Nicotine and chromium co-exposure lead to hepatotoxicity in male albino rats
Автор: Dey S., Nandi A., Das S., Sinha S.K., Dey S.K.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 3 т.19, 2023 года.
Бесплатный доступ
Nicotine is one of the major constituents of different types of smoking and is the more toxic part also. Thirty metals including chromium and some chromium compounds have been detected in tobacco smoke are causally associated with cancer in humans. In the present investigation, we evaluate the individual and combined effect of nicotine and chromium (VI) on the toxicity of liver in animal. In this study, a group of male albino rats (80-100 g) were induced by intraperitonial injection of vehicle (0.9% NaCl), nicotine tartrate (0.2 mg / 100 g body weight / day), K2Cr2O7 (0.8 mg / 100 g body weight / day), and combined exposure of K2Cr2O7 and nicotine tartrate at an interval of six hours for a period of 28 days. After the treatment the liver tissues were collected to measure the hepatotoxicity. It was showed that individual and combined exposure of chromium (VI) and nicotine marked decreased the activities of GOT, GPT, ALP and LDH in liver tissue. It was also noted that the level of MDA, CD and NO production increased significantly in response to individual and combined exposure of nicotine and chromium. On the other hand, it was observed that individual and combined exposure of nicotine and chromium (VI) marked decreased the GSH and GSSG content, and also the antioxidant enzymes (SOD, CAT, GPx, GR and GST) in tested organ. The present study suggests that nicotine and chromium exhibited significant changes during individual exposure whereas co-exposure showed a marked alteration of the toxicity of liver in male albino rats.
Nicotine, chromium, liver, toxicity, oxidative stress
Короткий адрес: https://sciup.org/143180571
IDR: 143180571
Текст научной статьи Nicotine and chromium co-exposure lead to hepatotoxicity in male albino rats
The liver is the key organ regulating homeostasis in the body. It involved with almost all the biochemical pathway related to growth, fight against disease, nutrient supply, energy provision and reproduction. It is expected not to only perform physiological functions but also to protect against hazards of harmful drugs and chemicals. In spite of tremendous scientific advancement in the field of chemology in recent years, liver problems are on rise. Tobacco smoking is the most socially spread habit and is considered as one of the leading causes of premature death in developed as well as developing countries. Epidemiological studies have shown that cigarette smoking may accelerate the progression of renal, pulmonary, and cardiac fibrosis (Zhang et al. , 2009), but whether it might cause organ damage in rather healthy tissues is an important question. The bad effects of nicotine on body function such as rise in heart rate, blood pressure, disturbed lipid profile, atherosclerosis and ischemic heart disease (Benowitz et al. , 1988) had been previously shown by direct administration of nicotine in human and animals (Schievelbein and Balfour 1984). Liver is considered to be the major site of nicotine biotransformation and nicotine exerts a number of adverse physiological effects on the liver (El-Zayadi 2006). icotine is absorbed through the lungs with smoking and is rapidly metabolized in the liver which induces three major adverse effects on the liver: Direct or indirect toxic effects, immunological effects, and oncogenic effects (Hukkanen et al. , 2005). Smoking causes liver cell injury and exerts genotoxic effect of rat liver (Bandyopadhyaya et al. , 2008).
Heavy metals are highly toxic compounds widely spread in the environment, due to their high degree of toxicity, arsenic, cadmium, chromium, lead, and mercury rank among the priority metals with high public health significance (Tchounwou et al. , 2012). Chromium is released in the environment from industrial processes, wood preservation, pigment, plating, welding, leather tanning, manufacture of stainless steel, and metal finishing (Blade et al. , 2007); therefore, its level in the industrial waste consists of an important health concern related to the environmental contamination (Mishra and
Bharagava 2016). Furthermore, chromium has been reported for its potential genotoxicity, cytotoxicity (Patlolla et al. , 2009), and carcinogenicity effects (Kim et al. , 2018). In the same way, its exposure has been linked to cell damages and D A disruption (Khalil et al. , 2013). The organs that are the most affected by chromium bioaccumulation are liver, kidney, and spleen. In fact, liver is one of the main toxicity targets as it is the biotransformation organ of the majority of xenobiotics.
It was noted that nicotine is one of the major constituents of different types of smoking and is the more toxic part also. Thirty metals have been detected in tobacco smoke, including nickel, arsenic, cadmium, chromium and lead. Arsenic and arsenic compounds and chromium and some chromium compounds are causally associated with cancer in humans, while nickel and cadmium and their compounds are probably carcinogenic to humans. So, the present study aims to evaluate the hepatotoxicity following individuals and coexposure of nicotine and chromium (VI) in male albino rat.
MATERIALS AND METHODS
Chemicals
Potassium dichromate, icotine tartrate and other fine chemicals were purchased from Sigma Chemical Company, USA. All other chemicals and reagents were purchased from Sisco Research Laboratory Pvt Ltd (SRL), India, and were of analytical grade.
Maintenance of Animals
Male albino rats of the Wistar strain (80-100 g) were obtained, divided into four groups, each group contain six animal, housed in polypropylene cages under standard conditions of temperature (25 ± 2.8 0 C) and humidity (60 ± 5%), with alternating 12 h light : 12 h dark cycles, and fed standard diet and water ad libitum. Animals were maintained in accordance with the guidelines of the ational Institute of utrition, Indian Council of Medical Research, Hyderabad, India, and approved by the ethical committee of Vidyasagar University (West Bengal, India).
Treatment of Animals
Laboratory acclimatized rats were divided into four groups of six animals each and almost equal average body weight. The animals of second group were induced by subcutaneous injection with nicotine tartrate (dissolved in 0.9% physiological saline) at a dose of 0.2 mg / 100 g body weight per day for 28 days, as described earlier (Dey and Roy 2010). The animals of first group was injected intraperitoneally (i.p.) with chromium(VI) as K2Cr2O7 at a dose of 0.8 mg per 100 g body weight per day (20% LD50) for 28 days, as described earlier (Dolai et al., 2016). The animal of third group was injected both nicotine and chromium as the previous doses at six hours interval for 28 days. The animals of the remaining group was received only the vehicle (0.9% aCl), served as control.
Tissue collection and homogenization
After the experiment period, the rats were sacrificed by cervical dislocation and liver tissue was immediately dissected out of the body, wiped off the blood and weighed. Then the liver tissue was homogenized (10%, w/v) in an appropriate buffer (pH = 7.4) and centrifuged. The resulting supernatants were then stored at - 200C for biochemical assays.
Analytical methods
The activities of transaminases (GOT and GPT) were estimated according to Reitman and Frankel (1957). Alkaline phosphatase (ALP) was measured according to Kind and King (1954). The activity of lactate dehydrogenase (LDH) was measured by the method of Young et al. , (1975).
Lipid peroxidation was measured according to the method of Ohkawa et al. , (1979). Malondialdehyde (MDA) was determined from the absorbance of the pink coloured product (TBARS) of thiobarbituric acid-MDA reaction, at 530 nm. The reaction of MDA with TBA has been widely adopted as a sensitive method of lipid peroxidation in animal tissues. Conjugated dienes was measured according to the method of Slater (1980). O release assays were done in liver and lungs mitochondria according to the method of Sanai et al. , (1998).
GSH was measured according to the method of Griffith (1980). GSSG was also assayed after derivatization of GSH with 2 vinylpyridine. GSSG was measured by the method of Griffith (1980).
Catalase activity was determined at room temperature by using a slightly modified version of Aebi (1984). The molar extinction coefficient of 43.6Mcm-1 was used to determine CAT activity. One unit of activity is equal to the millimoles of H 2 O 2 degraded per minute per milligram of protein. SOD activity was estimated by measuring the percentage inhibition of the pyrogallol auto- oxidation by SOD according to the method Marklund & Marklund (1974).
The rate of oxidation of reduced glutathione (GSH) by H 2 O 2 as catalyzed by the glutathione peroxides (GPx) present in the homogenate is assayed for the measurement of enzyme activity. Glutathione peroxidase activity was measured according to method of Paglia and Valentine (1967). The activity of glutathione reductase was measured by the method of Miwa (1972). Glutathione S-transferase activity was measured according to the method of Habig et al. , (1974).
Total protein of plasma and tissues was estimated according to the method of Lowry et al. , (1951).
Statistical Analysis
The data were expressed as mean ± S.E.M. Comparisons of the means of control, nicotine and nicotine with sodium selenite group were made by twoway A OVA with multiple comparison ‘t’-test, P< 0.05 as a limit of significance.
RESULTS AND DISCUSSION
In our present experiment it was found that the activity of GOT, GPT, ALP and LDH remarkably decreased in liver tissues after individual and combined exposure of nicotine and chromium [Figure-1(A-B); Figure-2 (A-B)]. It was noted that inhibition of acid phosphatase, adenosine triphosphatase and succinic dehydrogenase after administration of trivalent and hexavalent chromium ( ehru and Kaushal 1993). On the other hand, it was observed that significant increase in alkaline phosphatise activity due to lead intoxication (Awadallah and Hanna 1980). The chromium and other heavy metals have been reported to raise the level of amino transferases. The serum AST activity was significantly higher in animals injected with chromium than cobalt, zinc and manganese, while serum ALT activity were higher in cobalt than in chromium, zinc and manganese (Bavazzano et al., 1981). It was reported that ALT and AST activities are higher in tannery workers as compared to workers in the shoe factory (Drotman 1978). Liver performance indices such as ALP, ALT, and AST are widely used to evaluate the liver damage (Wang et al., 2009). ecrosis or cell membrane damage can trigger the release of these enzymes into the blood circulation (Wang et al., 2009). It seems that the increased level of serum enzymes indicate cellular leakage, structural damage, and performance dysfunction of membrane markers in the liver due to nicotine administration (Guyton and Hall 2001). On the other hand, it was known that LDH is an enzyme that is present in almost all body tissues. Conditions that can cause increased LDH in the blood may include liver disease, anemia, heart attack, bone fractures, muscle trauma, cancers, and infections. LDH activity in the serum is considered as marker of membrane damage, and it was found elevated on arsenic or nicotine exposure (Muthukumaran et al, 2008). So, the marked changes of LDH in liver indicated the hepatic damage.
Enhanced lipid peroxidation, conjugated diene and O production associated with antioxidant depletion in liver is a characteristic observation in response to individual and combined exposure of nicotine and chromium [Figure-3 (A-C)]. icotine, a potent carcinogen, used in the present study has been reported to be oxidizing into its metabolite cotinine mainly in liver and to a significant extent in lung and kidney and plays a key role in the pathogenesis of tissues (Husain et al., 2001; Yildiz et al., 1998). Previous studies have shown that endothelial and epithelial membrane damage, increased vascular permeability, membrane lipid peroxidation and the influx of polymorphonuclear leucocytes to the site of lung injury in nicotine treated rats are the hallmark of inflammation (Kirkham et al., 2003). The mechanism of free radical generation by nicotine is not clear. Chromium, a potent carcinogen, used in the present study has been reported to be oxidizing into its metabolite cotinine mainly in liver and to a significant extent in lung and plays a key role in the pathogenesis of tissues. Rana and Kumar (1984) reported enhancement of lipid peroxidation in rat liver after heavy metal poisoning with mercury, molybdenum, copper, chromium and manganese. It has been demonstrated that the chromium (V) complexes which are produced following reduction of chromium (VI) by cellular biological reductants, react with hydrogen peroxide to generate hydroxyl radicals which in turn act as the initiators of primary events in chromium (VI) cytotoxicity (Shi and Dalal 1990a). Bagchi et al., (1995) showed that chromium (VI) induces increases in hepatic mitochondrial and microsomal lipid peroxidation. Thus, the increased the level of MDA, conjugate diene and nitric oxide production in liver of individual and combined treated rats in the present study may be due to excessive generation of free radicals.
The excessive generation of oxygen free radicals can be prevented or scavenged by host antioxidant defense mechanism. In effective scavenging of free radicals due to depletion of antioxidants plays a crucial role in cell injury (Lima and Savin 2002). Previous studies have suggested that superoxide anion and hydrogen peroxide are the main source of nicotine-induced free radicals depleting the cellular antioxidant (Helen et al., 2000). GSH plays a crucial role in protecting the liver and kidney from oxidative stress by detoxifying exogenous toxicants and quenching reactive oxygen species (ROS). High concentration of GSH is found in cells as the major antioxidants defense, especially in regulating the extent and duration of oxidative ’burst’ (Abidi et al., 1999). GPx has a well-established role in protecting cells against oxidative injury. GPx utilizes GSH as a substrate to catalyses the reduction of organic hydroperoxides and hydrogen peroxide (Ray and Husain 2002). Therefore the excess H2O2 and lipid peroxides generated during nicotine ingestion are efficiently scavenged by GPx activity. The depression of this enzyme activity reflects perturbations in normal oxidative mechanisms during nicotine ingestion. On the other hand, it was found that GR activity inducing the production of GSH from GSSG. There are alternative functions for GSH in cellular metabolism independent of its antioxidant properties. GSH also participates in the detoxification of xenobiotics as a substrate for the enzyme glutathione-S-transferase. Superoxide dismutase is the antioxidant enzyme that catalyses the dismutation
of the highly reactive
superoxide anion to O 2 and to the less reactive species H 2 O 2 (Okado-Matsumoto and Fridovich 2001).
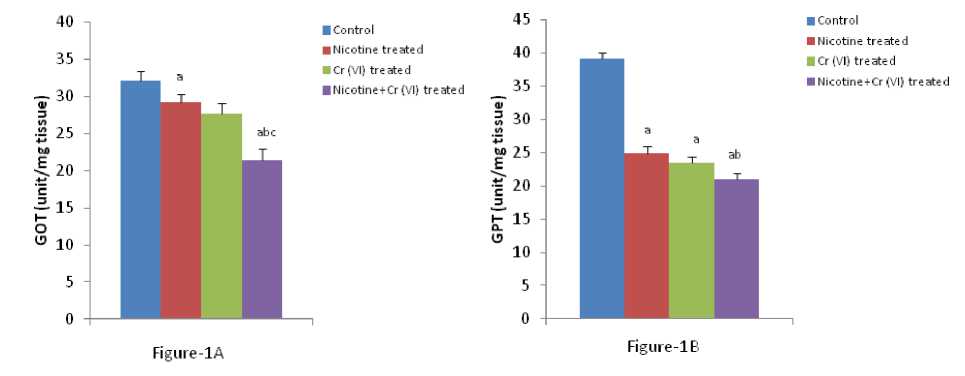
Figure 1 (A & B) : Shows the changes the activities of liver GOT and GPT in different experimental groups of rat. Data represents Mean ± SE.
‘a’ indicate significant difference (P<0.05) when compared with control.
‘b’ indicate significant difference (P<0.05) when compared with nicotine treated group.
‘c’ indicate significant difference (P<0.05) when compared with chromium treated group.
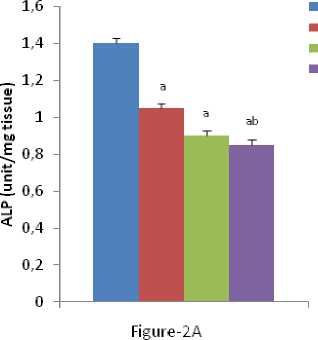
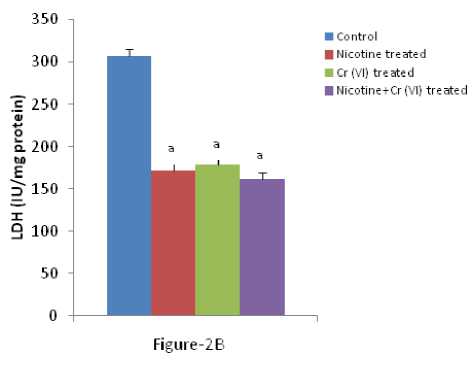
Figure 2 (A & B): Shows the changes the activities of liver ALP and LDH in different experimental groups of rat. Data represents Mean ± SE.
‘a’ indicate significant difference (P<0.05) when compared with control.
‘b’ indicate significant difference (P<0.05) when compared with nicotine treated group.
‘c’ indicate significant difference (P<0.05) when compared with chromium treated group.
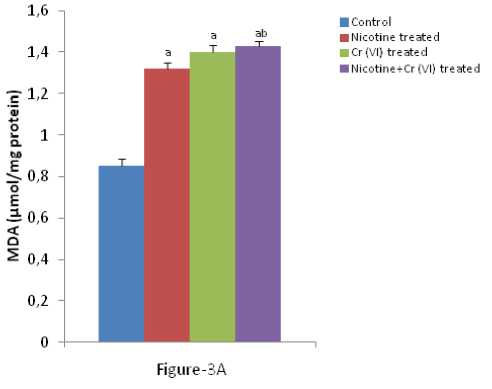
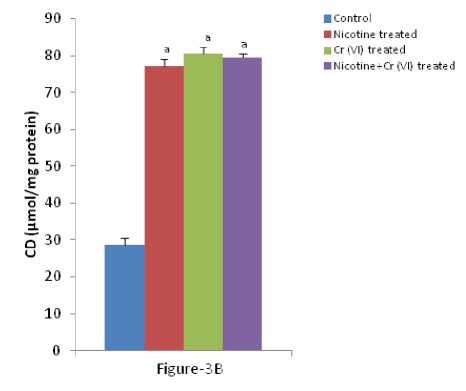
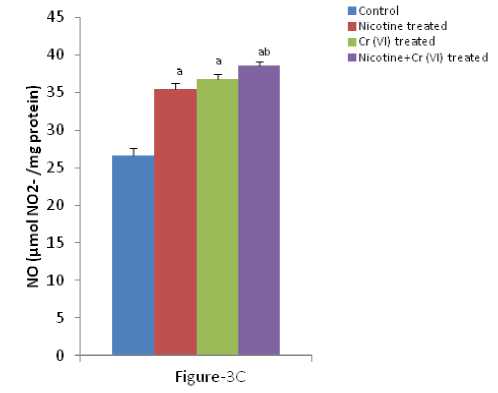
Figure 3 (A-C): Shows the changes the levels of liver MDA, CD and O production in different experimental groups of
rat. Data represents Mean ± SE.
a’ indicate significant difference (P<0.05) when compared with control.
b’ indicate significant difference (P<0.05) when compared with nicotine treated group.
c’ indicate significant difference (P<0.05) when compared with chromium treated group.
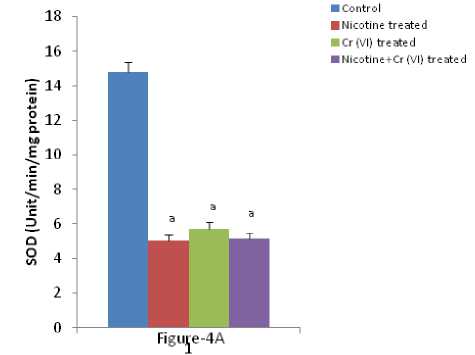
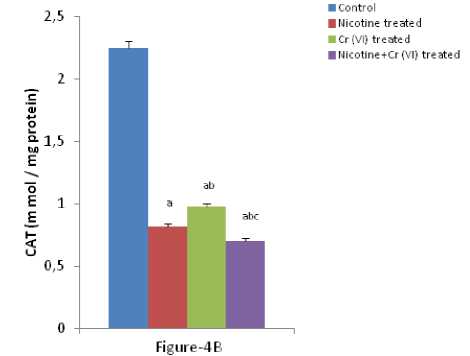
Figure 4 (A & B): Shows the changes the activities of liver SOD and CAT in different experimental groups of rat. Data represents Mean ± SE.
‘a’ indicate significant difference (P<0.05) when compared with control.
‘b’ indicate significant difference (P<0.05) when compared with nicotine treated group.
‘c’ indicate significant difference (P<0.05) when compared with chromium treated group.
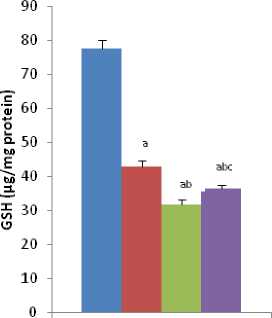
Figure-5A
■ Control
■ Nicotine treated
■ Cr (VI) treated
■ Nicotine+Cr (VI) treated
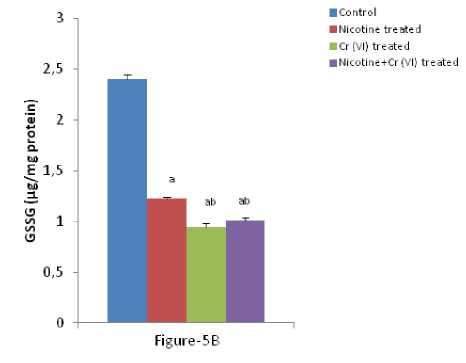
Figure-5 (A & B): Shows the changes the levels of liver GSH and GSSG in different experimental groups of rat. Data represents Mean ± SE.
‘a’ indicate significant difference (P<0.05) when compared with control.
‘b’ indicate significant difference (P<0.05) when compared with nicotine treated group.
‘c’ indicate significant difference (P<0.05) when compared with chromium treated group.
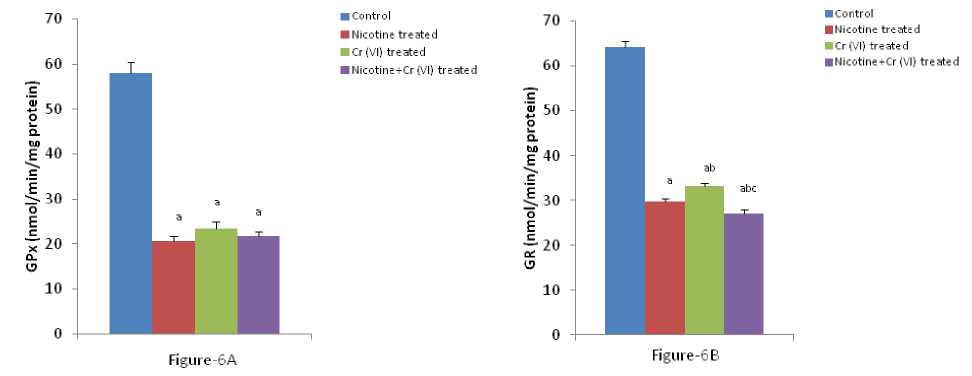
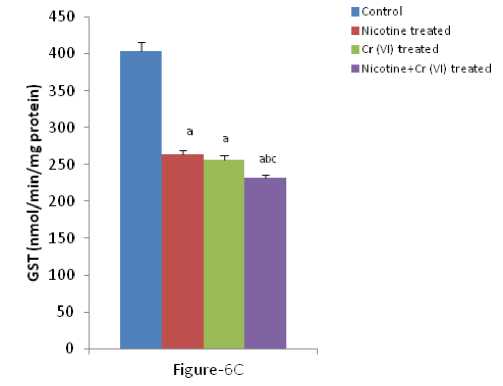
Figure-6 (A-C): Shows the changes the activities of liver GPx, GR and GST in different experimental groups of rat. Data represents Mean ± SE.
‘a’ indicate significant difference (P<0.05) when compared with control.
‘b’ indicate significant difference (P<0.05) when compared with nicotine treated group.
‘c’ indicate significant difference (P<0.05) when compared with chromium treated group.
umerous studies have shown the importance of SOD in protecting cells against oxidative stress (Huang et al , 1997). Thus decrease in the activity of SOD (Figure-4A) observed in the present study could be due to a feedback inhibition or oxidative inactivation of enzyme protein due to excess ROS generation. Catalase, which acts as preventative antioxidant plays an important role in protection against the deleterious effects of lipid peroxidation (Pigeolot et al , 1990). The inhibition of CAT activity (Figure-4B) is suggestive of enhanced synthesis of superoxide anion during the ingestion of combined exposure since superoxide anion is a powerful inhibitor of catalase (Ashakumary and Vijayammal 1996). Previous reports have shown the decreased activity of SOD and CAT in the tissues of experimental rats (Ashakumary and Vijayammal 1991). Husain et al , (2001) have reported that chronic administration of ethanol and nicotine decreased the level of GSH and activities of GPx, SOD and CAT in the lung and kidney. In the present study, it was found that the level of GSH and GSSG are decreased significantly in liver tissues [Figure- 5(A-B)] in response to individual and co-exposure of nicotine and chromium in male albino rats. On the other hand, the activities of GPx, GR and GST are also significantly decreased [Figure-16(A-C)]. This depletion of GSH, GSSG, GPx, GR, GST, SOD and CAT activities in liver in response to individual and co-exposure of nicotine and chromium treated rats may be due to enhanced utilization during detoxification.
CONCLUSION
To our knowledge this is the first study to suggest possible synergism between nicotine and chromium coexposed animals. However, this was established based only on few variables. Thus there is need to have a more detailed study to establish mechanism. There were significant alterations of the status of liver but the mechanisms behind these changes are not known. It can thus be concluded that both nicotine and chromium if given individually or in combination are toxic at the present dose and duration. Further studies are needed to be done in this direction to study the exact mechanism behind synergism between nicotine and chromium on the status liver functions.
ACKNOWLEDGEMENTS
CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest.
Список литературы Nicotine and chromium co-exposure lead to hepatotoxicity in male albino rats
- Abidi, P., Afaq, F., Arif, J.M., Lohani, M., Rahman, Q., (1999). Chrysotile-mediated imbalance in the glutathione redox system in the development of pulmonary injury. Toxicology Letters 106(1), 31-39.
- Aebi, H., (1984). Catalase in vitro. Methods in Enzymology 105, 121-126.
- Ashakumary, L., Vijayammal, P.L., (1991). Lipid peroxidation in nicotine treated rats. Journal of Ecotoxicoogy andl Environmental Monitoring 1, 283-290.
- Ashakumary, L., Vijayammal, P.L., (1996). Additive effect of alcohol and nicotine on lipid peroxidation and antioxidant defence mechanism in rats. Journal of Applied Toxicology 16, 305-308.
- Awadallah, R., Hanna, A., (1980). Serum enzyme changes due to trace amounts of some transition metal ions on the induction of experimental diabetes. Zeitschrift für Ernährungswissenschaft 19(2), 103-110.
- Bagchi, D., Hossoun, E.A., Bagchi, M., Stohs, S.J., (1995). Chromium-induced excretion of urinary lipid metabolites, DNA damage, nitric oxide production and generation of reactive oxygen species in Sprague-Dawley rats. Comparative Biochemistry and Physiology Part C: Pharmacology, Toxicology and Endocrinology 110(2), 177-187.
- Bandyopadhyaya, G., Sinha, S., Chattopadhyay, B. D., Chakraborty, A., (2008). Protective role of curcumin against nicotine-induced genotoxicity on rat liver under restricted dietary protein. European Journal of Pharmacology 588, 151-157.
- Bavazzano, P., Benassi, S., Forzieri, R., Petrioli, G., (1981). Levels of aspartate aminotransferase and alanine aminotransferase in two factories with various hepato-toxic risks. Quad Sclavo Diagn 17, 407-420.
- Benowitz, N.L., Porchet, H., Sheiner, L., Jacob, P., (1988). Nicotine absorption and cardiovascular effects with smokeless tobacco use: Comparison with cigarettes and nicotine gum. Clinical Pharmacology and Therapeutics 4, 23-28.
- Blade, L.M., Yencken, M.S., Wallace, M.E., Catalano, J.D., Khan, A., Topmiller, J.L., Shulman, S.A., Martinez, A., Crouch, K.G., Bennett, J.S., (2007). Hexavalent chromium exposures and exposure-control technologies in American enterprise: results of a NIOSH field research study. Journal of Occupational and Environmental Hygiene 4, 596618.
- Dey, S.K., Roy, S., (2010). Role of reduced glutathione in the amelioration of nicotine-induced oxidative stress. Bulletin of Environmental Contamination and Toxicology 84(4), 385-389.
- Dolai, D.P., Dey, S.K., Dash, S.K., Roy, S., (2016). Study the Chromium-Induced Oxidative Stress on Mitochondria from Liver and Lungs Origin. American Journal of Applied Scientific Research 2(6), 59-64.
- Drotman, R., (1978). Serum enzymes are indications of chemical induced liver damage. Drug and Chemical Toxicology 1, 163-171.
- El-Zayadi, A,R., (2006). Heavy smoking and liver. World Journal of Gastroenterology 12, 6098-101.
- Grifith, O.W., (1980). Determination of glutathione and glutathione sulfide using glutathione reductase and 2-vinyl pyridine. Analytical Biochemistry 106, 207212.
- Guyton, A.C., Hall, J.E., (2001). Text Book of Medical Physiology, 10th Ed, W.B.Saunders Company, NewYork..
- Habig, W.H., Pabst, M. J., Jakoby, W. B., (1974). Glutathone S-transferases, the first enzymatic step in mercapturic acid formation. Journal of Biological Chemistry 249, 7130-7139.
- Helen, A., Krishnakumar, K., Viajayammal, P.L., Augusti, K.T., (2000). Antioxidant effect of onion oil (Allium cepa. Linn) on the damages induced by nicotine in rats as compared to alpha-tocopherol. Toxicological Letters 116, 61-68.
- Huang, T.T., Carlson, E.J., Gillespie, A.M., Reaume, A.G., Hoffman, E.K., Chan, P.H., Scott, R.W., and C.J, Epstein., (1997). Superoxide mediated cytotoxicity in superoxide dismutase deficient fetal fibroblasts. Archives of Biochemistry Biophysics 344(2), 424-432.
- Hukkanen, J., P. 3rd Jacob, Benowitz, N.L., (2005). Metabolism and disposition kinetics of nicotine. Pharmacol Reviews 57, 79-115.
- Husain, K., Scott, B. R., Reddy, S.K., Somani, S.M., (2001). Chronic ethanol and nicotine interaction on rat tissue antioxidant defense system. Alcohol 25(2), 89 -97.
- Khalil, S., Awad, A., Elewa, Y.., (2013). Antidotal impact of extra virgin olive oil against genotoxicity, cytotoxicity and immunotoxicity induced by hexavalent chromium in rat. International Journal Veterinary Science and Medicine 1, 65-73.
- Kim, J., Seo, S., Kim, Y., Kim, D.H.., (2018). Review of carcinogenicity of hexavalent chrome and proposal of revising approval standards for an occupational cancer in Korea. Annals of Occupational and Environmental Medicine 30, 7.
- Kind, P.R.N., King, E. J.., (1954). Estimation of Plasma Phosphatase by Determination of Hydrolysed Phenol with Amino-antipyrine, Journal of Clinical Pathology 7, 322-326.
- Kirkham, P.A., Spooner, G., Ffoulkes-Jones, C., Calvez, R..,(2003). Cigarette smoke triggers macrophage adhesion and activation: role of lipid peroxidation products and scavenger receptor. Free Radical Biology and Medicne 35, 697-710.
- Lima, M.H., Savin, T.Z.., (2002). Animal response to drastic changes in oxygen availability and physiological oxidative stress. Comparative Biochemistry and Physiology 133, 537-556.
- Lowry, O.H., Roseborough, N.J., Farr, A.L., Randll, A.J.. , (1951). Protein measurement with Folin's phenol reagent. Journal of Biological Chemistry193, 265-275.
- Marklund, S., Marklund, G., (1974). Involvement of superoxide anion radical in autoxidation of pyrogallol and a convenient assay of superoxide dismutase. European Journal of Biochemistry 47, 469-474.
- Mishra, S., Bharagava, R.N., (2016). Toxic and genotoxic effects of hexavalent chromium in environment and its bioremediation strategies. Journal of Environmental Science and Health Part C Environmental Carcinogenesis & Ecotoxicology Reviews 34,1-32
- Miwa, S., (1972). Hematology, In: Modern Medical Techonology, 3 , 306-310.
- Muthukumaran, S., Sudheer, A.R., Menon V.P., Nalini, N., (2008). Protective effect of quercetin on nicotine-induced prooxidant and antioxidant imbalance and DNA damage in Wistar rats. Toxicology 243,207-215.
- Nehru, B., Kaushal, S.., (1993). Alterations in the hepatic enzymes following experimental lead poisoning. Biological Trace Element Research 38(1), 27-34.
- Ohkawa, H., Ohisi, N., Yagi. K.., (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry 95, 351-358.
- Okado-Matsumoto, A., Fridovich, I., (2001). Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu, Zn-SOD in mitochondria. Journal of Biological Chemistry 276, 38388-38393.
- Paglia, D.E., Valentine, W.N.., (1967). Studies on quantitative and qualitative characterization of erythrocyte glutathione peroxidase. Journal of Laboratory and Clinical Medicine 70, 158-169.
- Patlolla, A.K., Barnes, C., Hackett, D., Paul, B.T., (2009). Potassium dichromate induced cytotoxicity, genotoxicity and oxidative stress in human liver carcinoma (HepG2) cells. International Journal of Environmental Research and Public Health 6, 643653.
- Pigeolot, E., Corbisier, P., Houbion, A., Lambert, D., Michiels, C., Raes, M., Zachary, M.D., Remacle, J., (1990). Glutathione peroxidase, superoxide dismutase and catalase inactivation by peroxides and oxygen derived radicals. Mechanisms of Ageing and Development 51, 283-297.
- Rana, S.V.S., Kumar, A., (1984). Significance of lipid peroxidation in liver-injury after heavy metal poisoning in rat. Current Science 53(17), 933-934.
- Ray, G., Husain, S.A., (2002). Oxidants, antioxidants and carcinogenesis. Indian Journal of Experimental Biology 42, 1213-1232.
- Reitman, S., Frankel, S., (1957). A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. American Journal of Clinical Pathology 28, 56-63.
- Sanai, S., Tomisato M., Shinsuka N., Mayoko Y., Mayoko H., Akio, N., (1998). Protective role of nitric oxide in S. aurues infection in mice. Infection and Immunity 66,1017-1028.
- Schievelbein, H., Balfour, D. J. K., (1984). Nicotine and the Tobacco Smoking Habit. Pergamon Press, Oxford, 1-15.
- Shi, X., Dalal, N.S., (1990a). On the hydroxyl radical formation in the reaction between hydrogen peroxide and biologically generated chromium (V) species. Archives of Biochemistry and Biophysics 277(2), 342-350.
- Slater, T.I., (1980). Overview of methods used for detecting lipid peroxidation. Methods in Enzymology 105, 283-293.
- Tchounwou, P.B., Yedjou, C.G., Patlolla, A.K., Sutton, D. J., (2012). Heavy metals toxicity and the environment. Molecular, Clinical and Environmental Toxicology 101,133-164.
- Wang, J.Q., Zou, J. Li, Y.H., Cheng, W.M., Zhang, C. Lu, L., Ge, J.F., Huang, C., (2009). Preventive effects of total flavonoids of Litsea coreana leve on hepatic steatosis in rats fed with high fat diet. Journal of Ethnopharmacology 121, 54-60.
- Yildiz, D., Ercal, N., Armstrong, D.W., (1998). Nicotine enantiomers and oxidative stress. Toxicology 130, 155-165.
- Young, D.S., Pestaner, L.C., Gibberman, V., (1975). Effects of drugs on clinical laboratory tests. Clinical Chemistry 21, 1D-432D.
- Zhang, G., Kernan, K.A., Thomas, A., Collins, S., Song, Y., Zhu, L. Li, W., Leboeuf, R.C., Eddy, A.A., (2009). A novel signaling pathway: fibroblast nicotinic receptor alpha1 binds urokinase and promotes renal fibrosis. Journal of Biological Chemistry 284, 29050-29064.


