regulators of nerve conduction - Lessons from inherited neuropathies and rodent genetic models. Exp Neurol. 2015;267:209-218. https://doi.org/10.1016/j.expneurol.2015.03.009
- Rosenbluth J. Multiple functions of the paranodal junction of myelinated nerve fibers. J Neurosci Res. 2009;87(15):3250-3258. https://doi.org/10.1002/jnr.22013.
- Revenko SV, Sotnikov OS, Hodorov BI. Sravnitel'nyj analiz morfologicheskih i fiziologicheskih harakteristik perekhvata Ranv'e. Nejrofiziologiya. 1978;10(4):400-405.
- De Mello UK. Mezhkletochnye vzaimodejstviya v serdechnoj myshce. V kn.: Mezhkletochnye vzaimodejstviya, Moskva: Medicina; 1980:95-131.
- Sotnikov OS, Laktionova AA. Membrane fusion and syncytial neuronal cytoplasmic connection. Switzerland: Trans Tech Publications, Ltd; 2016. 159s.
- Lim HY, Bao H, Liu Y, Wang W. Select Septate Junction Proteins Direct ROS-Mediated Paracrine Regulation of Drosophila Cardiac Function. Cell Rep. 2019;28(6):1455-1470.e4.
- https://doi.org/10.1016/j.celrep.2019.07.004.
- Khadilkar RJ, Tanentzapf G.Septate junction components control Drosophila hematopoiesis through the Hippo pathway. Development. 2019;146(7):pii: dev166819. https://doi.org/10.1242/dev.166819.
- Ganot P, Zoccola D, Tambutté E, Voolstra CR, Aranda M, Allemand D, Tambutté S. Structural molecular components of septate junctions in cnidarians point to the origin of epithelial junctions in eukaryotes. Mol Biol Evol. https://doi.org/;32(1):44-62.DOI: 10.1093/molbev/msu265.
- Izumi Y, Furuse K, Furuse M. Septate junctions regulate gut homeostasis through regulation of stem cell proliferation and enterocyte behavior in Drosophila. J Cell Sci. 2019;132(18):pii: jcs232108. https://doi.org/10.1242/jcs.232108.
- Komada M, Soriano P. [Beta] IV-spectrin regulates sodium channel clustering through ankyrin-G at axon initial segments and nodes of Ranvier. J Cell Biol. 2002;156(2):337-348. https://doi.org/10.1083/jcb.200110003.
- Lacas-Gervais S, Guo J, Strenzke N, Scarfone E, Kolpe M, Jahkel M, De Camilli P, Moser T, Rasband MN, Solimena M. BetaIVSigma1 spectrin stabilizes the nodes of Ranvier and axon initial segments. J Cell Biol. 2004;166(7):983-990. https://doi.org/10.1083/jcb.200408007.
- Politov AL. Belkovaya poluprovodimost: alternativnoe tolkovanie elektricheskogo soedineniya. V kn.: Mezhkletochnye vzaimodejstviya. Moskva: Medicina; 1980:132-147.
- Hodorov BI. Obshchaya fiziologiya vozbudimyh membran. Rukovodstvo po fiziologii. Moskva: Nauka; 1975. 406s.
- Levin SV. Strukturnye izmeneniya kletochnyh membran. Leningrad: Nauka; 1976. 224s.
- Braun G, Uolker Dzh. Zhidkie kristally i biologicheskie struktury. Moskva: Mir; 1982. 198s.
- Vvedenskij NE. Vozbuzhdenie, tormozhenie, narkoz. V kn.: Izbrannye proizvedeniya. Moskva: Medgiz; 1952:291-395.
- Mironov AA, Komissarchik YAYU, Mironov VA. Metody elektronnoj mikroskopii v biologii i medicine. SPb: Nauka; 1994. 400s.
- Conese M, Carbone A, Beccia E, Angiolillo A. 2017. The Fountain of Youth: A Tale of Parabiosis, Stem Cells, and Rejuvenation. Open Med (Wars). 2017;12():376-383. https://doi.org/10.1515/med-2017-0053.
- Nasonov DN, Aleksandrov VYA. Reakciya zhivogo veshchestva na vneshnie vozdejstviya. Moskva-Leningrad: Izdatelstvo AN SSSR; 1940. 252s.
- Nasonov DN. Mestnaya reakciya protoplazmy i rasprostranyayushcheesya vozbuzhdenie. Moskva-Leningrad. Izdatel'stvo AN SSSR; 1962. 437s.
- Ling G. Fizicheskaya teoriya zhivoj kletki. SPb: Nauka; 2008. 375s.
- Perez-Puyana V, Ostos FJ, Lópes-Cornejo F, Romero A, Guerrero A. Assessment of the denaturation of collagen protein concentrates using different techniques. Biol Chem. 2019;400(12):1583-1591. https://doi.org/10.1515/hsz-2019-0206.
- Poghosyan AH, Schafer NP, Lyngsø J, Shahinyan AA, Pedersen JS, Otzen DE. Molecular dynamics study of ACBP denaturation in alkyl sulfates demonstrates possible pathways of unfolding through fused surfactant clusters. Protein Eng Des Sel. 2019;32(4):175-190. https://doi.org/10.1093/protein/gzz037.
- Srivastava R, Alam MS. Influence of micelles on protein's denaturation. Int J Biol Macromol. 2020; 145:252-261. https://doi.org/10.1016/j.ijbiomac.2019.12.154.
- Slivko-Koltchik GA, Kuznetsov VP, Panchin YV. Are there gap junctions without connexins or pannexins? BMC Evol Biol. 2019;19(1):46. https://doi.org/10.1186/s12862-019-1369-4.
- Mueller P, Rudin DO. Action potentials induced in biomolecular lipid membranes. Nature. 1968;217(5130):713-719. https://doi.org/10.1038/217713a0.
- Rosenberg B, Postow E. Semiconductivity in proteins and nucleic acids. In book: Experimental Methods in Biophysical Chemistry (C. Nicolau), New York: Wiley; 1973. 315s.
- Belousov AB, Wang Y, Song JH, Denisova JV, Berman NE, Fontes JD. Neuronal gap junctions play a role in the secondary neuronal death following controlled cortical impact. Neurosci. Lett. 2012;524(1):16-19. https://doi.org/10.1016/j.neulet.2012.06.065.
- Belousov AB, Fontes JD. Neuronal gap junctions: making and breaking connections during development and injury. Trends Neurosci. 2013; 36(4):227-236. https://doi.org/10.1016/j.tins.2012.11.001.
- Battefeld A, Klooster J, Kole MH. Myelinating satellite oligodendrocytes are integrated in a glial syncytium constraining neuronal high-frequency activity. Nat Commun. 2016;7():11298. https://doi.org/10.1038/ncomms11298.
- Ma D, Feng L, Cheng Y, Xin M, You J, Yin X, Hao Y, Cui L, Feng J. Astrocytic gap junction inhibition by carbenoxolone enhances the protective effects of ischemic preconditioning following cerebral ischemia. J Neuroinflammation. 2018;15(1):198. https://doi.org/10.1186/s12974-018-1230-5.
- Menichella DM., Majdan M, Awatramani R, Goodenough DA, Sirkowski E, Scherer SS, Paul DL. Genetic and physiological evidence that oligodendrocyte gap junctions contribute to spatial buffering of potassium released during neuronal activity. J Neurosci. 2006;26(43):10984-10991. https://doi.org/10.1523/JNEUROSCI.0304-06.2006.
- Pannasch U, Derangeon M, Chever O, Rouach N. Astroglial gap junctions shape neuronal network activity. Commun Integr Biol. 2012;5(3):248-254. https://doi.org/10.4161/cib.19410.
- Ioannou MS, Liu Z, Lippincott-Schwartz J. A Neuron-Glia Co-culture System for Studying Intercellular Lipid Transport. Curr Protoc Cell Biol. 2019;84(1):e95. https://doi.org/10.1002/cpcb.95.
- Sun EW, Guillen-Samander A, Bian X, Wu Y, Cai Y, Messa M, De Camilli P. Lipid transporter TMEM24/C2CD2L is a Ca2+-regulated component of ER-plasma membrane contacts in mammalian neurons. Proc Natl Acad Sci USA. 2019;116(12):5775-5784. https://doi.org/10.1073/pnas.1820156116.
- Buzsáki G. Electrical wiring of the oscillating brain. Neuron. 2001;31(3):342-344.
- Draguhn A, Traub RD, Bibbig A, Schmitz D. Ripple (approximately 200-Hz) oscillations in temporal structures. J. Clin. Neurophysiol. 2000;17(4):361-376. https://doi.org/10.1097/00004691-200007000-00003.
- Deans MR, Gibson JR, Sellitto C, Connors BW, Paul DL. Synchronous activity of inhibitory networks in neocortex requires electrical synapses containing connexin36. Neuron. 2001;31():477-485. https://doi.org/10.1016/s0896-6273(01)00373-7.
- Traub RD, Pais I, Bibbig A, Le Beau FEN, Buhl EH, Hormuzdi SG, Monyer H, Whittington MA. 2003. Contrasting roles of axonal (pyramidal cell) and dendritic (interneuron) electrical coupling in the generation of neuronal network oscillations. Proc. Natl. Acad. Sci. USA. 2003;100(3):1370-1374. https://doi.org/10.1073/pnas.0337529100.
- Berkinblit MB, Bozhkova VN, Bojcova LYU. Vysokopronicaemye kontaktnye membrany i ih rol v mezhkletochnyh vzaimodejstviyah. Moskva: Nauka; 1981. 464s.
- Ben-Yaakov K, Dagan SY, Segal-Ruder Y, Shalem O, Vuppalanchi D, Willis DE, Yudin D, Rishal I, Rother F, Bader M, Blesch A, Pilpel Y, Twiss JL, Fainzilber M. 2012. Axonal transcription factors signal retrogradely in lesioned peripheral nerve. EMBO J. 2012;31(6):1350-1363. https://doi.org/10.1038/emboj.2011.494.
- Lopez-Verrilli MA, Picou F, Court FA. Schwann cell-derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia. 2013;61(11):1795-1806. https://doi.org/10.1002/glia.22558.


