Новый тип наноструктур атомарного разрешения в алмазоподобных и других углеродных веществах
Автор: Шумилова Т.Г., Майер Е., Герварц К.
Журнал: Вестник геонаук @vestnik-geo
Рубрика: Научные статьи
Статья в выпуске: 5 (245), 2015 года.
Бесплатный доступ
К настоящему времени уже опубликованы многочисленные данные о наноструктурах атомарного разрешения (НСАР) углеродных веществ. Развитие системного описания наноструктурированных веществ, включая синтетические и природные, имеет важное значение для фундаментальной и прикладной науки. В данной статье мы предлагаем классификацию углеродных наноструктур и описание нового смешанного (промежуточного) типа наноструктуры атомарного разрешения помимо известных упорядоченных и разупорядоченных наноструктур. Смешанные НСАР в природном алмазоподобном углероде характеризуются одновременным присутствием (совмещением в пространстве) упорядоченного и неупорядоченного строения с малой дальностью структурного порядка, но с сохраняющимся дальним характером кристаллографического направления упорядоченных областей. Экспериментально установленный новый тип наноструктур атомарного разрешения представляет новый принцип строения вещества не только для углеродных веществ, но и для твердых материалов в целом. Обнаружение нового типа НСАР дает возможность для развития материалов, для теоретического и экспериментального моделирования эволюции состояния твердого вещества.
Углерод, алмаз, алмазоподобный углерод наноструктуры, высокоразрешающая просвечивающая электронная микроскопия
Короткий адрес: https://sciup.org/149129165
IDR: 149129165
Текст научной статьи Новый тип наноструктур атомарного разрешения в алмазоподобных и других углеродных веществах
The study of materials and mineral substances at the nano-level has become one of the most relevant materials related research areas and has drawn great attention both in the fields of fundamental and applied science — possibly resulting in new nanotechnologies and nanomaterials. The investigation of carbon-based mineral substances at atomic resolution has just started [1, 2], while in the field of materials science, rather rich data have already been accumulated on carbonbased nanostructures [3—11 and many others]. Analyzing an abundant number of carbon-based nanostructures it is very important to understand the main principles of their formation. In this context, the development of a systematic description of synthetic and natural nanostructured substances is of great importance. Here we present: 1) a detailed study of natural diamond-like carbon, 2) the results of a systematic categorization of atomic scale nanostructures over a wide range of natural carbon substances, and 3) describe the finding of mixed nanostructure types that represent a new state of carbon-based materials.
Material and methods
For the detailed study of ASNS by high resolution transmission electron microscopy, we used native carbon from the Kudymkol microdiamond deposit (Kazakhstan) with abundant diamond and co-existing graphite mineralization. The diamonds in the deposit occur in metasomatically intensively altered crustal-derived metamorphic rocks of Precambrian age in Kokchetav Massif (Northern Kazakhstan), the geological and petrological features were described in detail by L. D. Lavrova with co-authors [12, 13], diamond mineralization is estimated to occur at 531±9 Ma [14]. The
Kudymkol deposit is interesting due to anomalous typomorphic diamond peculiarities [15-20], the presence of diamond within all types of metasomatically altered rocks of the massif, and huge diamond concentrations (up to hundreds carats per ton). Altered gneisses contitute 89.6 % of diamond productivity with a total storage capacity of about 3 billion carats, according to industrial studies [12]. Contrasting hypothesizes of diamond formation have been published and hotly discussed up to present [12—14, 17, 18, 20—25 and others]. Regardless of formation process, the deposit belongs to a special type with no direct relation to magmatic processes.
The investigations of carbon mineralization of the Kudymkol diamond deposit have been carried out on a high-resolution transmission electron microscope FEI TECNAI F20 equipped with an energy-dispersive X-ray spectrometer (EDX), an energy filter (GATAN imaging filter), a scanning transmission electron microscopy (STEM) unit, and a digital CCD-Camera. The operating voltage of the microscope was 200 kV. The samples for the study have generally been prepared by applying the powdered material onto a copper grid with a holey carbon film. For the following results, it is important to note that the analysis of the carbon particles was performed in regions which were lying above holes of the supporting carbon films. The experimental conditions were carefully chosen in such a way that radiation damage by the high energy electrons could be avoided.
As a first step, carbon particles for the analysis were chosen in the “bright field” mode (BF), at intermediate magnification, controlling chemical purity and the absence of overlapping parts with STEM and EDX. Subsequently, high resolution transmission electron microscopy (HRTEM) studies were performed at mainly magnifications of 600,000— 900,000 times, which allowed the recording of images of the crystal lattice at near atomic resolution. The analysis of carbon particles within TEM-specimens was carried out while monitoring their structural properties by means of electron diffraction, Fourier transformation of high resolution images, and also by monitoring the chemical composition with EDX, STEM, and in some cases electron energy loss spectroscopy (EELS). The selected areas for diffraction from a particle were about 500 nm in diameter. The spectroscopic data were collected from areas with diameters of about 100—200 nm.
Results
Among natural carbon phases, we have analyzed particles that optically looked like diamonds and graphite. However, according to preliminary X-ray data, they exhibit different levels of ordering from well crystallized up to almost X-ray amorphous (Shumilova, 1996), which had been even observed for particles possessing clear crystalline morphological elements. In order to gain more insight into structural details at the nano-level, we performed HRTEM studies and have observed numerous varieties of ASNS. In our analysis, we found very special types of structures that were completely different from the carbon materials known to date.
For the definition and description of ASNS we apply the morphological principle of spatial arrangement of structural elements — atoms and their agglomerates — for constructing different morphological crystalline order and noncrystalline arrangements at the atomic scale. In this way, we can compose the different nanoscale morphological elements identified in our studies.
The real crystals are characterized by a well developed atomic lattice (Fig. 1a),
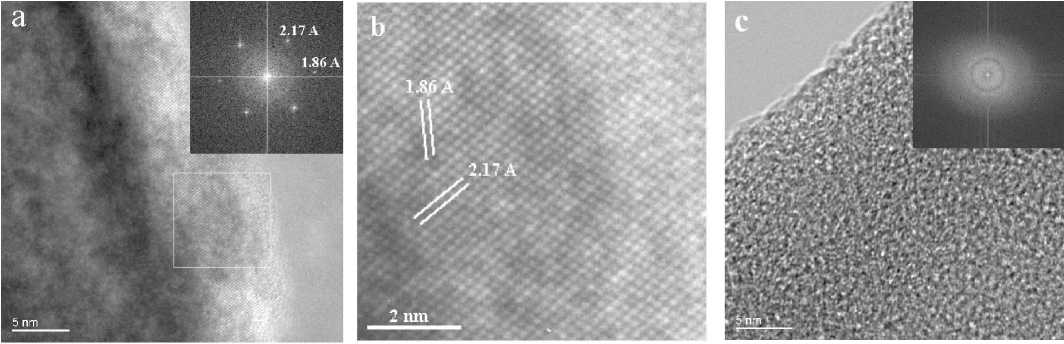
Fig. 1. HRTEM images of ASNS and corresponding Fast Fourier Transformation (FFT) patterns in the inserts (right top corners) with d-spacings of diamond in [110]: a — 3D-ordered crystal ASNS with diamond lattice; b — magnified diamond lattice from 1a image (selected square area); c — amorphous ASNS.
where atomic columns seen in projection are arranged on parallel lattice planes with translational symmetry. In contrast to crystalline ASNS, fully amorphous substances do not possess structural or chemical order even on the nanoscale (Fig. 1b). In addition to these two extreme structural types, amongst natural diamond-like and graphite-like particles, we found ASNS in which crystalline and amorphous aggregates were fully mixed on the atomic level (Fig. 2).
The depicted nanostructures exhibit lattice fringes with locally crystallographically well-defined translational symmetries characteristic of graphite or diamond, and with lattice plane spacings close to the typical interplanar distances of the corresponding crystalline phases. This observation is further supported by
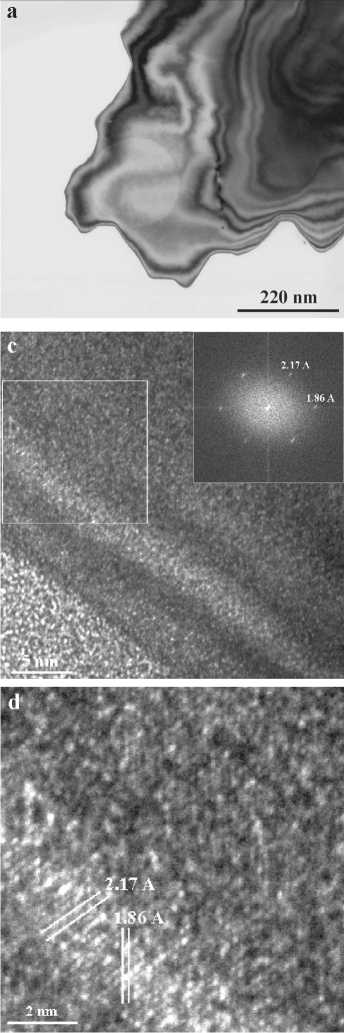
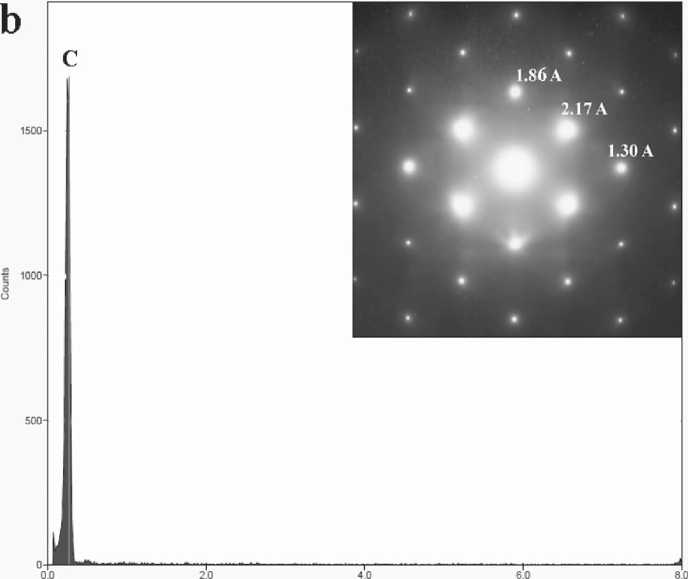
the distinct reflections in the FFT patterns (Fig. 2 c, d) clearly demonstrating the crystalline nature of the phases. The chemical purity of the carbon patterns was supported by EDX data (Fig. 2b). Alignment of nano-crystallites along crystallographic directions was proved by electron diffraction patterns (Fig 2b, right corner inset, ED region is pointed by cycle).
The observed structures are distinctly different from just a multiphase mixture of nanocrystals and amorphous material. Although the typical dimensions of the ordered regions with irregular morphology are not larger than 1 nm, it can be recognized, either visually in the HRTEM images or by inspecting their FFT, that there exists a definite tendency of spatial alignment of the nanocrystals extending over the whole image area. If the volume fraction of the crystallites within a chosen area of a new structure (ASNS) becomes smaller, the extent of spatial ordering of the nanocrystals reduces and the FFT spots weaken. Unfortunately, we do not have information about the 3D distribution of crystalline and amorphous regions.
In Figure 3, three different variants of the ordered ASNS from the same sample are shown, which display a decreasing content of the crystalline phase and an increasing content of the amorphous
Energy (KeV)
Fig. 2. TEM data for carbon particle with mixed ASNS: a — bright field observation image with the ED region (the faint circled area in a center); b — corresponding EDX spectrum, presenting pure carbon content; c — HRTEM image with corresponding full square FFT and; d — magnified image from the boxed area (left) in image 2c. The beam direction is [110] for diamond
phase. In particular in the first two cases, the FFT clearly demonstrates a distinct mutual alignment of nano-crystallites along chosen crystallographic directions.
Similar observations have also been made in more structurally ordered variants, where the arrangement of the graphitic planes is non-planar (Fig. 4).
Discussion
According to the results of our present studies, we propose the existence of a new type of atomic scale nanostructures that is represented by an intermediate state of solid carbon, between ordered and disordered, and is thus constituted by a metastable mixture of both. Following these findings we present a new categorization scheme of ASNS of carbon substances, which is defined by the morphological principle of spatial composition of structural elements — atoms and their ordered arrangements forming nanoscale morphological elements (Table 1).
According to the classification offered by us, ASNS are subdivided into 3 groups which specify the general ordering principles: ordered, disordered and mixed ASNS. As specific characteristics of these three groups, we use the level of dimensionality of the inherent order, the prevailing general structural ordering
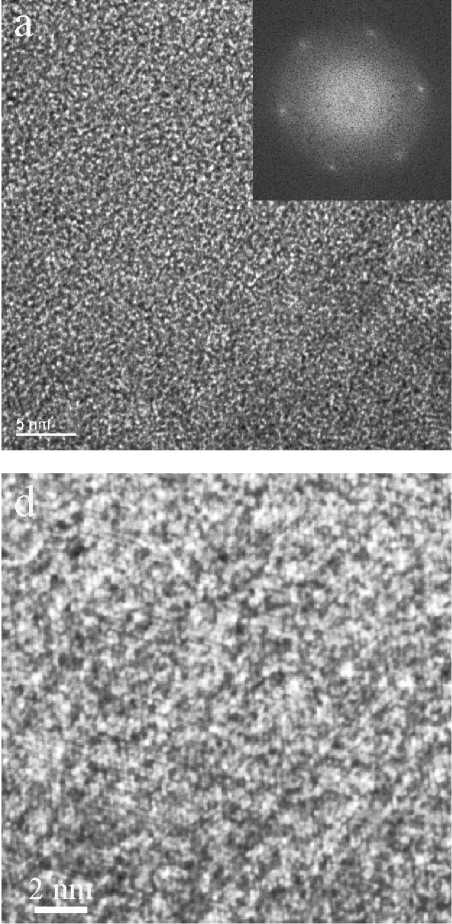
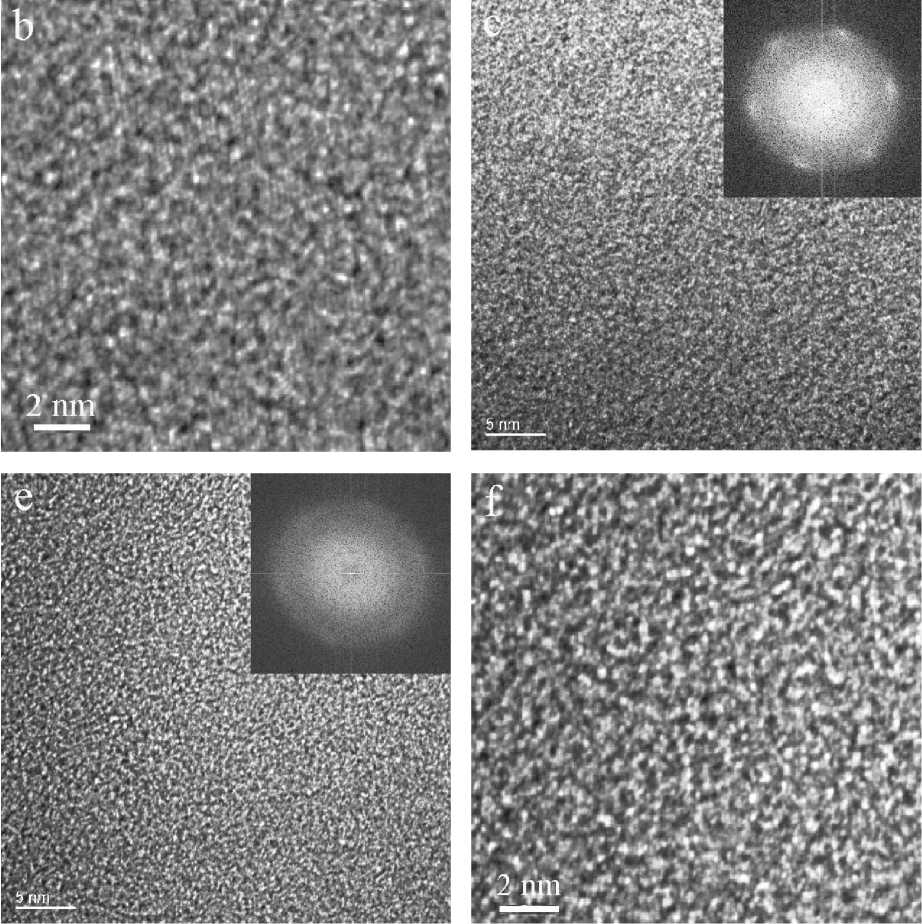
Fig. 3. HRTEM images at medium and high magnification and corresponding FFT of intermixed ASNS of the experimentally observed pseudo-diamond with d-spacing 1.26 (220) down the [111] direction: a, b — with predominating ordered structural elements; c, d — with equal ordered and amorphous elements; e, f — predominating amorphous structural elements
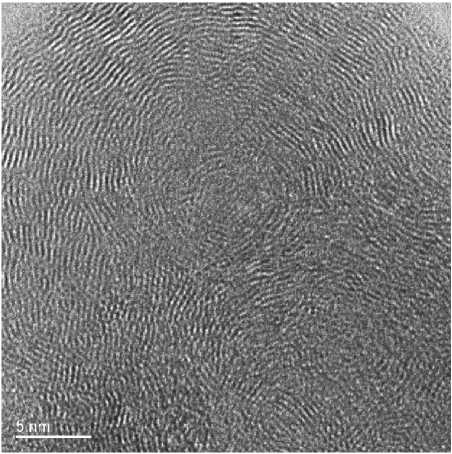
Fig. 4. HRTEM image of mixed ASNS of carbon with the non-planar arrangement of the graphitic planes
principle and the hybridization state of the carbon atoms. It should be noted that carbon atoms can not only have sp-, sp2-, and sp3-hybridization states, as widely accepted, but also intermediate states — spx, where 1 Ordered ASNS The ordered ASNS type is characterized by two variants — 3D-ordered crystals and low-dimensional nanostructures. In HRTEM images, the ASNS are represented by well recognizable atomic fringes, forming a three-dimensional lat- tice for the first variant and planar, linear and curved structural arrangements for the second variant, respectively. The ordered ASNS possess a mono-variant hybridization state of the carbon atoms. The carbon substances having the ASNS of the described type are listed in Table 2. Disordered ASNS The substances with disordered ASNS are comprised of irregular arrangements of carbon atoms. These ASNS are characterized by random positions of atoms and small clusters and correspondingly by the absence of sharp reflection spots in the FFT patterns. The structural composition principle of disordered ASNS can be described by the random Classification of atomic scale nanostructures of carbon substances Table 1 Type Ordered Level of 3D-ordered dimensional crystals Low-dimensional order nanostructures Structural Planar (2D) ordering Lattice (3D) Curved (2D) principle Linear (ID) Hybridization ,. • . 3 Monovariant state Mixed Disordered Amorphous Amorphous with 3D-ordered with low-dimensional Amorphous elements ordered elements Pseudo-layer Pseudo-lattice Pseudo-chain Cluster (OD) Pseudo-sphere Mono+polyvariant Polyvariant Table 2 Structural Carbon hybridization state state sp3 sp2 sp spx, x ^ L 2, 3 Admixture Ordered Diamond Hexagonal graphite Chaoite Fullerites Peapods Rhombohedral graphite ct-carbyne Cubic graphite P-carbyne Lonsdaleite Graphene Linear-polyyne Fullerenes Nanotubes/nanofibers Linear-polycumulene Onions Ultrathin nanotubes Mixed Pseudodiamond Pseudo-graphite Pseudo-carbyne Soot-like carbon Diamond-like carbon Graphite-like carbon Carbyne-like carbon Glassy-like carbon Amorphous Carbon glass Amorphous carbon *Theoretically probable phases which have not been discovered in natural or artificial materials are set in italics. Carbon substances agglomeration of clusters without any mutual crystallographic coordination between them. In terms of the hybridization state, the substances with the disordered ASNS type have to be represented by poly-variant carbon atoms. Mixed ASNS The type of mixed (intermediate) ASNS reported by us in the present studies, is characterized by the simultaneous presence (overlapping) of both ordered and disordered structural motives which can be identified in the HRTEM images and their Fourier transformations. According to the level of dimensional order we divide the ASNS into two variants: amorphous with either three-dimensional or low-dimensional ordered elements (Table 1). The mixed ASNS type is represented by different systems of nanoscale regions with crystalline order which, at the same time, have long-range order with respect to their crystallographic orientation, as is evidenced by the presence of corresponding reflection spots in the FFT images. The nanoscale ordered regions are surrounded by disordered (amorphous) matter as a dense atomic-scale intercalation. The nanoscale order in this case consists of pseudolattice, -layer, -chain and -sphere arrangements of atoms, which are the general structural principles of the mixed ASNS variant (Table 1). At present, we use only a qualitative estimation of the prevalence of ordered or amorphous structural elements; however in future, the development of quantitative description criteria is also possible. As the pseudolattice connecting the crystallographic orientation of individual nanoscale regions with crystalline order is charac- terized by distances corresponding well to highly ordered macroscopic substances, we propose to use the terms pseudo-diamond, pseudo-graphite and pseudo-carbyne (Table 2) to name substances with the mixed ASNS. As can be demonstrated by the present experimental data, the definition of the mixed ASNS can be only made on the basis atomic scale characterization results, since other methods with lower spatial resolution, such as X-ray or electron diffraction, cannot identify the occurrence of structural ordering in a mixture of amorphous and nanocrystalline intercalated structural elements. Conclusion On the basis of a detailed study of natural carbon substances by HRTEM, a new type of atomic scale nanostructures — the mixed ASNS type has been established. It can be considered as a new structural ordering principle not only for carbon substances but also for any other similar type of solid matter. We suppose that the new ASNS type can have a fundamental value for nanoscience, as well as for theoretical and experimental modeling of solid matter based on the principles established in natural minerals. Acknowledgments The authors greatly thank A. Solo-gubenko and Th. E. Weirich for help in TEM study and A. A. Zayachkovsky for providing the mineral material from the Kumdykol diamond deposit, and the German Academic Exchange Service DAAD (project # 325), the UB RAS (the projects # 12-C-5-1035) for financial support.
Список литературы Новый тип наноструктур атомарного разрешения в алмазоподобных и других углеродных веществах
- Shumilova T. G., Akai J. Natural carbon nanophases. Syktyvkar: Geoprint, 2004. 20 p..
- Kovalevski V. V., Buseck P. R., Cowley J. M. Comparison of Carbon in shungite rocks to other natural Carbons: An X-ray and TEM study//Carbon, 2001. # 39, 243-256.
- Banhart Z. M. Radiation-Induced Transformation of Graphite to Diamond//Phys. Rev. Let., 1997. 79, # 19, 3680-3683.
- Dresselhaus M. S. Future directions in carbon science//Annu. Rev. Mater. Sci., 1997. 27, 1-34.
- El-Barbary A. A., Trasobares S., Ewels C. P. Electron spectroscopy of carbon materials: experiment and theory//Journal of Physics. Conference Series, 2006. # 26, 149-152.


