Numerical simulation of radiotherapy beam interaction with soft tissues and pla plastic for 3D printing of dosimetric phantoms
Автор: Miloichikova I.A., Bulavskaya A.A., Polomoshnova D.A., Saburov V.O., Stuchebrov S.G.
Журнал: Сибирский онкологический журнал @siboncoj
Рубрика: Лабораторные и экспериментальные исследования
Статья в выпуске: 6 т.23, 2024 года.
Бесплатный доступ
Introduction. In the development of new methods of radiotherapy, studies of the biological effects of sparsely (photons, electrons) and densely (protons, ions) ionizing radiation are relevant. Reproducibility is a challenge in preclinical studies. Dosimetric phantoms of laboratory animals are an effective tool for dose assessment, facilitating standardization of tests conducted under different conditions. Existing phantoms often fail to address radiobiological issues like placing of biological samples or dosimetry detectors. A method for manufacturing dosimetric phantoms must be developed to accurately manufacturing products and modify their design in accordance with the task.
Dosimetric phantom, preclinical studies, numerical simulation, monte carlo method, percentage depth dose distribution, 3d printing technologies, pla plastic
Короткий адрес: https://sciup.org/140308738
IDR: 140308738 | УДК: 615.849.12:53.043 | DOI: 10.21294/1814-4861-2024-23-6-62-69
Текст научной статьи Numerical simulation of radiotherapy beam interaction with soft tissues and pla plastic for 3D printing of dosimetric phantoms
In the contemporary medical community, the issue of oncological diseases continues to be a significant concern [1]. It is established that approximately half of all cancer patients undergo radiotherapy at some stage of their treatment [2]. The development of new therapeutic approaches relies heavily on the results of preclinical studies.
The objective of large-scale multicentre preclinical studies is to enhance the efficacy of radiotherapy for malignant neoplasms. Of particular interest are studies of the biological effects of protons and ions, methods for increasing the conformity of irradiation with electron and photon irradiation, and a combination of photons and densely ionizing radiation in one course [3, 4]. In the context of preclinical studies, the question of reproducibility of experimental results obtained on different systems and with different types of ionizing radiation, specifically the assessment of the radiation dose, is a significant consideration [5]. To address these challenges, dosimetric phantoms offer a valuable solution. Their design enables the placement of a cell culture in the area of interest or the simulate of a laboratory animal’s anatomy, with the option of incorporating ionizing radiation detectors [6]. However, existing standard phantoms frequently fail to satisfy the requisite criteria for addressing specific radiobiological issues. Consequently, there is a pressing need to devise a methodology for the fabrication of tissue-equivalent phantoms that are suitable for the experimental validation of radiation dose in preclinical studies.
Three-dimensional printing technology has the potential to revolutionize the field of radiation medicine by enhancing precision, effectiveness, and personalization, ultimately improving patient outcomes and the quality of care provided [7, 8]. The creation of bespoke treatment plans entails the generation of 3D-printed anatomical models derived from imaging data, thereby enabling oncologists to visualize tumors and adapt therapies to the specific anatomy of each individual [9]. The fused filament fabrication method has been demonstrated to be an effective approach for the creation of dosimetry phantoms [10]. This method is distinguished by its relatively low cost in comparison to other fabrication techniques, its capacity to create models derived from tomographic three-dimensional data sets, its high degree of accuracy in reproducing complex phantom geometries, and its versatility in simulating a wide range of tissue properties through the use of diverse materials [11].
One of the most commonly used materials for the creation of objects via 3D printing methods is PLA plastic (polylactide) [12]. PLA is derived from renewable resources, such as cornstarch, which renders it more biocompatible than some petroleum-based plastics [13]. Due to its relatively low atomic number, PLA is an appropriate material for simulating soft tissue in radiotherapy [14]. The capacity to modify the settings for manufacturing products using the fused deposition method permits the fabrication of objects with varying physical density by altering the fill factor during printing [15]. A solid-state dosimetric phantom for preclinical studies, produced using the fused filament fabrication method, should replicate the characteristics of biological tissues with a specified degree of accuracy in regard to their interaction with sparsely (photons and electrons) and densely (protons and ions) ionizing radiation.
In order to develop new dosimetric phantoms, it is necessary to evaluate the possibility of using PLA plastic for their production, which will be carried out using the fused filament fabrication method. Numerical simulation methods may be employed for this purpose. Monte Carlo method is widely used to calculate the dose distribution of radiotherapy [16]. This method is integral to advancing medical technology and improving patient care through their applications in radiotherapy, medical imaging, drug delivery, and epidemiology [17, 18].
The objective of this study is to develop a numerical model to simulate the interaction between PLA plastic objects manufactured by fused filament fabrication and various types of ionizing radiation used in radiotherapy. Additionally, the study aims to determine the optimal 3D printing parameters of the PLA plastic samples for imitating soft tissues.
Material and Methods
Software for numerical simulation
A numerical simulation is conducted utilising the Geant4 toolkit (version 10.2p02) [19], which processes the parameters of the beam and the nature of particle interaction with a range of materials. The Geant4 toolkit is based on the Monte Carlo method, which relies on random sampling to obtain numerical results. This method is particularly useful for complex systems where deterministic methods may be insufficient. It is used to simulate the radiation transport in monitoring systems, beam-shaping devices, dosimetry phantoms, and biological tissues [18, 20]. In this work, the QBBC Physics List was applied, which is the most widely used in medical physics simulation [21, 22].
Materials under study
In the context of preclinical testing, products that imitate soft tissues are of particular practical interest [23, 24]. In the presented research, the most common biological tissues, namely adipose and muscle, were selected for analysis. In order to create numerical models of biological tissues, the chemical composition and physical density were determined on the basis of data sourced from the literature [25].
In this study, PLA plastic was selected as the material for investigation in the context of threedimensional printing. PLA plastic offers a number of advantages for 3D printing. PLA is relatively straightforward to print with, as it adheres well to the print bed and typically requires lower temperatures compared to other materials, thereby reducing the risk of warping. Furthermore, the production of odour is minimal during the printing process, and the resulting finish is smooth, which makes it the optimal choice for detailed models. Furthermore, PLA plastic is biodegradable under the appropriate conditions, which contributes to the broader objective of sustainable manufacturing practices [13, 14]. The parameters for modelling PLA plastic were determined based on a review of the literature [14].
The creation of the PLA plastic simulation model involved the utilisation of varying densities, given table/Таблица
Parameters of the investigated materials used for the numerical simulation [14, 25, 27]
Параметры исследуемых материалов, используемых для создания модели [14, 25, 27]
|
Parameter/Параметр |
Natural PLA plastic/ Натуральный ПЛА пластик |
Adipose/ Жировая ткань |
Muscle/ Мышечная ткань |
|
Content of elements (by weight)/ Элементный состав (по весу), % |
C – 50.0; H – 5.6; O – 44.4 |
H – 11.1; C – 29.7; N – 0.9; O – 58.0; Na – 0.1; P – 0.1; S – 0.1 |
H – 10.4; C – 10.3; N – 2.4; O – 76.2; Na – 0.1; P – 0.1; S – 0.1; Cl – 0.2; K – 0.2 |
|
Density, g/cm3/Плотность, г/см3 |
0.85–1.18 |
0.91 |
1.06 |
|
Mean excitation energy, eV/ Средняя энергия возбуждения, эВ |
77.9 |
70.2 |
74.7 |
Примечание: таблица составлена авторами.
Note: created by the authors.
that the density of the printed sample can be modified through alterations to the fill factor during the manufacturing by 3D printing. In order to ascertain the physical densities of printed products derived from PLA plastic, cubic objects with dimensions of 2×2×2 cm were produced on an Original Prusa i3 MK3s 3D printer utilising the fused filament fabrication method. The following 3D printing parameters were employed during the printing process: nozzle diameter, 0.4 mm; layer thickness, 0.3 mm; number of perimeters, 1; number of lower layers, 0; number of upper layers, 0; print speed, 60 mm/s; extruder temperature, 195 °C; table working surface temperature, 65 °C; fill factor, 70 % to 100 %. It was determined that a fill factor of at least 70 % was necessary to avoid the formation of excessive air voids within the product, which would be undesirable for the fabrication of phantoms [15]. Consequently, the range of sample densities that was printed from natural PLA plastic was established, spanning from 0.85 g/cm³ to 1.18 g/cm³ with the step of 0.01 g/cm³. The relationship between the fill factor and sample densities was established.
Models of adipose and muscle tissues, as well as the PLA plastic, were created in accordance with the parameters that must be taken into account in numerical modelling using the Geant4 toolkit. These parameters include density, chemical composition, and average excitation energy during interaction with ionizing radiation. Based on the available data on the density and chemical composition of biological tissues and PLA plastic, the average excitation energy was determined using the international database [26].
The resulting values were then used to create models of all the studied materials, as presented in Table.
Simulated geometry
A numerical model was constructed for photons with an energy of 1.25 MeV (representative of the average energy of gamma radiation emitted by the Co-60 isotope), electrons with an energy of 6 MeV, protons with an energy of 150 MeV, and carbon ions with an energy of 300 MeV/nucleon. The selected types of ionizing radiation and beam energies align with the parameters typically employed in radiotherapy [27].
The geometry illustrated in Figure 1 was utilised as the basis for the ensuing numerical experiments. A flat square 10×10 cm beam with uniform distribution of particles over the source area (No. 1 in Fig. 1) was selected as the radiation source for photon and electron beams simulation. The source was located in a vacuum chamber at a distance of 1 mm from the surface of a phantom measuring 30×30×30 cm, constructed from the material under study (No. 2 in Fig. 1), in accordance with the standard irradiation parameters. In the case of proton and carbon ion beams simulation, a circular beam with a diameter of 1 cm was employed. The geometry of the calculation phantom was selected to align with that of a standard solid-state tissue-equivalent plate phantom. In the calculation, the phantom was divided into elementary sensitive
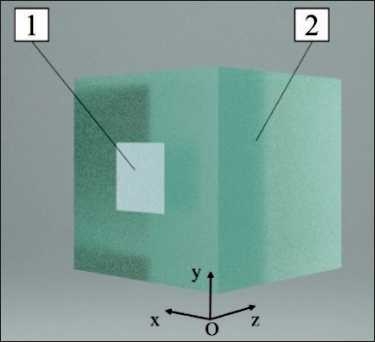
Fig. 1. The numerical simulation geometry. Notes: 1 – ionizing radiation source; 2 – investigated material; created by the authors Рис. 1. Геометрия численного моделирования.
Список литературы Numerical simulation of radiotherapy beam interaction with soft tissues and pla plastic for 3D printing of dosimetric phantoms
- Zhuĭkova L.D., Choĭnzonov E.L., Ananina O.A., Odintsova I.N. Onkologicheskaya zabolevaemost' v Cibirskom i Dal'nevostochnom federal'nykh okrugakh. Sibirskii onkologicheskii zhurnal. 2019; 18(6): 5-11. https://doi.org/10.21294/1814-4861-2019-18-6-5-11.
- Sostoyanie onkologicheskoĭ pomoshchi naseleniyu Rossii v 2023 godu. Pod red. A.D. Kaprina, V.V. Starinskogo, A.O. Shakhzadovoi. M., 2024. 262 s.
- Koryakina E.V., Potetnya V.I., Troshina M.V., Efimova M.N., Baĭkuzina R.M., Koryakin S.N., Lychagin A.A., Pikalov V.A., Ul'yanenko S.E. Sravnenie biologicheskoi effektivnosti uskorennykh ionov ugleroda i tyazhelykh yader otdachi na kletkakh kitaiskogo khomyachka. Radiatsiya i risk. 2019; 28(3): 96-106.
- Suckert T., Müller J., Beyreuther E., Azadegan B., Brüggemann A., Bütof R., Dietrich A., Gotz M., Haase R., Schürer M., Tillner F., von Neubeck C., Krause M., Lühr A. High-precision image-guided proton irradiation of mouse brain sub-volumes. Radiother Oncol. 2020; 146: 205-12. https://doi.org/10.1016/j.radonc.2020.02.023.
- Desrosiers M., DeWerd L., Deye J., Lindsay P., Murphy M.K., Mitch M., Macchiarini F., Stojadinovic S., Stone H. The Importance of Dosimetry Standardization in Radiobiology. J Res Natl Inst Stand Technol. 2013; 118: 403-18. https://doi.org/10.6028/jres.118.021.
- Esplen N., Therriault-Proulx F., Beaulieu L., Bazalova-Carter M. Preclinical dose verification using a 3D printed mouse phantom for radiobiology experiments. Med Phys. 2019; 46(11): 5294-303. https://doi.org/10.1002/mp.13790.
- Manmadhachary A., Malyala S.K., Alwala A.M. Medical applications of additive manufacturing. Proceedings of the International Conference on ISMAC in Computational Vision and Bio-Engineering. Springer International Publishing. 2019; 1643-53. https://doi.org/10.1007/978-3-020-00665-5_152.
- Tino R., Yeo A., Leary M., Brandt M., Kron T. A Systematic Review on 3D-Printed Imaging and Dosimetry Phantoms in Radiation Therapy. Technol Cancer Res Treat. 2019; 18. https://doi.org/10.1177/1533033819870208.
- Jusufbegović M., Pandžić A., Šehić A., Jašić R., Julardžija F., Vegar-Zubović S., Beganović A. Computed tomography tissue equivalence of 3D printing materials. Radiography (Lond). 2022; 28(3): 788-92. https://doi.org/10.1016/j.radi.2022.02.008.
- Okkalidis N. 3D printing methods for radiological anthropomorphic phantoms. Phys Med Biol. 2022; 67(15). https://doi.org/10.1088/1361-6560/ac80e7.
- Salmi M. Additive Manufacturing Processes in Medical Applications. Materials (Basel). 2021; 14(1): 191. https://doi.org/10.3390/ma14010191.
- Savi M., Andrade M.A.B., Potiens M.P.A. Commercial filament testing for use in 3D printed phantoms. Radiation Physics and Chemistry. 2020; 174(9). https://doi.org/10.1016/j.radphyschem.2020.108906.
- Cojocaru V., Frunzaverde D., Miclosina C.O., Marginean G. The Influence of the Process Parameters on the Mechanical Properties of PLA Specimens Produced by Fused Filament Fabrication-A Review. Polymers (Basel). 2022; 14(5): 886. https://doi.org/10.3390/polym14050886.
- Mille M.M., Griffin T.K., Maass-Moreno R., Lee C. Fabrication of a pediatric torso phantom with multiple tissues represented using a dual nozzle thermoplastic 3D printer. Journal of Applied Clinical Medical Physics. 2020; 21:(11): 226-36. https://doi.org/10.1002/acm2.13064.
- Auffray L., Gouge P.A., Hattali L. Design of experiment analysis on tensile properties of PLA samples produced by fused filament fabrication. The International. Journal of Advanced Manufacturing Technology. 2022; 118(3): 4123-37. https://doi.org/10.1007/s00170-021-08216-7.
- Larsson E., Ljungberg M., Strand S.E., Jönsson B.A. Monte Carlo calculations of absorbed doses in tumours using a modified MOBY mouse phantom for pre-clinical dosimetry studies. Acta Oncol. 2011; 50(6): 973-80. https://doi.org/10.3109/0284186X.2011.582517.
- Özseven A., Ümit K. Verification of Percentage Depth-Doses with Monte Carlo Simulation and Calculation of Mass Attenuation Coefficients for Various Patient Tissues in Radiation Therapy. Süleyman Demirel Üniversitesi Sağlık Bilimleri Dergisi. 2020; 11(2): 224-30. https://doi.org/10.22312/sdusbed.705468.
- Radiation Therapy Dosimetry: A Practical Handbook. Ed by A. Darafsheh. CRC Press. 2021. 504 p.
- Geant 4 [Internet]. CERN, Geneva, c1998-2024 [cited 2024 Sep 1]. URL: https://geant4.web.cern.ch.
- Mettivier G., Guatelli S., Brown J., Incerti S. Advances in Geant4 application in Physics, Medicine and Biology frontiers. Phys Med. 2024; 124. https://doi.org/10.1016/j.ejmp.2024.103371.
- Ribon A., Apostolakis J., Dotti A., Folger G., Ivanchenko V., Kosov M., Uzhinsky V., Wright D.H. Status of GEANT4 hadronic physics for the simulation of LHC experiments at the start of LHC physics program. CERN-LCGAPP. 2010; 2.
- Miloichikova I., Bulavskaya, A., Bushmina E., Dusaev R., Gargioni E., Gavrikov B, Grigorieva, A., Stuchebrov S. Development and verification of a Geant4 model of the electron beam mode in a clinical linear accelerator. Journal of Instrumentation. 2024; 19(7). https://doi.org/10.1088/1748-0221/19/07/C07007.
- DeWerd L.A., Kunugi K. Accurate Dosimetry for Radiobiology. Int J Radiat Oncol Biol Phys. 2021; 111(5): 75-81. https://doi.org/10.1016/j.ijrobp.2021.09.002.
- De Almeida C.E., Salata C. Absolute, reference, and relative dosimetry in radiotherapy. Dosimetry. IntechOpen. 2022. https://doi.org/10.5772/intechopen.101806.
- ICRP [Internet]. [cited 2024 Sep 10]. URL: https://www.icrp.org/index.asp.
- National Institute of Standards and Technology [Internet]. ESTAR [cited 2024 Sep 10]. URL: https://physics.nist.gov/PhysRefData/Star/Text/ESTAR.html.
- Khan F.M., Gibbons J.P. Khan’s the physics of radiation therapy. 5th ed. Lippincott Williams & Wilkins. 2014. 624 p.
- Absorbed Dose Determination in External Beam Radiotherapy: An International Code of Practice for Dosimetry Based on Standards of Absorbed Dose To Water. Vienna: International Atomic Energy Agency, 2024. https://doi.org/10.61092/iaea.ve7q-y94k.


