North East India rice genotypes: screening of arsenic tolerant and sensitive rice at germinating stage
Автор: Thounaojam Thorny Chanu, Meetei Thounaojam Thomas, Panda Sanjib Kumar, Upadhyaya Hrishikesh
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 4 т.15, 2019 года.
Бесплатный доступ
Arsenic (As) accumulation in rice is hazardous to both plant and human because of its toxicity and carcinogenic properties. North-East (NE) rice genotypes (76) were screened at germination stage under As treatments (0, 50 and 100 µM) in order to discover As tolerant and sensitive NE rice genotypes. Results showed a differential response to As with different degree of impact that can be categorized as tolerant, moderately tolerant and sensitive rice genotypes. This study reveals a significant background for the selection of the highest tolerant and sensitive rice genotypes.
Arsenic, germination, north east rice, sensitive, tolerant
Короткий адрес: https://sciup.org/143168574
IDR: 143168574
Текст научной статьи North East India rice genotypes: screening of arsenic tolerant and sensitive rice at germinating stage
The toxic and carcinogen As is ubiquitous in the environment through natural sources and human activities, causing serious problems to the ecosystem. The two most common inorganic forms of As are arsenite (AsIII) and arsenate (AsV), dominant in submerged soil and aerobic soil respectively, where factors like pH, redox conditions, and microbial activities influence the interconversion of these two forms. As enters in plant from soil and water and then enters food chain affecting both plant and animal. Several studies revealed that As stress in plant results in the generation of reactive oxygen species (ROS) which induced oxidative stress to the biomolecules and finally resulted into cell death as reviewed by Abbas et al. (2018). Among the various crops, the major staple crop rice ( Oryza sativa L. ) causes a big impact on human health through consumption as rice can accumulate high concentration of As (Bakhat et al., 2017).
Rice ( Oryza sativa L.), one of the most important cereal crops, being a paddy field crop particularly susceptible to As stress. Accumulation of As occurs higher in rice than other cereals, affecting the yield, moreover, becomes one of the major routes of As exposure in humans through food chain (Davis et al., 2017) and so, rice contribute a substantial amount to the total arsenic consumption in contaminated area (Roxane Guillod-Magnin et al., 2018). Availability of As in soil and physiological properties of plant, these two factors control the accumulation of As in plants (Santra et al., 2013). It has been found that As species and their concentration differ with soil condition, and rice cultivars (Awasthi et al., 2017).
In North East (NE) India, rice is the primary staple food crop but being an efficient accumulator of As, rice greatly imparts a big impact on human health on consumption. Moreover, rice quality and yield are also greatly affected by As contamination. In order to counteract these problems, there is an urgent need to produce locally adapted or tolerant rice which have low arsenic accumulation. Thus, the study was undertaken to screen NE rice genotypes in germination stage to discover As tolerant and sensitive genotype. This was studied by analysing germination percentage, length of plumule and radical, fresh mass and dry mass and arsenic tolerant index (%) of 76 NE rice under As treatment.
MATERIALS AND METHODS
Viable rice seeds (76 genotypes) were procured from Rice Research Station, Wangbal, Manipur, India; Regional Rainfed Lowland Rice Research Station (RRLRRS) Gerua, Guwahati, Assam, India, ICAR-National Bereau of Plant Genetic Resources (NBPGR) Regional Station, Umiam, Meghalaya, India and ICAR-Research complex North Eastern Hills region, Mizoram, Kolasib, Mizoram, India. The seeds were surface sterilized with 0.1% mercuric chloride (HgCl 2 ) solution and set for germination in petri dishes having filter paper moistened with 10 ml of 50 µM and 100 µM As at 28 ± 2ºC for 3 days. For control (0 µM) distilled water was used. On the fourth day of germination uniformly germinated seeds were selected and done the analysis.
Phenotype of the rice seeds
Seed colour and seed shape were determined on the basis of visual observation. Seed length and breadth were measured by using a scale of ten seeds and took mean value. Seed weight also measured.
Germination percentage (GP)
20 sterilized seeds of each variety were set for germination separately and using the following formula (Abdul et al., 2014), germination percentage was analysed
GP = (Number of total germinated seeds/ Total number of seeds tested) × 100
Growth of seedlings
Growth was determined by measuring the length of the radical and plumule and fresh and dry mass of the seedlings. Dry mass of the seedlings was determined after the seedlings were oven dried for 72 h at 80ºC. Reduction percentage of radical length, plumule length and fresh and dry mass of the seedlings were determined by the formulas (Vibhuti et al., 2015):
Radical length percentage reduction (RLPR) = 100 × [1 - (Radical length As stress/ Radical length control)]
Plumule length percentage reduction (PLPR) = 100 × [1 - (Plumule length As stress/ Plumule length control)]
Fresh weight percentage reduction (DWPR) = 100 ×
[1 - (Fresh weight As stress/ Fresh weight control)]
Dry weight percentage reduction (DWPR) = 100 × [1 - (Dry weight As stress/ Dry weight control)]
Arsenic tolerant index (ATI%)
Using the formula described by Wilkins (1978), ATI (%) was calculated.
ATI % = [( Dry weight of treated plants)/( Dry weight of control plants)] x 100.
Statistical analysis
All data obtained were subjected to one-way analyses of variance (ANOVA) and LSD test was used for comparison between mean of treatments and between rice genotypes. All the experiments were repeated three times and each bar represents mean ± standard error of the three experiments. Asterisk and letters indicate a significant mean difference at P = 0.05.
RESULTS AND DISCUSSION
Phenotype of the 76 rice genotypes were studied which is given in Table 1. Most of the rice genotypes bear straw yellow colour, only few of them have different colours like blackish brown of R43, R49 and R56, reddish yellow of R21, R28, R35, R46, R55 and brownish yellow of R24, R50, R51, R52 and R73. Long bold is the common shaped of the rice seeds but unusually, slight spindle shaped in R16 and oval shaped in R32 are also observed.
From the analysis, ten highest sensitive and ten highest tolerant rice cultivars to As were selected (Fig 1). Germination percentage of the 20 selected rice genotypes under As treatment is given in Fig 2A which showed differential responses of the rice to As. RT3 and RT6 rice genotypes germinated at the rate of 100% with respect to control in both 50 and 100 µM, revealing 100% germination tolerant percentage (GT%) whereas in genotypes like RS11, RS12 and RS16, germination was inhibited almost by 50% in 100 µM, of which the GT % are 54.21%, 51.66 % and 55.55% respectively. The inhibition of germination might be due to the toxic effect of As on seed metabolic activities that resulted into inhibition of certain enzymes require for seed germination and growth. Previous studies also reported inhibition of seed germination under heavy metal stress by lowering of carbohydrate-metabolizing enzymes thereby affecting digestion and mobilization of reserved carbohydrate of seeds (Singh et al., 2011, Pena et al 2011). The study of Adrees et al. (2015) also demonstrated inhibition of seed germination by heavy metal stress including As. The genotype that germinated 100% with respect to control may be due to proper activation of internal As detoxification mechanisms or restriction of As uptake inside the plant thus less toxic effect. Plant operates different mechanisms against stress, different plant response differently. The response to As differs with the different genotypes.
Table 1. Phenotype of the 76 NE rice genotypes
|
Code No. |
Name |
Seed colour |
Seed shape |
Seed length in mm |
Seed breath in mm |
Seed weight in g |
|
R1. |
Anjali |
Straw yellow |
Medium bold |
8 |
3 |
0.025 |
|
R2. |
Bahadur |
Straw yellow |
Medium bold |
8 |
3 |
0.023 |
|
R3. |
Bias muthi |
Straw yellow |
Medium bold |
8 |
3 |
0.021 |
|
R4. |
Bio 25 |
Straw yellow |
Medium bold |
8.3 |
2.8 |
0.025 |
|
R5. |
Chandan |
Straw yellow |
Long bold |
9.3 |
3 |
0.024 |
|
R6. |
CR 310 |
Straw yellow |
Medium bold |
7.6 |
3 |
0.022 |
|
R7. |
CR 311 |
Straw yellow |
Medium bold |
8.6 |
3 |
0.024 |
|
R8. |
CR 601 |
Straw yellow |
Long bold |
9 |
3 |
0.025 |
|
R9. |
CR 909 |
Straw yellow |
Long bold |
9.6 |
3 |
0.025 |
|
R10. |
Dhusari |
Straw yellow |
Long bold |
10 |
3 |
0.028 |
|
R11. |
Hawai |
Straw yellow |
Medium bold |
8 |
3 |
0.027 |
|
R12. |
Ikora |
Reddish brown |
Medium bold |
8 |
3.1 |
0.028 |
|
R13. |
Kanchan |
Straw yellow |
Medium bold |
7.3 |
2.1 |
0.016 |
|
R14. |
Mia |
Straw yellow |
Medium bold |
8 |
3.8 |
0.028 |
|
R15. |
Naveen |
Straw yellow |
Medium bold |
8.3 |
3 |
0.022 |
|
R16. |
Padmini |
Straw yellow |
Slender (Slightly spindle) |
8.3 |
2 |
0.016 |
|
R17. |
Panchnum |
Yellow brown |
Slender |
8 |
2.1 |
0.018 |
|
R18. |
Pankaj |
Straw yellow |
Medium bold |
7.6 |
3 |
0.022 |
|
R19. |
Parijat |
Straw yellow |
Long bold |
9 |
2.8 |
0.023 |
|
R20. |
Prasad |
Straw yellow (dark) |
Medium bold |
8 |
3.3 |
0.023 |
|
R21. |
Sahibhagi dhan |
Reddish yellow |
Slender |
9 |
2.3 |
0.023 |
|
R22. |
Satyabhama |
Straw yellow |
Medium bold |
8.3 |
3 |
0.026 |
|
R23. |
Subnum |
Straw yellow (light) |
Slender |
8.3 |
2 |
0.014 |
|
R24. |
Tarkekoo |
Brownish yellow |
Medium bold |
8.6 |
3.6 |
0.023 |
|
R25. |
TN1 |
Straw yellow |
Medium bold |
7.6 |
3 |
0.019 |
|
R26. |
Chakhao |
Brown |
Long bold |
9.6 |
3 |
0.025 |
|
R27. |
Chakhao Amubi |
Brown |
Long bold |
9.6 |
3 |
0.031 |
|
R28. |
Chakhao Anangbi |
Reddish yellow |
Long bold |
10 |
3.3 |
0.028 |
|
R29. |
Chakhao Angaobi |
Straw yellow |
Medium bold |
8.6 |
3 |
0.033 |
|
R30. |
Changlei |
Straw yellow |
Long bold |
9.7 |
3 |
0.25 |
|
R31. |
Changmen chakhao |
Straw yellow |
Medium bold |
7.6 |
2.8 |
0.019 |
|
R32. |
Heimang |
Straw yellow (dark) |
Oval |
5.6 |
3.6 |
0.021 |
|
R33. |
Heitup |
Straw yellow |
Long bold & slender |
10 |
4 & 2.5 |
0.029 |
|
R34. |
Huikap |
Straw yellow |
Medium bold |
7.3 |
3.1 |
0.025 |
|
R35. |
Kabok |
Reddish yellow |
Long bold |
9 |
3 |
0.024 |
|
R36. |
Katan chakhao |
Straw yellow (blacken at tips) |
Long bold |
9.6 |
4 |
0.032 |
|
R37. |
Keibi |
Straw yellow |
Medium bold |
8.3 |
3.8 |
0.026 |
|
R38. |
Kono aro |
Straw yellow |
Long bold |
10.3 |
3 |
0.029 |
|
R39. |
Kumbi |
Straw yellow |
Long bold |
10 |
3 |
0.027 |
|
R40. |
Moirang phaongaoba |
Straw yellow |
Medium bold |
8.6 |
3 |
0.022 |
|
R41. |
Moirang phou |
Straw yellow (dark) |
Long bold |
9.6 |
3.8 |
0.030 |
|
R42. |
Pat |
Straw yellow |
Long bold |
9.6 |
3.1 |
0.028 |
|
R43. |
Phaugak |
Blackish brown |
Medium bold |
7.4 |
3 |
0.23 |
|
R44. |
Phauren amubi |
Blackish yellow |
Long bold |
10 |
3 |
0.28 |
|
R45. |
Phoudum |
Straw yellow |
Medium bold |
8.3 |
3.8 |
0.028 |
|
R46. |
Phoungang |
Reddish yellow |
Long bold |
9.3 |
3.1 |
0.031 |
|
R47. |
Phouren |
Straw yellow |
Long bold |
9.6 |
3.1 |
0.028 |
|
R48. |
Phouren khongNgangbi |
Straw yellow |
Long bold |
9.6 |
3 |
0.027 |
|
R49. |
Poireiton chakhao |
Blackish brown |
Long bold |
9.3 |
3 |
0.023 |
|
R50. |
Sangsangba |
Brownish yellow |
Long bold |
9.3 |
3.7 |
0.29 |
|
R51. |
Taothabi |
Brownish yellow |
Medium bold |
8.6 |
3.1 |
0.023 |
|
R52. |
Tungoo |
Brownish yellow |
Long bold |
9 |
3.3 |
0.029 |
|
R53. |
Wangoo |
Straw yellow |
Long bold |
9.8 |
3 |
0.029 |
|
R54. |
Yenthik |
Straw yellow (light) |
Long bold |
10 |
3 |
0.031 |
|
R55. |
137492 |
Reddish yellow |
Medium bold |
8.3 |
3 |
0.021 |
|
R56. |
140000 |
Blackish brown |
Long bold |
10 |
3.1 |
0.024 |
|
R57. |
146079 |
Straw yellow |
Medium bold |
8 |
3 |
0.020 |
|
R58. |
200507 |
Straw yellow |
Medium bold |
8.3 |
3 |
0.020 |
|
R59. |
200536 |
Straw yellow |
Medium bold |
8.3 |
2.7 |
0.025 |
|
R60. |
207937 |
Straw yellow |
Medium bold |
8 |
2.65 |
0.021 |
|
R61. |
207949 |
Straw yellow |
Medium bold |
7.3 |
3 |
0.024 |
|
R62. |
264486 |
Straw yellow |
Medium bold |
7.3 |
2.9 |
0.021 |
|
R63. |
324307 |
Straw yellow |
Long bold |
9.1 |
2.35 |
0.025 |
|
R64. |
330250 |
Straw yellow (dark) |
Medium bold |
7.3 |
3 |
0.020 |
|
R65. |
350076 |
Straw yellow |
Medium bold |
8.3 |
3 |
0.022 |
|
R66. |
350818 |
Straw yellow |
Medium bold |
7.7 |
2.9 |
0.017 |
|
R67. |
380488 |
Straw yellow |
Long bold |
10 |
3 |
0.02 |
|
R68. |
463756 |
Straw yellow (light) |
Medium bold |
7.3 |
3.85 |
0.031 |
|
R69. |
466632 |
Straw yellow |
Medium bold |
7.3 |
2.7 |
0.019 |
|
R70. |
466717 |
Straw yellow |
Long bold |
10 |
4 |
0.035 |
|
R71. |
Jwain |
Straw yellow |
Long bold |
9.3 |
3 |
0.029 |
|
R72. |
Myari |
Straw yellow |
Long bold |
9.3 |
3 |
0.030 |
|
R73. |
Pnet |
Brownish yellow |
Long bold |
10 |
3.65 |
0.035 |
|
R74. |
Saw |
Straw yellow |
Medium bold |
8.7 |
3 |
0.032 |
|
R75. |
Hawangsen |
Brownish yellow |
Long bold |
9.3 |
3 |
0.026 |
|
R76. |
Rokamlova |
Straw yellow |
Long bold |
10 |
3 |
0.027 |
О рМ 50 рМ 100 рМ ОрМ 50 рМ 100 рМ 0 рМ 50 рМ 100 рМ 0 рМ 50 рМ 100 рМ

Figure 1. Effect of As (0, 50 and 100 µM) in germinating seedlings of the 20 NE rice genotypes
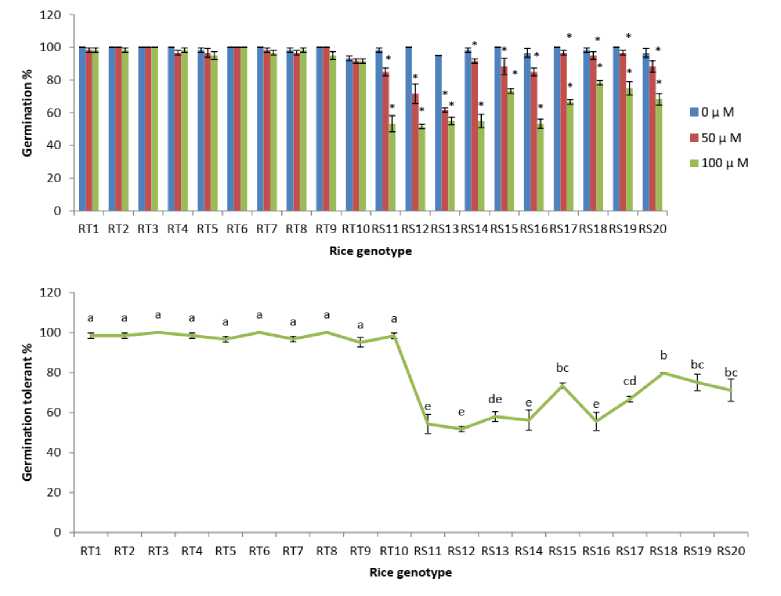
Figure 2. Changes in germination under As treatments (0, 50 and 100 µM) and germination tolerant % at 100 µM As of the rice genotypes. The data presented are mean ± SE (n = 3). * and letters superscripted indicate significant mean difference from control and between the genotypes respectively at 5% level of significance in multiple comparison by LSD test
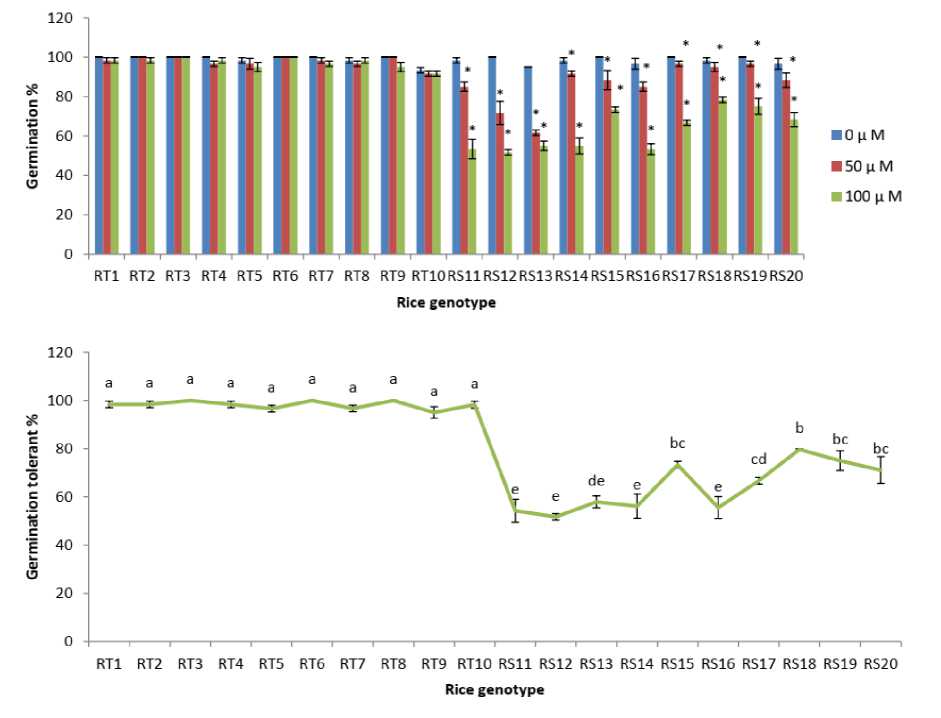
Figure 3. Changes in length of radical and plumule under As treatments (0, 50 and 100 µM) and radical reduction % and plumule reduction % at 100 µM As of rice genotypes. The data presented are mean ± SE (n = 3). * and letters superscripted indicate significant mean difference from control and between the genotypes respectively at 5% level of significance in multiple comparison by LSD test
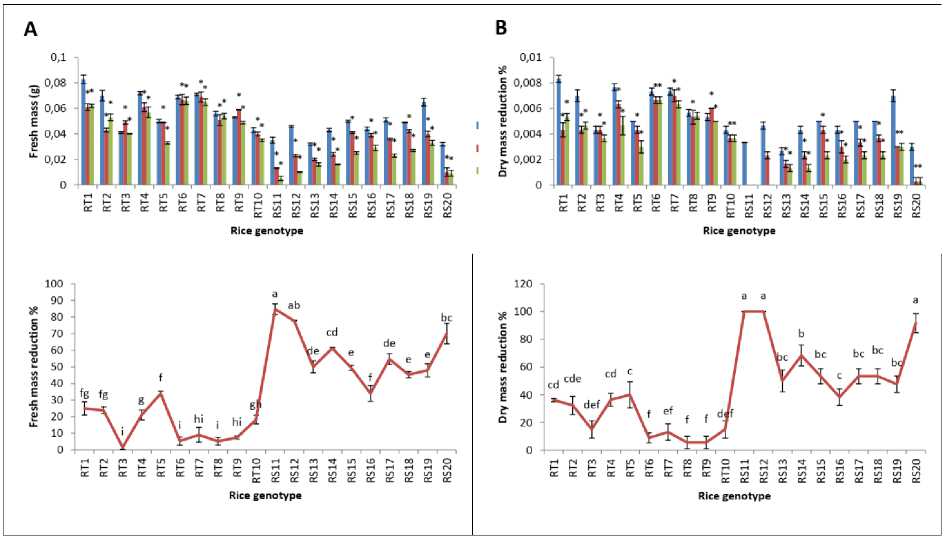
Figure 4. Changes in fresh and dry mass of rice seedlings under As treatments (0, 50 and 100 µM) and reduction % of fresh and dry mass at 100 µM As of rice genotypes. The data presented are mean ± SE (n = 3). * and letters superscripted indicate significant mean difference from control and between the genotypes respectively at 5% level of significance in multiple comparison by LSD test.
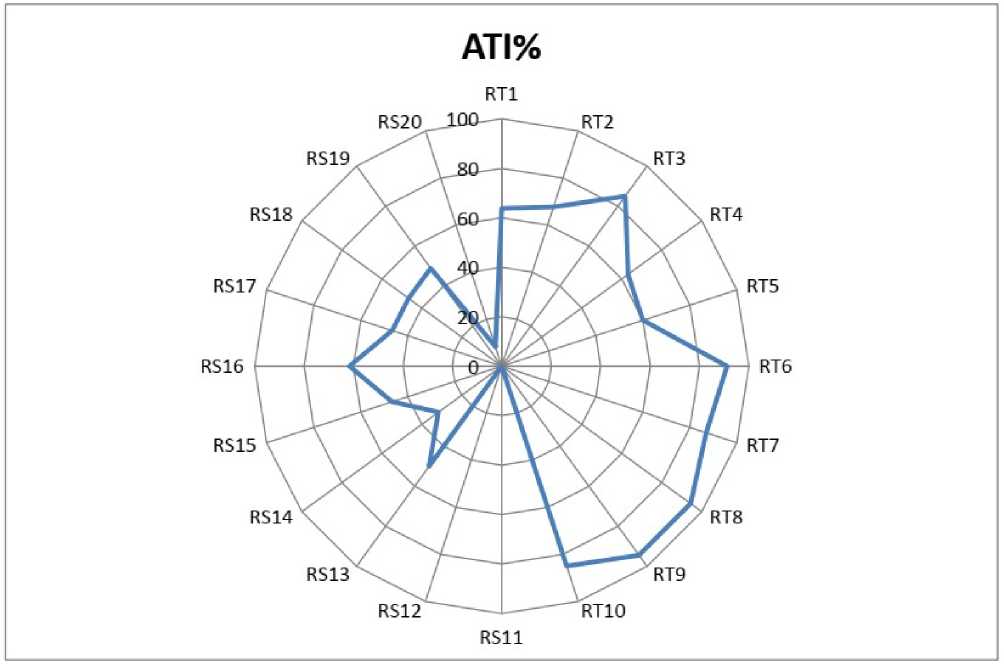
Figure 5. Arsenic tolerant index % (ATI%) of the 20 NE rice seedlings under 100 µM As. The data presented are mean ± SE (n = 3). Different letters superscripted indicates significant mean difference between the genotypes at 5% level of significance in multiple comparison by LSD test.
Fresh mass of the 20 rice genotypes is given in Fig. 4A, revealing differential response of the rice genotypes under As treatments. A gradual decrease in fresh mass is observed with the increased of As concentration where highest reduction is observed in the genotype RS11 with fresh mass reduction of 84.68% in comparison with control, which is followed by RS12 (77.70%), and RS20 (70.03%). Reduction may be due to the induction of oxidative stress to rice the seedlings by the As induced reactive oxygen species (ROS) production which lead to disturbances in various metabolisms (Liang 2018).
The rice genotype with lowest fresh mass reduction % with respect to control is RT3 (1.58%) followed by RT8 (5.26%), RT6 (5.39%), RT9 (7.47%) and RT7 (9.12%). The result of the dry mass analysis also revealed the alteration of rice plant growth under As treatment (Fig. 4B). Highest reduction of dry mass was observed in rice genotypes RS11 and RS12 at 100 µM As with respect to the control. However, it is seen that As showed less effect in the rice genotypes RT8 and
RT9, with only 5.5 DWPR with respect to control. The result of the ATI% at 100 µM As also clearly revealed the variation in response to As stress in the NE rice genotypes. In the study, lowest ATI% is observed in RS11 and RS12 rice genotypes with 0 tolerant with As whereas, RT8 and RT9 showed highest tolerant index with 94.44 ATI% in each genotype, followed by RT6 (91.07%), RT7 (86.90%) and RT10 (85%) with respect to control. The variation in response to As of the rice genotypes may be due to genetic differences which resulted into difference protective mechanisms.
CONCLUSION
ACKNOWLEDGEMENT
The financial support from the Department of Science and Technology (DST), Government of India, under DST Women Scientist A scheme (Reference No: SR/WOS-A/ LS-159/2017), is greatly acknowledged and the Rice Research Station, Wangbal, Manipur, India; Regional Rainfed Lowland Rice Research Station (RRLRRS) Gerua, Guwahati, Assam, India, ICAR-National Bureau of Plant Genetic Resources (NBPGR) Regional Station, Umiam, Meghalaya, India and ICAR- Research complex North Eastern Hills region, Mizoram, Kolasib, Mizoram, India, are too highly acknowledge for providing rice seeds for the research.
Список литературы North East India rice genotypes: screening of arsenic tolerant and sensitive rice at germinating stage
- Abbas G., Murtaza B., Bibi I., Shahid M., Niazi NK., Khan MI., Amjad M., Hussain M., Natasha. (2018) Arsenic uptake, toxicity, detoxification, and speciation in plants: Physiological, biochemical, and molecular aspects. Int. J. Environ. Res. Public Health, 15, 5
- Adrees M., Ali S., Rizwan M., Ibrahim M., Abbas F., Farid M. (2015) The effect of excess copper on growth and physiology of important food crops: A review. Environ. Sci. Pollut. R., 22, 8148-8162
- Awasthi S., Chauhan R., Srivastava S., Tripathi R.D. (2017) The Journey of arsenic from soil to grain in rice. Front. Plant Sci., 8, 1007
- Bakhat HF., Zia Z., Fahad S., Abbas S., Hammad H.M., Shahzad A.N., Abbas F., Alharby H., Shahid M. (2017) Arsenic uptake, accumulation and toxicity in rice plants: Possible remedies for its detoxification: A review. Environ. Sci. Pollut. Res., 24, 9142-9158
- Davis M.A., SignesPastor A.J., Argos M., Slaughter F., Pendergrast C., Punshon G.A., Ahsan H., Karagas M.R. (2017) Assessment of human dietary exposure to arsenic through rice. Sci Total Environ., 586, 1237-124
- Liang D. (2018) A salutary role of reactive oxygen species in intercellular tunnel-mediated communication. Front. Cell Dev Biol., 6, 2
- Md. Abdul Halim., Ghosh M., Nigar M., Hossain F., Akhter N. (2014) Screening of arsenic tolerance in rice at germination and early seedling stage as influenced by sodium arsenate. Jahangirnagar Univ. J. Biol. Sci., 3, 17-26
- Pena L.B., Azpilicueta C.E., Gallego S.M. (2011) Sunflower cotyledons cope with copper stress by inducing catalase subunits less sensitive to oxidation. J. Trace Elem. Med. Biol., 25, 125 129
- Roxane Guillod-Magnin R., Brüschweiler B.J., Aubert R., Haldimann M. (2018) Arsenic species in rice and rice-based products consumed by toddlers in Switzerland. Food Addit Contam Part A, 35, 1164-1178
- Santra S.C., Samal A.C., Bhattacharya P., Banerjee S., Biswas A., Majumdar J. (2013) Arsenic in food chain and community health risk: A study in Gangetic West Bengal. Procedia Environ. Sci., 18, 2-13
- Singh H.P., Kaur G., Batish D.R., Kohli R.K. (2011) Lead (Pb) inhibited radical emergence in Brassica campestris involves alterations in starch metabolizing enzymes. Biol. Trace. Elem. Res., 144, 295 301
- Tiwari S., Lata C. (2018) Heavy metal stress, signaling, and tolerance due to plant-associated microbes: An Overview. Front. Plant Sci., 9, 452
- Vibhuti, Shahi C., Bargali K., Bargali S.S. (2015) Seed germination and seedling growth parameters of rice (Oryza sativa) varieties as affected by salt and water stress. Indian J. Agric. Sci., 85, 102-108
- Wilkins D.A. (1978) The measurement of tolerance to edaphic factors by means of root growth. New Phytol., 80, 623-633


