NMR 13C spectra of the 1, 1, 3-trimethyl-3-(4-methylphenyl)butyl hydroperoxide in various solvents: molecular modeling
Автор: Turovskiy Nikolay Antonovich, Raksha Elena Vladimirovna, Berestneva Yuliya Vasilyevna
Журнал: НБИ технологии @nbi-technologies
Рубрика: Технико-технологические инновации
Статья в выпуске: 3 (18), 2015 года.
Бесплатный доступ
GIAO-ca lculat ed NM R 13C chemical shifts as obtained at various computational levels are reported for the 1,1,3-trimethyl-3-(4-methylphenyl)butyl hydroperoxide. The data are compared with experimental solution data in chloroform-d, acetonitrile-d 3, and DMSO-d 6, focusing on the agreement with spectral patterns and spectral trends. Calculation of magnetic shielding tensors and chemical shifts for 13C nuclei of the 1,1,3-trimethyl-3-(4- methylphenyl)butyl hydroperoxide molecule in the approximation of an isolated particle and considering the solvent influence in the framework of the continuum polarization model (PCM) was carried out. Comparative analysis of experimental and computer NMR spectroscopy results revealed that the GIAO method with MP2/6-31G(d,p) level of theory and the PCM approach can be used to estimate the NMR 13C chemical shifts of the 1,1,3-trimethyl-3-(4- methylphenyl)butyl hydroperoxide.
Nmr spectroscopy, 3-trimethyl-3-(4-methylphenyl)butyl hydroperoxide, chemical shift, magnetic shielding constant, giao, molecular modeling, nmr-спектроскопия
Короткий адрес: https://sciup.org/14968405
IDR: 14968405 | УДК: 669.017 | DOI: 10.15688/jvolsu10.2015.3.7
Текст научной статьи NMR 13C spectra of the 1, 1, 3-trimethyl-3-(4-methylphenyl)butyl hydroperoxide in various solvents: molecular modeling
DOI:
Arylalkyl hydroperoxides are useful starting reagents in the synthesis of surface-active peroxide initiators for the preparation of polymeric colloidal systems with improved stability [5]. Thermolysis of arylalkyl hydroperoxides was studied in acetonitrile [11]. NMR 1Н spectroscopy has been already used successfully for the experimental evidence of the complex formation between a 1,1,3-trimethyl-3-(4-methylphenyl)butyl hydroperoxide and tetraalkylammonium bromides in acetonitrile [2; 9; 12] and chloroform solution [9]. The aim of this work is a comprehensive study of the 1,1,3-trimethyl-3-(4-methylphenyl)butyl hydroperoxide (ROOH) by experimental NMR 13C spectroscopy and molecular modeling methods.
Experimental
The 1,1,3-trimethyl-3-(4-methylphenyl)butyl hydroperoxide (ROOH) was purified according to [5]. Its purity (99 %) was controlled by iodometry method as well as by NMR spectroscopy. Experimental NMR 13C spectra of the hydroperoxide solutions were obtained by using the Bruker Avance II 400 spectrometer (NMR 1Н – 400 MHz, NMR 13C – 100 MHz) at 297 K. Solvents, chloroform-d, acetonitrile-d3, and DMSO-d6, were Sigma-Aldrich reagents and were used without additional purification but were stored above molecular sieves before using. Tetramethylsilane (TMS) was internal standard. The hydroperoxide concentration in solutions was 0.03 mol ∙ dm-3.
Molecular geometry and electronic structure parameters, as well as harmonic vibrational frequencies of the 1,1,3-trimethyl-3-(4-methylphenyl)butyl hydroperoxide molecule were calculated after full geometry optimization in the framework of B3LYP/6-31G(d,p) and MP2/ 6-31G(d,p) methods. The resulting equilibrium molecular geometry was used for total electronic energy calculations by the B3LYP/6-31G(d,p) and MP2/6-31G(d,p) methods. All calculations have been carried out using the Gaussian03 [4] program.
The magnetic shielding tensors (c, ppm) for 13С nuclei of the hydroperoxide and the reference molecule were calculated with the МР2/6-31G(d,p) and B3LYP/6-31G(d,p) equilibrium geometries by standard GIAO (Gauge-Independent Atomic Orbital) approach [13]. The calculated magnetic isotropic shielding tensors, χ i, were transformed to chemical shifts relative to TMS molecule, δ i , by δ i = χ ref - χ i , where both, χ ref and χ i , were taken from calculations at the same computational level. Table 1 illustrates c values for TMS molecule used for the hydroperoxide 13C nuclei chemical shifts calculations.
χ values were also estimated in the framework of 6-311G(d,p) and 6-311++G(d,p) basis sets on the base of МР2/6-31G(d,p) and B3LYP/6-31G(d,p) equilibrium geometries. The solvent effect was considered in the РСМ approximation [3; 7]. χ values for magnetically equivalent nuclei were averaged.
Inspecting the overall agreement between experimental and theoretical spectra RMS errors ( σ ) were used to consider the quality of the 13С nuclei chemical shifts calculations. Correlation coefficients ( R ) were calculated to estimate the agreement between spectral patterns and trends.
Results and Discussions
Experimental NMR 13С spectra of the 1,1,3-trimethyl-3-(4-methylphenyl)butyl hydroperoxide
Experimental NMR 13C spectra of the 1,1,3-trimethyl-3-(4-methylphenyl)butyl hydroperoxide
Table 1
Magnetic shielding tensors for 13С nuclei of the TMS
|
Solvent |
MP2 |
B3LYP |
||||
|
1 |
2 |
3 |
1 |
2 |
3 |
|
|
- |
207.54 |
199.71 |
199.37 |
191.80 |
184.13 |
183.72 |
|
Chloroform |
207.86 |
200.13 |
199.79 |
192.08 |
184.53 |
184.13 |
|
Acetonitrile |
208.01 |
200.32 |
199.99 |
192.19 |
184.70 |
184.30 |
|
DMSO |
208.01 |
200.33 |
200.00 |
192.30 |
184.81 |
184.40 |
Note . 1 - 6-31G(d,p); 2 - 6-311G(d,p); 3 - 6-311++G(d,p).
(ROOH) were obtained from chloroform-d, acetonitrile-d3, and DMSO-d6 solutions. The hydroperoxide concentration in all samples was 0.03 mol ∙ dm-3. The experimental NMR 13C spectra of the ROOH are presented in Fig. 1.
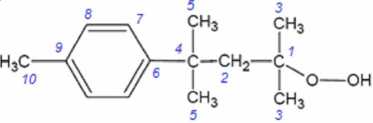
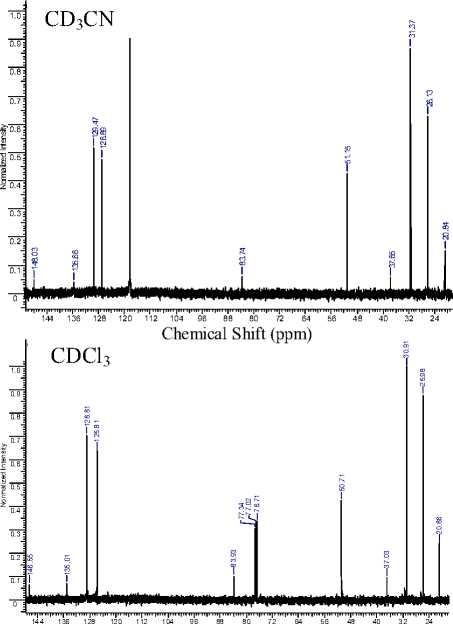
Ten signals for the hydroperoxide carbon atoms are observed in the ROOH 13C NMR spectrum. Signal of the carbon atom bonded with a hydroperoxide group shifts slightly to the stronger fields with the solvent polarity increasing, while the remaining signals are shifted to weak fields. A linear dependences between the 13C chemical shifts values of the hydroperoxide are observed in the studied solvents (Fig. 1). This is consistent with the authors [1], who showed linear correlation between the chemical shifts values in chloroform-d and dimethylsulphoxide-d6 for a large number of organic compounds of different
Chemical Shift (ppm)
(CD 3 ) 2 SO
1.0
0.9
0.8
0.7
0.6
0.5
0.4
0.3
0.2
0.1
ймйи^^
δ CDCl3
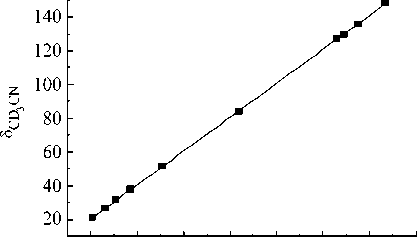
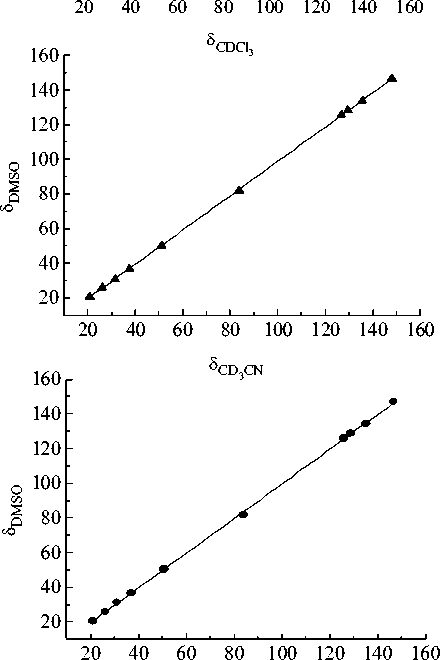
Fig. 1. The relationship between the e xperimental NMR 13C chemical shifts (relative to TMS) of the 1,1,3-trimethyl-3-(4-methylphenyl)butyl hydroperoxide in different solvents
144 136 128 120 112 104 96 88 80 72 64 56 48 40 32 24 16
Chemical Shift (ppm)
classes. Equations corresponded to the obtained relationships (Fig. 1) are listed below.
5 cd 3 cn = ( 0.02±0.23 ) + ( 1.006±0.002 ) 5^ 5 DMso-d6 = ( -0.46±0.41 ) + ( 0.999±0.004 ) 5^ 5 DMso—d6 = ( -0.48±0.24 ) + ( 0.993±0.003 ) 5 cd 3 cn
Molecular modeling of the 1,1,3-trimethyl-3-(4-methylphenyl)butyl hydroperoxide NMR 13С spectra by МР2 and B3LYP methods
The hydroperoxide molecule geometry optimization in the framework of МР2/6-31G(d,p) and B3LYP/6-31G(d,p) methods was carried out as the first step of the hydroperoxide NMR 13С spectra modeling. Initial hydroperoxide configuration chosen for calculations was the one obtained by semiempirical AM1 method and used recently for the hydroperoxide O-O bond homolysis [11] as well as complexation with Et4NBr [2; 10] modeling. The main parameters of the hydroperoxide fragment molecular geometry obtained in the isolated particle approximation within the framework of MP2/6-31G(d,p) (Fig. 2) and B3LYP/6-31G(d,p) levels of theory are presented in Table 2. Peroxide bond O-O is a reaction centre in this type of chemical initiators. Thus, the main attention was focused on the geometry of -CO-OH fragment. The calculation results were compared with known experimental values for the tert-butyl hydroperoxide [6], and appropriate agreement between calculated and experimental parameters can be seen in the case of МР2/6-31G(d,p) method.
Calculation of 13C chemical shifts of the hydroperoxide was carried out by GIAO method in the approximation of an isolated particle as well as in studied solvents within the PCM model, which takes into account the nonspecific solvation. Equilibrium hydroperoxide geometries obtained in the framework of MP2/6-31G(d,p) and B3LYP/ 6-31G(d,p) levels of theory for the isolated particle approximation were used in all cases.
The chemical shift values ( 8 , ppm) for 13C nuclei in the hydroperoxide molecule were evaluated on the base of calculated magnetic shielding constants ( x , ppm). TMS was used as
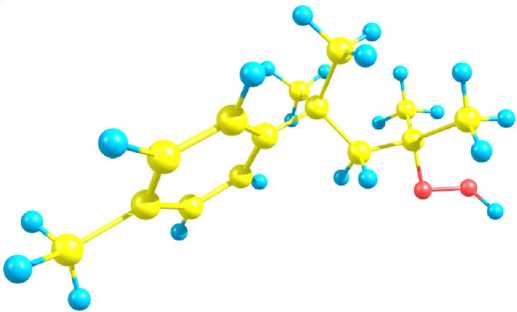
Fig. 2. The 1,1,3-trimethyl-3-(4-methylphenyl)butyl hydroperoxide structural model (МР2/6-31G(d,p) method)
Table 2
Molecular geometry parameters of the 1,1,3-trimethyl-3-(4-methylphenyl)butyl hydroperoxide -СО-ОН moiety
|
Parameter |
МР2/6-31G(d,p) |
B3LYP/6-31G(d,p) |
Experiment |
|
l O-O , Å |
1.473 |
1.456 |
1.473 |
|
l C-O , Å |
1.459 |
1.465 |
1.443 |
|
l O-H , Å |
0.970 |
0.971 |
0.990 |
|
C-O-O, º |
108.6 |
110.0 |
109.6 |
|
O-O-H, º |
98.2 |
99.9 |
100.0 |
|
C-O-O-H, º |
112.4 |
109.1 |
114.0 |
Note. Experimental values are those for tert -butyl hydroperoxide from [13].
standard, for which the molecular geometry optimization and χ calculation were performed using the same level of theory and basis set. Values of the 13C chemical shifts were found as the difference of the magnetic shielding tensors of the corresponding TMS and hydroperoxide nuclei (Tables 3 and 4).
The correct spectral pattern for the hydroperoxide NMR 13C spectrum was obtained for all methods and basis sets used within the isolated molecule approximation (see Table 3) as well as solvation accounting (see Table 4). Exceptions are aromatic C8 and C9 carbons, which signals are interchanged for all calculations.
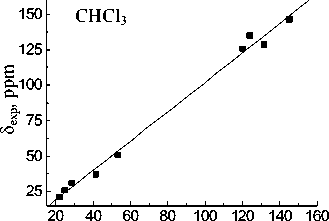
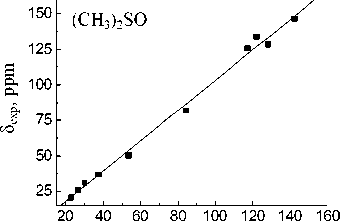
The best reproduced experimental chemical shift value for the carbon atom of the CO-OH group is observed in the case of MP2/6-31G(d,p) approximation in all used solvents whereas B3LYP with the same basis set gives slightly worse values.
Basis set extension to 6-311++G(d,p) leads to a deterioration of the calculation results. Calculated value for the carbon of CO-OH group (83.61 ppm) within the isolated molecule approximation is closest to experimental one in acetonitrile (83.74 ppm). When passing to the calculations in the PCM mode solvation accounting leads to more correct results for the MP2 and B3LYP methods. The lowest σ values for all solvents are obtained with 6-31G(d,p) basis set. Linear relationships between the experimental NMR 13C chemical shifts and the calculated values δ calc for the hydroperoxide 13C nuclei (see Fig. 3) have been obtained for both methods and all basis sets. The correlation coefficients ( R ) corresponding to obtained dependences are shown in Table 4. Joint account of σ and R values indicates possibility of MP2 method with 6-31G(d,p) basis set using for the calculation of the hydroperoxide 13C nuclei chemical shifts.
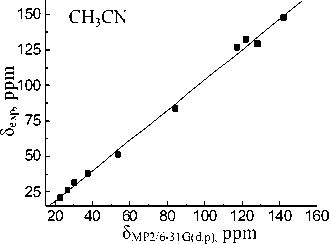
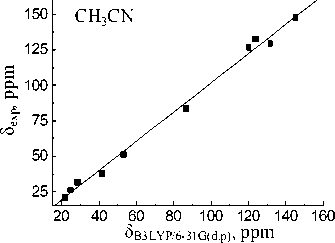
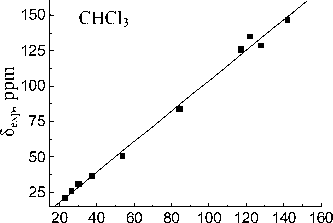
δ МР2/6-31G(d.p), ppm
δ МР2/6-31G(d.p), ppm
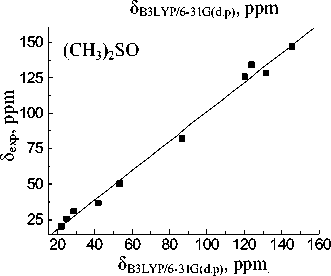
Fig. 3. Experimental ( δ exp) versus GIAO calculated 13С chemical shifts (relative to TMS) of the 1,1,3-trimethyl-3-(4-methylphenyl)butyl hydroperoxide
Table 3
NMR 13С chemical shifts ( δ , ppm) of the 1,1,3-trimethyl-3-(4-methylphenyl)butyl hydroperoxide (the isolated particle approximation)
|
Nuclei |
MP2 |
B3LYP |
||||
|
1 |
2 |
3 |
1 |
2 |
3 |
|
|
C1 |
83.61 |
86.87 |
88.24 |
85.77 |
90.90 |
92.04 |
|
C2 |
53.50 |
57.48 |
57.42 |
53.23 |
57.70 |
57.00 |
|
C3 |
26.46 |
26.71 |
26.62 |
24.62 |
25.27 |
24.93 |
|
C4 |
37.04 |
40.23 |
40.37 |
41.14 |
44.66 |
44.49 |
|
C5 |
30.10 |
30.93 |
31.03 |
28.57 |
29.78 |
29.75 |
|
C6 |
141.39 |
153.47 |
153.93 |
144.46 |
158.38 |
158.37 |
|
C7 |
116.91 |
125.90 |
126.29 |
119.80 |
130.58 |
131.03 |
|
C8 |
127.07 |
137.37 |
137.97 |
130.75 |
142.40 |
143.32 |
|
C9 |
121.55 |
130.79 |
131.41 |
123.68 |
134.44 |
135.07 |
|
C10 |
22.98 |
23.82 |
23.75 |
21.84 |
23.25 |
22.84 |
Note. 1 - 6-31G(d,p); 2 - 6-311G(d,p); 3 - 6-311++G(d,p).
Table 4
NMR 13С chemical shifts (δ, ppm) of the 1,1,3-trimethyl-3-(4-methylphenyl)butyl hydroperoxide in different solvents
|
Nuclei |
MP2 |
B3LYP |
4 |
||||
|
1 \ |
2 1 |
3 |
1 1 |
2 1 |
3 |
||
|
Chloroform |
|||||||
|
C1 |
84.29 |
87.74 |
89.27 |
86.44 |
91.86 |
93.14 |
83.93 |
|
C2 |
53.69 |
57.73 |
57.64 |
53.37 |
57.89 |
57.17 |
50.71 |
|
C3 |
26.62 |
26.99 |
26.89 |
24.73 |
25.52 |
25.17 |
25.98 |
|
C4 |
37.46 |
40.74 |
40.92 |
41.54 |
45.20 |
45.07 |
37.03 |
|
C5 |
30.18 |
31.10 |
31.20 |
28.59 |
29.90 |
29.88 |
30.91 |
|
C6 |
142.14 |
154.40 |
154.90 |
145.12 |
159.23 |
159.25 |
146.55 |
|
C7 |
117.33 |
126.51 |
126.91 |
120.11 |
131.14 |
131.60 |
125.81 |
|
C8 |
127.92 |
138.43 |
139.03 |
131.56 |
143.43 |
144.34 |
128.81 |
|
C9 |
121.90 |
131.35 |
131.91 |
123.85 |
134.81 |
135.37 |
135.01 |
|
C10 |
23.01 |
23.96 |
23.88 |
21.81 |
23.36 |
22.93 |
20.86 |
|
σ |
27.86 |
25.63 |
28.66 |
20.82 |
59.16 |
63.32 |
- |
|
R |
0.997 |
0.997 |
0.997 |
0.996 |
0.996 |
0.996 |
- |
|
Acetonitrile |
|||||||
|
C1 |
84.58 |
88.13 |
89.73 |
86.72 |
92.27 |
93.61 |
83.74 |
|
C2 |
53.80 |
57.88 |
57.78 |
53.44 |
57.98 |
57.27 |
51.15 |
|
C3 |
26.70 |
27.13 |
27.02 |
24.77 |
25.63 |
25.28 |
26.13 |
|
C4 |
37.64 |
40.99 |
41.18 |
41.71 |
45.43 |
45.32 |
37.65 |
|
C5 |
30.22 |
31.19 |
31.29 |
28.59 |
29.95 |
29.93 |
31.37 |
|
C6 |
142.50 |
154.84 |
155.37 |
145.42 |
159.62 |
159.66 |
148.03 |
|
C7 |
117.52 |
126.80 |
127.20 |
120.24 |
131.37 |
131.84 |
126.89 |
|
C8 |
128.29 |
138.90 |
139.49 |
131.88 |
143.85 |
144.75 |
129.47 |
|
C9 |
122.08 |
131.63 |
132.17 |
123.94 |
135.00 |
135.52 |
132.66 |
|
C10 |
23.03 |
24.04 |
23.96 |
21.80 |
23.41 |
22.97 |
20.84 |
|
σ |
24.571 |
22.325 |
25.741 |
17.395 |
55.560 |
60.243 |
|
|
R |
0.998 |
0.998 |
0.998 |
0.997 |
0.997 |
0.996 |
|
|
DMSO |
|||||||
|
C1 |
84.60 |
88.15 |
89.75 |
86.84 |
92.38 |
93.72 |
81.79 |
|
C2 |
53.80 |
57.88 |
57.78 |
53.54 |
58.09 |
57.37 |
50.18 |
|
C3 |
26.70 |
27.13 |
27.03 |
24.87 |
25.74 |
25.37 |
25.76 |
|
C4 |
37.65 |
41.00 |
41.19 |
41.82 |
45.54 |
45.43 |
36.64 |
|
C5 |
30.22 |
31.19 |
31.30 |
28.69 |
30.06 |
30.03 |
30.85 |
|
C6 |
142.52 |
154.86 |
155.39 |
145.54 |
159.74 |
159.77 |
146.73 |
|
C7 |
117.53 |
126.81 |
127.22 |
120.35 |
131.48 |
131.94 |
125.65 |
|
C8 |
128.31 |
138.92 |
139.52 |
131.99 |
143.97 |
144.86 |
128.43 |
|
C9 |
122.09 |
131.64 |
132.18 |
124.05 |
135.11 |
135.62 |
133.98 |
|
C10 |
23.03 |
24.04 |
23.96 |
21.90 |
23.51 |
23.06 |
20.45 |
|
σ |
25.496 |
31.656 |
35.949 |
21.206 |
70.994 |
76.092 |
|
|
R |
0.997 |
0.997 |
0.997 |
0.995 |
0.996 |
0.995 |
|
Note. 1 - 6-31G(d,p); 2 - 6-311G(d,p); 3 - 6-311++G(d,p); 4 - experimental data.
Conclusions
A comprehensive study of the 1,1,3-trimethyl-3-(4-methyl-phenyl)butyl hydroperoxide by experimental NMR 13C spectroscopy and molecular modeling methods was performed. A comparative assessment of the 13C nuclei chemical shifts calculated by GIAO in various approximations. For NMR 13C spectra of the hydroperoxide in different solvents MP2 and B3LYP methods approximations with 6-31G(d,p), 6-311G(d,p), and 6-311++G(d,p) basis sets allow to obtain the correct spectral pattern. A linear correlations between the calculated and experimental values of the 13C chemical shifts for the studied hydroperoxide molecule were obtained for all solvents studied. In all cases, the MP method combined with 6-31G(d,p) basis set allows to get a better agreement between the calculated and experimental data as compared to the B3LYP results.
Список литературы NMR 13C spectra of the 1, 1, 3-trimethyl-3-(4-methylphenyl)butyl hydroperoxide in various solvents: molecular modeling
- Abraham R.J., Byrne J.J., Griffiths L., Perez M. 1H Chemical Shifts in NMR: Part 23. The Effect of Dimethyl Sulphoxide Versus Chloroform Solvent on 1H Chemical Shifts. Magn. Reson. Chem., 2006, vol. 44, pp. 491-509.
- Berestneva Yu.V., Raksha E.V., Тurovskiy N.А., Zubritskiy M.Yu. Interaction of the 1,1,3-Trimethyl-3-(4-Methylphenyl)Butyl Hydroperoxide With Tetraethylammonium Bromide. Tagirov M.S., Zhikharev V.A., eds. Current Problems of Magnetic Resonance and Its Application: Program Lecture Notes Proceedings of the 17th International Youth Scientific School (Kazan, June 22-27, 2014). Kazan, Kazan State University, 2014, pp. 113-116.
- Cossi M., Scalmani G., Rega N., Barone V. New Developments in the Polarizable Continuum Model for Quantum Mechanical and Classical Calculations on Molecules in Solution. J. Chem. Phys., 2002, vol. 117, pp. 43-54.
- Frisch M.J., et al. Gaussian 03, Revision B.01. Gaussian, Inc., Pittsburgh PA, 2003.
- Kinash N.I., Vostres V.B. Synthesis of the Aryl-Containing Peroxides -2-Methyl-4-Pentane Derivatives. Scientific Journal of Lviv Polytechnic National University, 2003, no. 529, pp. 124-128.
- Kosnikov A.Yu., Antonovskiy V.L., Lindeman S.V., Antipin M.Yu., Struchkov Yu.T., Turovskiy N.A., Zyatkov I.P. X-ray Crystallographic and Quantum-Chemical Investigation of Tert-Butyl Hydroperoxide. Theoretical and Experimental Chemistry, 1989, vol. 25, iss. 1, pp. 73-77.
- Mennucci B., Tomasi J. Continuum Solvation Models: A New Approach to the Problem of Solute’s Charge Distribution and Cavity Boundaries. J. Chem. Phys., 1997, vol. 106, pp. 5151-5158.
- Raksha E.V., Berestneva Yu.V., Тurovskiy N.А., Zubritskiy М.Yu. Quantum Chemical Modeling of the 1,1,3-T r imeth yl-3-(4 -Met h yl-P h en yl)Bu tyl Hydroperoxide NMR 1H and 13C Spectra. Scientific Publications of Donetsk National Technological University, Chemical and Chemical Technology Series, Donetsk, Donetsk National Technological University, 2014, iss. 1 (22), pp. 150-156.
- Turovskiy N.А., Berestneva Yu.V., Raksha Е.V., Opeida J.А, Zubritskiy M.Yu. Complex Formation Between Hydroperoxides and Alk4NBr on the Base of NMR Spectroscopy Investigations. Russian Chemical Bulletin, 2014, no. 8, pp. 1717-1721..
- Тurovskiy N.А., Raksha E.V., Berestneva Yu.V., Pasternak E.N., Zubritskiy M.Yu., Opeida I.A., Zaikov G.Е. Supramolecular Decomposition of the Aralkyl Hydroperoxides in the Presence of Et4NBr. Pethrick R.A., Pearce E.M., Zaikov G.E., eds. Polymer Products and Chemical Processes: Techniques, Analysis Applications. Toronto, New Jersey, Apple Academic Press, Inc., 2013. 270 p.
- Turovskiy M.A., Raksha O.V., Opeida I.O., Turovskaya O.M., Zaikоv G. E. Molecu lar Modelling of Aralkyl Hydroperoxides Homolysis. Oxidation Communications, 2007, vol. 30, no. 3, pp. 504-512.
- Turovskiy N.A., Raksha E.V., Berestneva Yu.V., Zubritskiy M.Yu. Formation of 1,1,3-Trimethyl-3-(4-Methylphenyl)Butyl Hydroperoxide Complex With Tetrabutylammonium Bromide. Russian J. Gen. Chem., 2014, vol. 84, no. 1, pp. 16-17.
- Wolinski K., Hilton J.F., Pulay P. Efficient Implementation of the Gauge-Independent Atomic Orbital Method for NMR Chemical Shift Calculations. Am. Chem. Soc., 1990, vol. 112, pp. 8251-8260.


