New polyvinylchloride plasticizers
Автор: Mazitova Aliya Karamovna, Aminova Guliya Karamovna, Maskova Albina Rafitovna, Sabitov Ildar Narimanovich, Nedoseko Igor Vadimovich
Журнал: Нанотехнологии в строительстве: научный интернет-журнал @nanobuild
Рубрика: Решение экологических проблем
Статья в выпуске: 6 т.9, 2017 года.
Бесплатный доступ
One of the main large-capacity polymers of modern chemical industry is polyvinylchloride (PVC). Polyvinylchloride is characterized by many useful engineering properties - chemical firmness in different environments, good electric properties, etc. It explains immensely various use of materials on the basis of PVC in different engineering industries. It is cable, building, light industries, mechanical engineering and automotive industry where PVC is widely applied. One of the reasons why PVC production is dramatically growing is that there is no yet other polymer which could be subjected to such various modifying as it is done with PVC. However under normal temperature this polymer is fragile and isn't elastic that limits the field of its application. Rapid growth of production of polyvinylchloride is explained by its ability to modify properties, due to introduction of special additives when processing. Introduction of plasticizers - mostlly esters of organic and inorganic acids - into PVC allows significant changing properties of polymer. Plasticizers facilitate process of receiving polymeric composition, increase flexibility and elasticity of the final polymeric product due to internal modification of polymeric molecule. This paper presents the results of research on production methods, physicochemical and mechanical properties of new chemical additives of polyvinylchloride - plasticizers based on oxyalkylated alcohols. It also describes the optimal conditions for the compounds synthesis and the results of experiments performed with these compounds as additives in PVC film compositions. It is noted that composition obtained by introducing developed plasticizers into PVC compositions meet the requirements of the existing standards, and their oil and petrol resistance exceed standard samples.
Oxyalkylated phenols and butanols, phthalates of oxyalkylated alcohols, pcv plasticizers, pvc film, oilresistance, petrolresistance, elongation at break, meltflow index, breaking strength, brittleness temperature, heat stability
Короткий адрес: https://sciup.org/142211961
IDR: 142211961 | УДК: 541.64:546.22 | DOI: 10.15828/2075-8545-2017-9-5-168-180
Текст научной статьи New polyvinylchloride plasticizers
M achine - readable information on CC- licenses (HTML- code ) in metadata of the paper
New polyvinylchloride plasticizers. by Mazitova A.K., Aminova G.K., Maskova A.R., Sabitov I.N. is licensed under a ...
P olyvinylchloride (PVC) is one of the most demanded large-capacity polymers made in Russia and abroad, following only polyethylene by production volume. The range of materials and products based on it continues to grow, because they meet the high requirements of modern processing and operating conditions [1–4].
Further development of polyvinylchloride production accompanied by continuous extention of scope first of all is determined by development of functional chemicals additives – stabilizers and plasticizers.
Stabilizers are introduced into composition of polymers to decelerate their ageing that caused mainly by destruction. A large number of chemical

SOLUTIONS FOR ECOLOGICAL PROBLEMS compounds are used as stabilizers: heat stabilizers reduce harmful effects of thermal and thermooxidizingdegradation of polymer; antiozonants protect polymers from effect of atmospheric oxygen and ozone; light stabilizers slow down aging of polymers under the influence of ultra-violet light; antirads protect polymers from destruction under the influence of high-energy radiations; passivators of polyvalent metals protect polymers from the destroying effect of metal «poisons»; antifatiguesprotect polymeric materials, mainly rubbers, from cracking at action of variable loadings [5–7].
The largest segment of the additives market is the market of plasticizer. Introduction of plasticizer into polymeric composition makes it possible to produce material with specified elasticity for the wide range of temperatures. The proper selection of polymer facilitates the polymer processing, greatly increases the frost resistance, flame resistance and improves many other properties of polymer soft products [8].
Various classes of chemical compounds are studied and used as plasticizers: esters ofterephthalic, aliphatic, dicarboxylic, trimellitic and pyromellitic acids; polyester and phosphoricesters; epoxidizedester of soy, palm oils, of tallow fatty acids and other compounds (for example, higher alcohols from C18, paraffin wax) [9, 10].
In recent years there has been a purposeful development of chemical additives. Thus complex stabilizers containing all necessary components, including lubricants have been already in use. Toxic cadmium, barium, leadcontaining heat stabilizers are replaced by non-toxic carboxylates of alkaline earth metals. New plasticizers, giving to plastic compound specific properties, are developed [11].
This paper presents the results of research on development of PVC films compositions with new oil and petrol-resistant plasticizers.
Preobtained calcium-zinc complex salts of oleic, stearic and alpha branched saturated monocarboxylic acids (VIC) were used as heat stabilizers [12, 13].
Phthalates of oxyalkylated alcohols (Fig. 1). Initial oxylated and hy-droxypropylated butanol and phenol obtained previously were used as plasticizers [14–17].
Conditions for obtaining the desired products are shown in Table 1, physicochemical parameters are given in the Table 2.
Obtained esters were tested as PVC plasticizers in compositions of PVC films.

SOLUTIONS FOR ECOLOGICAL PROBLEMS
Ri
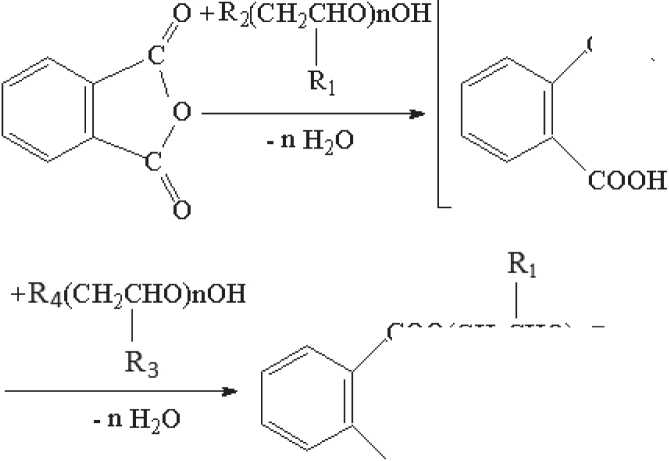
COO(CH3CHO)nR2
COO(CH3CHO)nR2
COO(CH3CHO)nR4
R3
where R1 = H, R2 = C4H9, R3 = H, R4 = C4H9 (I); R1 = CH3, R2 = C4H9, R3 = CH3, R4 = C4H9 (II); R1 = H, R2 = C6H5, R3 = H, R4 = C6H5 (III); R1 = CH3, R2 = C6H5, R3 = CH3, R4 = C6H5 (IV); R1 = H, R2 = C6H5, R3 = H, R4 = C4H9 (V); R1 = CH3, R2 = C6H5, R3 = CH3, R4 = C4H9 (VI); R1 = CH3, R2 = C6H5, R3 = H, R4 = C4H9 (VII); R1 = H, R2 = C6H5, R3 = CH3, R4 = C4H9 (VIII).
Fig. 1
Compositions of the films and the experiment result of obtained esters as plasticizers in commercial formulations of PVC films are shown in Table 3 (Formulation (wt parts): PVC – 100; plasticizer – 50; Ca–Zn complex stabilizer – 3.0).
Prepared compositions were milled in laboratory mill 320 PD at the temperature of 160–162оC for 10 minutes. There were no any difficulties during rolling of composition: the film didn’t stick to the rolls, holes, chips and cracks were absent in the obtained samples of plastic. Moreover, during the production of PVC compositions in the mixer and their processing on rollers technological difficulties did not arise.
Physical, physicochemical and physico-mechanical properties were determined in accordance with standard methods. The strength and elongation
SOLUTIONS FOR ECOLOGICAL PROBLEMS
The optimum conditions for obtaining chemical additives
Physicochemical parameters of synthesized esters
Table 1
|
№ |
Reagent molecularratio |
Temperature, оС |
Note |
|
|
1 |
alcohol : ethylene (propylene) oxide |
1:1.3-2.2 |
110–180 |
amount of catalyst 0.5–3% (wt. by loading) |
|
2 |
phthalic anhydride: oxyalkylated alcohol |
1:2 |
121–170 |
amount of catalyst 0.1–2% (wt. by loading) + activated carbon in an amount of 1% (wt. of weight of the loaded components) |
Table 2
|
Indicator name |
Plasticizers |
|||||||
|
I |
II |
III |
IV |
V |
VI |
VII |
VIII |
|
|
Oxyalkylation degree, n |
2.0 |
2.2 |
1.0 |
2.1 |
2.0* |
2.2* |
2.0* |
2.2* |
|
Density, d204 |
1.4816 |
1.4745 |
1.1086 |
1.1002 |
1.1081 |
1.1050 |
1.1062 |
1.1060 |
|
Index of refraction, 20 nD |
1.0757 |
1.02991 |
1.5194 |
1.4924 |
1.5183 |
1.5174 |
1.5178 |
1.5178 |
|
Acid number, mg КОН/g |
0.1 |
0.4 |
0.1 |
0.1 |
0.2 |
0,2 |
0,2 |
0.2 |
|
Ether index, mg КОН/g |
243 |
207 |
273 |
198 |
257 |
203 |
211 |
235 |
|
Molecular mass, found |
461 |
541 |
411 |
566 |
436 |
553 |
514 |
476 |
|
Freezing temperature, о С |
–50 |
–44 |
–40 |
–39 |
–40 |
–37 |
–39 |
–40 |
|
Weight fraction of volatile compound (100оС, 6 h ),% |
0.25 |
0.25 |
0.10 |
0.15 |
0.10 |
0.10 |
0.12 |
0.10 |
|
Flash-point, оС |
200 |
Higher than 200 |
200 |
199 |
200 |
200 |
200 |
200 |
* phenol ethoxylation degree = 1.0; phenol hydroxypropylation degree = 2.1
SOLUTIONS FOR ECOLOGICAL PROBLEMS
Physicochemical parameters of synthesized esters
Table 3
SOLUTIONS FOR ECOLOGICAL PROBLEMS
All tested samples of synthesized esters provided PVC films with relevant technical properties. Important technological parameters, such as time of heat stability, melt flow rate, in all cases of the use of test plasticizer samples were much higher, that indicates facilitated processing of the PVC compositions. It should be also noted that the PVC films have improved oil and petrol resistance. In addition, preliminary tests showed that obtained esters belong to the 3 hazard class. As it is known, DOP is a toxic plasticizer, which belongs to the 2 hazard class.
Thus, PVC compounds that contain the new plasticizers by all characteristics meet the existing standards and are recommended for further testing.
Список литературы New polyvinylchloride plasticizers
- Braginsky O.B. Syr'evaja baza neftehimii: sovremennoe sostojanie i perspektivy razvitija . Materialy seminara «Hlororganicheskij sintez, tendencii rynka i tehnologij» . Moscow. 2006. p. 4..
- Ulyanov V.M., Rybkin E.P., Gudkovich A.D., Pishin GA. Polivinilhlorid . Moscow. Himija , 1992. 288 p..
- Wilkie Ch., Summers J., Daniels Ch. Polivinilhlorid . SPb. Pro-fessia , 2007. 728 p..
- Chalaya N.M. Proizvodstvo produkcii iz PVH -real'nost' i perspektivy . Plasticheskie massy , 2006. № 1. pp. 4-7..
- Minsker K.S., Fedoseyev G.T. Destrukcija i stabilizacija polivinilhlorida . Moscow. Himija , 1972. 420 p..
- Foigt I. Stabilizacija sinteticheskih polimerov protiv dejstvija sveta i tepla . SPb. Himija , 1972. 544 p..
- Maslova I.P. Himicheskie dobavki k polimeram. Spravochnik . Moscow. Himija , 1981. 264 p..
- Mazitova A.K., Aminova G.K., Nafikova R.F., Deberdeev R.T. Osnovnye polivinil-hloridnye kompozicii stroitel'nogo naznachenija . Ufa, 2013. 130 p..
- Barshteyn R.S., Kirilovich V.I., Nosovskiy Y.E. Plastifikatory dlja polimerov . Moscow. Himija , 1982. 196 p..
- Tinius K. Plastifikatory . Moscow. Himija , 1964. 915 p..
- Karimov F.Ch., Mazitova A.K., Khamaev V.Kh, Minsker K.S., Zaikov G.E. Stabilization of plasticized polyvinyl chloride by 3-mercapto-1,2,4-triazine-5-one derivatives. Oxidation Communications. 1997. Vol. 20, № 2. pp. 286-289. (In English).
- Aminova G.K., Nafikova R.F., Maskova A.R., Stepanova L.B., Buylova EA. Stabi-lizatory polivinilhlorida /Nauka i jepoha: mono-grafija/; pod obshhej red. prof. O.I. Kirikova ; under the general editorship. prof. O.I. Kirikova]. Kniga 9. Voronezh: VGPU , 2012. Ch. XVIII, Vol. 500. pp. 275-295..
- Mazitova A.K., Stepanova L.B., Aminova G.K., Maskova A.R. Razrabotka funkcional'nyh dobavok dlja polivinilhloridnyh kompozicij stroitel'nogo naznachenija . Promyshlennoe proizvodstvo i ispol'zovanie jelastomerov . 2015. № 2. pp. 27-31..
- Aminova G.F., Gabitov A.I., Maskova A.R., Gorelov V.S. Plastifikatory na osnove ok-sialkilirovannyh fenolov /V sb.: The problems of the construction complex of Russia XVIII. International scientific-technical conference . 2014. pp. 271-273..
- Mazitova A.K., Aminova G.K., Gabitov A.I., Maskova A.R., Rahmatullina R.G. Novye plastifikatory PVH-kompozicij special'nogo naznachenija . Bashkir chemical journal . 2015. Vol. 22, № 3. pp. 23-26..
- Mazitova A.K., Aminova G.F. Gabitov A.I., Maskova, A.R., Khusnutdinov B.R., Fattakhova A.M. Razrabotka novyh plastifikatorov polivinilhlorida . Jelektronnyj nauchnyj zhurnal «Neftegazovoe delo» . 2014. № 12-1. pp. 120-127.
- Mazitova A.K., Aminova G.K., Maskova A.R., Builova EA., Nedopekin D.V. Dife-noksijetilftalaty i butoksijetilfenoksijetilftalaty -novye plastifikatory polivini-lhlorida . Jelektronnyj nauchnyj zhurnal «Neftegazovoe delo» . 2015. № 5. pp. 376-397.


